
Research Article
Austin J Med Oncol. 2021; 8(3): 1069.
Single Voxel ¹H-MR Spectroscopy in the Human Spinal Cord at 3T -Preliminary Results
Wawrzyniak P¹, Hebda A¹, Heinze S²* and Bobek-Billewicz B¹
¹Department of Radiology, Maria Sklodowska-Curie National Research Institute of Oncology, Gliwice, Poland
²Department of Radiology, Maria Sklodowska-Curie National Research Institute of Oncology, Cracow Branch, Poland
*Corresponding author: Sylwia Heinze, Department of Radiology, Maria Sklodowska-Curie National Research Institute of Oncology, Cracow Branch, Poland
Received: October 16, 2021; Accepted: November 08, 2021; Published: November 15, 2021
Abstract
Purpose: ¹H-Magnetic Resonance Spectroscopy is a non-invasive technique that provides information on tissue metabolism and biochemistry. Because of technical difficulties, this method is rarely used in the spinal cord examination. The main goal of this study was to develop a routine protocol for MRS of intramedullary lesions.
Material and methods: ¹H-MRS protocol was set on a group of healthy volunteers. 48 spectra were acquired in total. 30 of them were acquired in cervical spinal cord and the remaining (18 spectra) were acquired in the thoracic spinal cord.
Results: In ¹H-MRS of the spinal cord one of the most important problem is small voxel size. Mean voxel size in this study was 7x9x29 mm - what is much smaller than in the brain examinations finally, almost 60% of spectra were of acceptable quality in volunteer examinations, what enabled the following patients’ examinations.
Conclusions: Challenges of spinal cord spectroscopy were discussed and the ability of providing additional diagnostic information was proved.
Keywords: Magnetic resonance spectroscopy (MRS); Spinal cord MRS; Single voxel MRS
Introduction
¹H-Magnetic Resonance Spectroscopy (¹H-MRS) is a noninvasive technique that provides information on tissue metabolism and biochemistry [1], what is important from a clinical point of view - it allows for better lesion differentiation and appropriate treatment application, with no need to perform invasive diagnostic procedure. It can be particularly useful in cases of the intramedullary lesions because of the high risk of serious biopsy and surgery complications [2]. The main goal of this study was to develop a routine protocol for MRS of intramedullary lesions.
Although the ¹H-MRS technique itself is known and successfully used for years in brain examinations (e.g. for lesion differentiation), to obtain diagnostic spectra of the spinal cord is not an easy task. Differential diagnosis of the intramedullary lesions based on medical imaging is more troublesome. Implementing ¹H MRS in spinal cord might bring additional parameter to differentiate these lesions with more confidence. There are however many technical difficulties associated with spinal cord ¹H-MRS to overcome: low signal to noise ratio associated with small voxel size (voxel size is limited by spinal cord dimensions), field inhomogeneity caused by Cerebrospinal Fluid (CSF) flow influenced by the cardiac and the respiratory movements, as well as the patient body motions.
The first approach to this topic task was taken in 1997 by Gómez- Ansón et al. [3] - described examinations were performed in a cervical spinal cord in healthy volunteers. Authors achieved good quality data and made an attempt to metabolite quantification, and proved that the concept of 1H-MRS in spinal cord is possible and technically achievable. Next, several studies in healthy volunteers were made, most of them aimed to overcome mentioned above difficulties. First major paper was published in 2004 by Cooke et al. [4]. Author’s meticulous approach was a real breakthrough in the ¹H-MRS of the spinal cord. With 2T spectrometer and purpose-built quadrature surface coil Cooke et al. tackled the most important problems of field homogeneity, voxel size, spinal cord movement and metabolite quantification. Several conference proceedings [5-7], and scientific papers [8-11] sprout to further explore the topic. Their main goal was to improve the methodological aspect, but studies with Multiple Sclerosis (MS) patients were also performed. Another milestone work was published by Henning et al. [12]. It not only explored in details other spinal cord regions, but was also the first work on spinal cord tumors (apart from Dydak’s et al. short paper [6]). Tumor group was small (2 patients only) however, metabolite ratios changes observed in brain tumors were present in cases of spinal cord tumor too. Hock et al. [13] reviewed all the mentioned above papers up to date and described what state of the art ¹H-MRS acquisition should include. Hock’s et al. other study was concerned on improvement the acquisition scheme to acquire higher spectra quality [14-17]. However, the state of the art protocol reported previously was still viable.
