
Case Report
Austin Med Sci. 2016; 1(1): 1002.
CD19-Positive Extramedullary Relapse of Acute Lymphoblastic Leukemia after Blinatumomab Therapy
Al Malki MM¹*, Aldoss IT¹, Song J² and Pullarkat V¹
¹Department of Hematology and Hematopoietic Cell Transplantation
²Department of Pathology, City of Hope Medical Center, USA
*Corresponding author: Al Malki MM, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope National Medical Center, 1800 E. Duarte Road, Duarte, CA 91010, USA
Received: December 18, 2015; Accepted: January 19, 2016; Published: January 21, 2016
Abstract
Background: Blinatumomab is a monoclonal, bispecific T-cell engaging (BiTE) antibody that targets the CD19 antigen on B-cells together with CD3 antigen on T-cells. Blinatumomab is active against CD19+ B- Acute Lymphoblastic Leukemia (B-ALL), with encouraging complete remission rates in relapsed/refractory ALL as well as in ALL with Minimal Residual Disease (MRD). The precise mechanism of blinatumumab resistance remains unclear and emergence of CD19-negative B-ALL clones has been postulated as a mechanism.
Case Report: We describe a case of relapsed/refractory CD19+ ALL treated with blinatumomab resulting in bone marrow remission with negative MRD status, who subsequently relapsed with CD19+ extramedullary disease in gastrointestinal tract as well as muscle and connective tissue.
Conclusion: Our case report suggests poor efficacy of blinatumomab in controlling ALL at extramedullary sites and such relapses may occur despite complete remission in bone marrow. Leukemic blasts at relapse sites after blinatumomab therapy may continue to express CD19 thereby suggesting mechanisms of resistance other than emergence of CD19 negative clones.
Keywords: Blinatumomab; Acute lymphoblastic Leukemia; Relapsed/ Refractory; Extramedullary; CD19
Case Report
We report the case of a 22-year-old Hispanic male with precursor B-ALL with normal karyotype who had first presented at 16 years of age with Central Nervous System (CNS) involvement. He was first treated on a Children’s Oncology Group protocol. He achieved complete remission but had isolated CNS relapse during maintenance therapy. He was re-induced with the UK-MRC ALL regimen using mitoxantrone and triple intrathecal chemotherapy. Once again he achieved a complete remission. He also received craniospinal radiation therapy followed by maintenance therapy with methotrexate and 6-mercaptopurine. While on maintenance therapy, he sustained his second isolated CNS relapse with negative Minimal Residual Disease (MRD) status in his bone marrow. He continued on the same maintenance therapy at that time and received triple intrathecal chemotherapy. After less than a year, he again presented with testicular and CNS-relapse. His bone marrow remained negative for MRD by Multicolor Flowcytometry (MCFC) assessment. He was treated with intrathecal liposomal cytarabine therapy as well as testicular radiation and achieved a fourth complete remission.
After 6 months, he presented with White Blood Cell (WBC) count of 75,000/μL with 80% circulating blasts. His bone marrow exam showed 95% lymphoblast’s expressing CD19, CD22, CD24, CD10, CD20, CD58, CD34, HLA-DR, and TdT. He was started on hydroxyurea and dexamethasone to control his disease. He was then enrolled in a clinical trial of blinatumomab for refractory/relapsed ALL and his treatment course was complicated by fevers, transient transaminase elevation, and capillary leak syndrome resulting from cytokine release syndrome associated with blinatumomab. He achieved complete bone marrow response with negative MRD status on MCFC with one cycle of therapy. One week after completion of cycle number 1, he complained of epigastric pain associated with nausea, anorexia and weight loss. He underwent an esophagogastroduodenoscopy which showed multiple nodular lesions in the gastric wall. A biopsy of one of the lesions showed involvement with B-ALL. The leukemic blasts continued to express CD19 by immunohistochemistry (Figure 1). He also complained of musculoskeletal pain in his back and arms. He underwent a Positron Emission Tomography (PET) scan (Figure 2), which showed diffuse subcutaneous and intra-muscular highly FDG-avid lesions. He refused further treatment and elected to receive hospice care. After 4 weeks, a complete blood count showed WBC of 106,000/μL with 85% CD19-positive lymphoblasts. He died shortly thereafter.
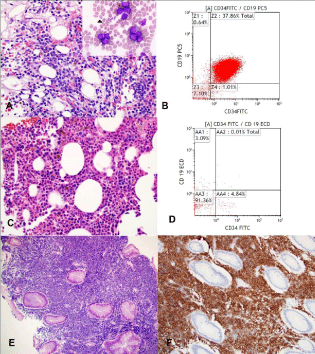
Figure 1: (A) The bone marrow shows numerous blasts on the core
biopsy and aspirate (inset) that are (B) positive for CD19 and CD34 by flow
cytometry. The follow-up bone marrow biopsy (C) after treatment shows
trilineage hematopoiesis with (D) no evidence of B lymphoblastic leukemia by
flow cytometry. (E) The gastric biopsy shows involvement by B lymphoblastic
lymphoma and was (F) CD19 positive.
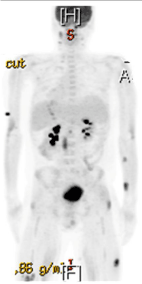
Figure 2: PET scan showing intra-abdominal, intramuscular and
subcutaneous lesions that were FDG-avid.
Discussion
Blinatumomab is a bispecific T-cell engaging monoclonal antibody consisting of 2 variable regions: one specific to CD3 for T-cell recruitment and activation and the other targeting the CD19 antigen [1]. It was initially tested in the setting of MRD-positive B-ALL. In the study by Topp et al., 16 of 20 MRD positive patients responded completely to blinatumomab. Their response was durable, with or without consolidation by hematopoietic cell transplantation, and the majority of them remained disease free for more than 24 months [2]. Subsequently, in an open-label, single-arm, multicenter phase 2 study of 189 patients with Philadelphia-negative relapsed/ refractory ALL; 43% achieved CR/CRh after median of 2 (range: 1-5) cycles of blinatumomab. Most of responses (79%) occurred after 1 cycle [3]. However, relapses occur frequently after blinatumomab therapy for overt relapsed/refractory disease, particularly when it is not followed by alloHCT consolidation.
The mechanisms of primary blinatumomab resistance as well as loss of response remain unclear at present. Loss of the CD19 target antigen expression by leukemic blasts has been observed and contributes to relapse in some cases [1,4]. However, our patient had strong expression of CD19 at extramedullary site suggesting a mechanism of relapse that does not involve CD19. In one study, relapses occurred in 8 of 12 initial responders who did not proceed to receive alloHCT, 3 cases were CD19-negative (one extramedullary) while 4 cases were CD19-positive (2 extramedullary) and one case had unknown CD19 status. Therefore, it appears that both extramedullary as well as bone marrow relapses can either be CD19 positive or negative [4].
It could be postulated that poor trafficking of the blinatumomab or T-cells to extramedullary sites may contribute to such relapses that continue to express CD19. The purpose of this report is to heighten awareness towards the possibility of extramedullary relapses in patients who have good bone marrow disease control with blinatumomab. Blinatumomab therefore appears to be a poor choice for treatment of patients who relapse at extramedullary sites.
In conclusion, although blinatumomab is effective in producing deep remissions in a substantial proportion of patients with relapsed/ refractory disease, it appears to have minimal efficacy in controlling disease in extramedullary sites as evidenced by multiple sites of extramedullary disease in our patient despite complete remission in bone marrow. Extramedullary relapses may continue to express CD19 target antigen suggesting a mechanism of resistance that does not involve expansion of CD19 negative clone.
References
- Ribera JM, Ferrer A, Ribera J, Genesca E. Profile of blinatumomab and its potential in the treatment of relapsed/refractory acute lymphoblastic leukemia. OncoTargets and therapy. 2015; 8: 1567-1574.
- Topp MS, Gökbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M, et al . Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. 2012; 120: 5185-5187.
- Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukemia: a multicentre, single-arm, phase 2 study. The Lancet Oncology. 2015; 16: 57-66.
- Topp MS, Gokbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014; 32: 4134-4140.