Materials and Methods
Study Population
To establish proper acquisition parameters, ¹H-MRS was tested on a group of healthy volunteers (n=12, 9 males and 3 females); 48 spectra were acquired in total. 30 of them were acquired in cervical spinal cord and the remaining (18 spectra) were acquired in the thoracic spinal cord. In the end, roughly all extent of spinal cord was tested for viability of spectroscopic acquisition. Volunteers had no history of spinal cord injury or disease. Written informed consent was obtained from all of them prior to MRI examination, and they had no contradictions to perform such a study. Examinations were performed on a 3T Siemens Prisma scanner with various acquisition parameters until desired spectra quality were achieved. Each study lasted approximately one hour. All the procedure was in accordance with the Helsinki Declaration and the ethical standards of the responsible local ethics committee.
Experimental
For every volunteer exam multiple ¹H-MRS acquisitions with varying parameters were performed, to check their impact on spectra quality. Base parameters were chosen according to guidelines presented in major methodological papers [4,12,13], but several adjustments were made to acquire the highest quality on 3T system. Single Voxel - Point Resolved Spectroscopy (SVS-PRESS) sequence with water suppression was used to acquire short and long echo time spectra (30 and 135 ms, respectively). RF coils were chosen depending on the examined region; for the cervical spinal cord - neck elements of the multichannel head coil array were used (20 channel head coil), for the thoracic region, elements from multichannel spinal coil array were used (32 channel spinal coil). For every acquired spectrum coil elements closest to MRS Voxels were used for signal reception, so only a part of coil array was used as described by Henning et al. [12]. Before final placement of the spectroscopy voxel, high resolution, isotropic T2 images were acquired (TR/TE 1500/131 ms, 0.8 mm³ voxel, 1.4 Nex, Flip Angle (FA) 140o, Field of View (FoV) 250x250, iPAT 2 GRAPPA). Hock et al. [13] outlined, that maximum effort must be applied to ensure adequate voxel placement, what is possible only if high resolution images showing boundaries between spinal cord and CSF are obtained. Due to small size of spinal cord, maximal voxel size was confined to its dimensions (voxel volume varied between 1.1 and 2.78 cm³ (mean 1.84 ± 0.48 cm³). Average dimensions in every direction were 7x9x29 mm. To keep the SNR at the sufficient level spectra were heavily averaged: 256 for TE 30 ms and 320 for TE 135 ms. ECG gating was used to avoid influence of the spinal cord movement due to cerebrospinal fluid (CSF) flow. Acquisition was delayed by 300 ms to the R wave of ECG signal to acquire spectra only in diastole, when CSF flow is slowest. Repetition Time (TR) of 2000 ms was used, but real time between excitation pulses was determined by subject Heart Rate (HR). To prevent CSF flow problem and eliminate spectra contamination by the lipid signal from the bones 6 saturation bands were used at top, bottom, Right, Left (R-L), Anterior and Posterior (A-P) directions. Top and bottom one fine-tuned to saturate water signal (to negate CSF flow) and R-L and A-P ones fine-tuned to saturate fat (to negate lipid contamination from the bones). Because of high number of averages and additional ECG gating total acquisition times were 10:52 min for TE 135 ms and 8:34 min for TE 30 ms. Soft pads were placed under subject legs and shoulder to provide highest level of comfort in supine position. Additionally, when requested, a flat soft pad was placed under subject lumbar spine. Blanket was provided for thermal comfort when needed. Every volunteer was also informed of crucial effect of any movement on ¹H-MRS acquisition quality. Before each measurement a manual shimming routine was performed to minimize Full Width at Half Maximum (FWHM) of the reference water peak and to ensure that spinal cord is on resonance as described by Cooke et al. [4]. During acquisition single spectral averages were observed, when a high, broad peak was seen at lipid and macromolecule part of the spectrum, the acquisition was stopped (in most cases that happened because of volunteer movements, so the signal was received from the bones). Next, high resolution T2 acquisition was repeated, with precise readjustment of voxel position.
Spectrum post processing
To improve the spectra quality, they were analyzed by an experienced user with the Syngo software (Siemens AG, version VE61A) to look for severe lipid contamination, peak overlap or other signs of bad spectra quality. After initial quality control, the chosen spectra were analyzed with LC-Model software (version 6.1-4F) [18] (Figure 1).

Figure 1: Example spectrum acquired in this study (post-processed with the LC-Model software).
Standard set of central nervous system Magnetic Resonance Spectroscopy metabolites were fitted to the spectra [20], ones with Crame’r-Rao lower bounds smaller than 25% were considered as present in acquired spectrum. Internal calibration water peak method was used for quantification of metabolite concentration however, no coil sensitivity adjustments were made [19] so calculated concentrations are in units specific to site and scanner used.
Discussion
MRI is a method of choice in spinal cord imaging. Its unique and robust tissue contrast capabilities are unmatched in intramedullary lesion differential diagnosis. Standard sequences are well suited to estimate lesion size and its contrast enhancement as well as any syrinx that is commonly associated with spinal cord tumors. However, newer methods are capable of imaging much more. Diffusion Weighted (DWI) and Diffusion Tensor Imaging (DTI) are one way of looking into tissue microstructure. DWI displays how well water molecules diffuse in the given tissue; it is restricted in area of cytotoxic oedema, high cellularity tumors and abscess. DTI with fibre tracking is great at preoperative planning, when knowledge of certain fibre bundles is crucial for positive surgery outcome. Another diagnostic method is ¹H-MRS. It is a gateway into tissue biochemistry. Knowledge of metabolites that are present only in certain type of tumors or of some level of one metabolite that is related to certain type of tumor can be very helpful in differential diagnosis.
¹H-MRS has an established position in brain lesion differential diagnosis. However, patient movements, low signal to noise ratio associated with small voxel size and field inhomogeneity caused by Cerebrospinal Fluid (CSF) flow and internal organs movement are factors to be dealt with in case of ¹H-MRS in spinal cord. Volunteer examinations are first step in establishing successful acquisition scheme. ¹H-MRS in spinal cord had been summarized in work by Hock et al. [13]. However, any hardware is a bit different, and software is too, sometimes minor, but in most cases major adjustments are needed.
Essential for successful ¹H-MRS in spinal cord is proper voxel positioning. Most papers are focused on cervical spinal cord spectroscopy, where positioning is less troublesome, and attention is drawn to voxel spanning across as few interspinous spaces as possible [4] because shim quality depends on it. In the cervical spinal cord it is relatively easy, but on the cervicothoracic border, where curvature is present, that task becomes much more difficult
In our study, ¹H-MRS Voxel was positioned on T2 isotropic sequence images. Reconstructions were made with planes chosen accordingly to desired spine level, so transversal plane was perpendicular to spinal cord on given level and sagittal/coronal planes were in parallel orientation. Saturation bands were used to reduce CSF flow artifacts, to suppress signals from outside the voxel and to avoid artifacts caused by the chemical shift. In final iteration they were placed as in Hocks et al. [13] review, overlapping with voxel volume. Four saturation bands were distributed to establish proper saturation of lipid signal from the spine. It covered anterior, posterior, left and right plane of positioned voxel. These saturation bands were fine tuned to saturate bone signal, which means they saturated part of spectra at 1.3 ppm. Additionally, two saturation bands, fine-tuned to saturate water (4.7 ppm), with intention to further negate CSF flow effects were placed at cranio-caudal sides of the voxel.
In ¹H-MRS of the spinal cord the most important problem is small voxel size. As mentioned in this paper, voxels dimensions in this study are much smaller than those in the brain examinations. Size of the voxel is crucial in achieving good SNR, because these two parameters have strong correlation, in other words voxel needs to have proper volume or acquisition will last too long. Right-left and anterior-posterior voxel dimensions are fixed for given subject and level of the spinal cord, they are limited by spinal cord dimensions. The cranio-caudal length in volunteers is adjustable, however in patients, the size of the voxel is determined by the lesion size. Cooke et al. [4] found volume of 9x7x35 mm to be optimal in cervical spinal cord, what is achievable in cervical region, but beyond is not. Henning et al. [12] suggested range 4x6x15 mm to 6.5x8.5x27 mm what is closer to average voxel dimensions used in this paper (7x9x29 mm) (Figure 2).
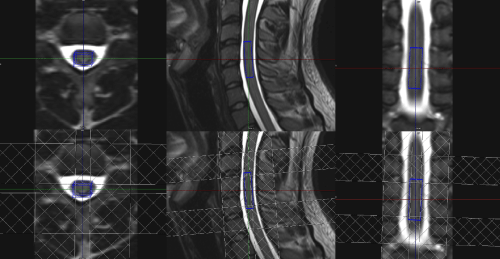
Figure 2: Example voxel positioning - volume and saturation bands.
Cho
Cr
NAA
SNR
Mean
2.2
5.0
7.1
2.2
SD
1.0
2.9
4.0
1.1
Table 1: Mean values of metabolite concentrations with standard deviation in site and scanner specific units.
If voxel size is very small, good SNR is achievable only with sufficient number of averages and good acquisition scheme. According to literature, we used PRESS voxel location method for spinal cord ¹H-MRS, because of its high SNR. When seeking robust acquisition that can be used on patients, number of averages cannot be too high, because of time factor. Every patient is able to be perfectly only still for limited amount of time. Henning et al. [4] proposed 512 averages what leads to acquisition time between 17 and 21 min (depending on HR). For majority of patients this is too long. In this study, 256 averages were used with short echo time (TE) and 320 averages were used with long Echo Time (TE) acquisitions, what is reasonable compromise between SNR and acquisition time. Acquisition time of one spectrum is 10 min, what was not too long to handle by all volunteers. Also, in clinical ¹H-MRS of the brain reference spectra are always acquired. In the spinal cord, because of too long acquisition time, they are not. Long scanning time in ¹H-MRS increases probability of patient movements, so we used all available methods that could improve patient’s comfort during the exam.
All major papers agreed upon necessity of cardiac gating. Cooke et al. [4] as first one found that ECG gating led to significant spectral line narrowing. Spectral line width is essential for metabolite quantification. The idea of ECG gating is to measure the signal from spinal cord only when it is still, what means when CSF pulsation is slowest, what corresponds to the heart diastole. We used an ECG device with 300ms delay in relation to an R wave so the pulse sequence was shifted to a diastole (Figure 3).
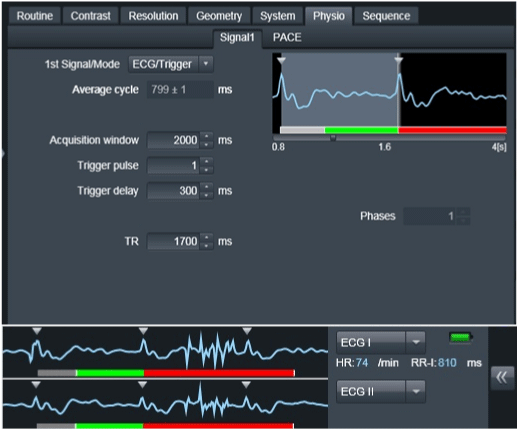
Figure 3: ECG - gating parameters used in acquisitions. Grey bar shows used delay of 300ms.
Monitoring single spectra averages in the inline display on the scanner was found to be very helpful. The scanner software allows observing a single average of ongoing acquisition. If the subject moved during the acquisition clear fat signal could be seen as the lipid and macromolecule part of the spectra (0-1.9 ppm). In that case acquisition should be stopped and rerun with a new localizing sequence. It enabled wasting less time on acquiring spectra that was doomed or to be of poor quality because of the patient movement what caused a fat contamination (Figure 4).

Figure 4: Peak rising in the marked area (1.3 ppm) during acquisition indicates lipid contamination of the spectra, what suggests the subject movement.
Overall, less than 60% of spectra were of acceptable quality in volunteer examinations, however for such a difficult task the learning curve is steep. Concentrations that were obtained are useful only internally, because no coil sensitivity adjustments were made. In case of preliminary studies, such as this one, this is acceptable but in future absolute concentrations are desired for precise information about lesion biochemistry.
In this study, 63% of spectra were measured in the cervical spinal cord and 37% in the thoracic spinal cord. Only two spectra in the thoracic spinal cord was considered of good quality. Breathing and heart motions are a major problem in thoracic region.
Conclusion
MR spectroscopy of the spinal cord is a promising tool for research and diagnosis because it can provide additional information complementary to other non-invasive imaging methods. Spectroscopic acquisition in the spinal cord should meet some challenging requirements of achieving sufficient SNR and avoid fat contamination, what is essential to accurately evaluate the metabolites. The proper positioning of the voxel is the most important aspect. For accurate placement, a good quality isotropic T2 sequence is needed. Fat contamination could be avoided if saturation bands are fine-tuned to saturate that component in the following examinations. Voxel size should be maximized and suited to spine dimensions in right-left and anterior-posterior directions. Craniocaudal size should be maximized according to spinal cord curvature or suspicious lesion shape. Spectra should be heavily averaged, but since time is a factor, single acquisition should not be longer than 10 min. Pulsation of cerebrospinal fluid leads to wider metabolite peaks and lower SNR, so its contribution should be eliminated by ECG gating. Lastly, single spectrum average should be monitored periodically on inline display for fat contamination. In case of the fat contamination spectra, acquisition should be repeated with another isotropic T2 localization sequence.
References
- Bertoldo D, Watcharakorn A, Castillo M. Brain proton magnetic resonance spectroscopy. Introduction and overview. Neuroimag Clin N Am. 2013; 23: 359-380.
- Samartzis D, Gillis C, Shih P, et al. Intramedullary Spinal Cord Tumors: Part II - Management Options and Outcomes. Global Spine J. 2016; 6: 176-185.
- Gómez-Ansón B, MacManus DG, Parker GJM, et al. In vivo 1H MRS of the spinal cord. Neuroradiology. 2000; 42: 515-517.
- Cooke FJ, Blamire AM, Manners DN, et al. Quantitative Proton Magnetic Resonance Spectroscopy of the Cervical Spinal Cord. Magnetic Resonance in Medicine 2004; 51: 1122-1128.
- Dubey P, Smith M, Banekamp D, et al. Proton MR Spectroscopic Imaging of the Human Cervical Spine at 3 Tesla. Proceedings of the International Society for Magnetic Resonance in Medicine 2005; 13.
- Dydak U, Kollias SS, Schar M, et al. MR Spectroscopy in Different Regions of the Spinal Cord and in Spinal Cord Tumours. Proceedings of the International Society for Magnetic Resonance in Medicine 2005; 13: 813.
- Rapalino O, Law M, Salibi N, et al. In vivo MRS in the human cervical spinal cord at 1.5 and 3T. Proceedings of the International Society for Magnetic Resonance in Medicine 2006; 14.
- Ciccarelli O, Wheeler-Kingshott CA, McLean MA, et al. Spinal cord spectroscopy and diffusion-based tractography to assess acute disability in multiple sclerosis. Brain. 2007; 130: 2220-2231.
- Edden R, Bonekamp D, Smith M, et al. Proton MR Spectroscopic Imaging of the Medulla and Cervical Spinal Cord. Journal of Magnetic Resonance Imaging. 2007; 26: 1101-1105.
- Marliani AF, Clementi V, Albini-Riccioli L, et al. Quantitative Proton Magnetic Resonance Spectroscopy of the Human Cervical Spinal Cord at 3 Tesla. Magnetic Resonance in Medicine 2007; 57: 160-163.
- Marliani AF, Clementi V, Albini-Riccioli L, et al. Quantitative Cervical Spinal Cord 3T Proton MRS in Multiple Sclerosis. AJNR Am J Neuroradiol. 2010; 31: 180-184.
- Henning A, Schar M, Kollias S, et al. Quantitative Magnetic Resonance Spectroscopy in the Entire Human Cervical Spinal Cord and Beyond at 3T. Megnetic Resonance in Medicine. 2008; 59: 1250-1258.
- Hock A, Henning A, Boesiger P, et al. 1H-MR Spectroscopy in the Human Spinal Cord. AJNR Am J Neuroradiol. 2013; 34: 1682-1689.
- Hock A, MacMillan E, Fuchs A, et al. Non-water suppressed proton MR spectroscopy allows spectral quality improvement in the human cervical spinal cord. Proc. Intl. Soc. Mag. Reson. Med. 2011; 19.
- Hock A, Fuchs A, Boesiger P, et al. Spinal cord MR spectroscopy in neoplastic lesion. Proceedings of the International Society for Magnetic Resonance in Medicine 2012; 20.
- Hock A, Kollias S, Boesiger P, et al. Motion detection and dual retrospective correction for MR spectroscopy in human spinal cord. Proceedings of the International Society for Magnetic Resonance in Medicine 2013; 21.
- P Wyss, A Hock, S Kollias. The Application of Human Spinal Cord Magnetic Resonance Spectroscopy to Clinical Studies: A Review, Seminars in Ultrasound, CT and MRI. 2017; 38: 153-162.
- Bobek-Billewicz B, Hebda A, Stasik-Pres G, et al. Measurement of glycine in a brain and brain tumors by means of 1H MRS. Folia Neuropathol. 2010; 48: 190-199.
- Provencher S. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993; 30: 672-679.
- Govindaraju V, Young K, Maudsley A. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000; 13: 129-153.