
Research Article
Austin Med Sci. 2016; 1(1): 1003.
A Role for Endovascular Intervention in Blunt Vertebral Injury: A Single Institutional Experience
Felbaum DR¹, Armonda RA², Liu H³*, Stemer AB4, Bell R5 and Aulisi EF2
¹Department of Neurosurgery, Medstar Georgetown University Hospital, USA
²Department of Neurosurgery, Medstar Washington Hospital Center, USA
³Department of Neuro-Interventional Radiology, Medstar Washington Hospital Center, USA
4Department of Neurology, Medstar Georgetown University Hospital, USA
5Department of Neurosurgery, National Naval Medical Center, USA
*Corresponding author: Ai-His Liu, Department of Radiology, Medstar Washington Hospital Center, USA
Received: February 04, 2016; Accepted: March 10, 2016; Published: March 14, 2016
Abstract
Introduction: Blunt carotid or vertebral artery injury occurs in up to 0.14% of all trauma admissions. Newer studies have delineated the efficacy of medical management of these injuries. Given polytrauma patients may present with a relative contraindication to antiplatelet therapy, alternative management strategies are necessary. In addition, the natural history of blunt vascular injury is not without morbidity and can compound the difficulty in managing trauma patients with multi-organ injuries at risk for hemorrhagic complications.
Methods: We performed a retrospective analysis from 2012 to 2015 of all endovascular procedures performed for patients with a diagnosis of carotid or vertebral artery dissection. CT Angiography (CTA) was performed in trauma patients meeting Modified Denver Screening protocol as an initial screening test for vascular injury. In patients with suspected Grade III or higher injuries, Digital Subtraction Angiography (DSA) was performed to better delineate the severity of injury.
Results: A total of 19 patients were identified: 6 patients with Biffl Grade 3 injury, 5 patients with Biffl Grade 4 injury, and 2 patients with Biffl Grade 5 injury. The remaining 6 patients were Grades 1 or 2. We performed parent artery occlusion for all patients with symptomatic or progressive Grade 3 injuries that were failing medical management and all patients with Grade 4 and Grade 5 injuries. In all patients whom endovascular intervention was performed, neurologic outcomes were stable or improved at a minimum of hospital discharge. There were no immediate or delayed complications from the endovascular intervention. In conservatively treated patients, there were no incidences of stroke or progression while medically managed.
Conclusion: Medical management should be first line therapy for treatment of blunt vascular injury. In a clinical setting of contraindication to anticoagulation or failed maximal medical therapy, parent artery occlusion for of select high grade vertebral artery injuries is a potential treatment avenue. Overall, an individualized treatment paradigm based on clinical and radiographic symptoms, in combination with multi-disciplinary experience and available resources may assist in providing better outcomes.
Keywords: Blunt vascular injury; Endovascular; Vertebral artery; Stroke
Introduction
Management of high grade traumatic carotid or vertebral artery injury remains controversial [1-4]. The incidence in all trauma patients ranges from 0.007 to 0.14. In these patients, the stroke rate can vary depending on initial presentation, laterality, and grade of blunt vascular injury. Diagnoses of these lesions are increasingly being made during screening CT angiography. The criteria for performing screening tests vary, but are commonly variations of Modified Denver Criteria [2]. More recently, cervical spine injury guidelines recommend performing CTA as a screening tool when indicated [5].
Intuitively, high grade injuries may be more likely to have neurologic sequelae from stroke [6,7]. This must be tempered with recent data suggesting a lower stroke incidence regardless of systemic anticoagulation or antiplatelet therapy [8]. In patients with Biffl Grade III and higher, the natural history of stroke is uncertain but not negligible. With recent endovascular advances, the role for intervention has not been defined. Given the heterogeneity and potential complexity of the trauma population, we present our experience from a neuro-interventional perspective.
Methods
We performed a retrospective analysis from 2012 to 2015 of all endovascular procedures performed for patients with a diagnosis of carotid or vertebral dissection. At our institution, the trauma protocol involves undergoing CT Angiography in patients meeting Modified Denver Screening as an initial radiographic test for detecting carotid or vertebral artery injury. In patients with suspected Grade III or higher injuries, Digital Subtraction Angiography (DSA) was performed to better delineate the severity of injury and potential endovascular intervention.
Results
A total of 19 patients were identified: 6 patients with Biffl Grade 3 injury, 5 patients with Biffl Grade 4 injury, and 2 patients with Biffl Grade 5 injury. The remainders were Biffl Grade 1 or 2. We performed Parent Artery Occlusion (PAO) for all patients with symptomatic or progressive Grade 3 injuries (3 of 18), and all patients with Grade 4 (5 of 18) and Grade 5 (2 of 18) injuries. In addition, patients were selected for PAO if they had progressive symptomatic disease despite medical therapy, absolute contraindication to anticoagulation, or we intended to prevent recanalization of the vessel and subject the patient to an unknown thromboembolic risk. In one grade 2 patient with persistent embolic injury despite several anticoagulation therapies, PAO was performed. PAO was performed primarily in vertebral artery injuries. Technically, the injured vessel was occluded primary from its proximal origin and distally, if access was deemed feasible without exposing added risk to the patient. Occlusion was generally performed using coils. In all patients whom endovascular intervention was neurologic outcomes were stable or improved at time of discharge or most recent clinical follow-up. There were no immediate or delayed complications from the endovascular intervention. In conservatively treated patients, there were no incidences of stroke or progression while on an anti-coagulation.
Discussion
With the advent of aggressive screening practices, blunt vascular injury of the carotid or vertebral arteries is becoming increasing recognized. In their landmark paper regarding vertebral vessel injury, Biffl et al. documented a 0.53% incidence of injury diagnosed via DSA in all trauma admissions. Their initial series showed a 24% rate of posterior circulation stroke, with an attributable death rate of 8% [6]. In particular, the incidence of stroke per grade was: Grade 1-19%, Grade 2-40%, Grade 3-13%, and Grade 4-33%. The use of heparin was implemented to treat these dissections and was associated with improved outcomes and low hemorrhagic complication rates. In a separate paper for carotid injuries, Biffl et al. had a 0.38% incidence of blunt carotid injury. At 7-10 day re-imaging, 70% of cases persisted despite management with heparinization. This prompted a recommendation of surgical repair for Grade II lesions or higher [7]. This was reversed after reviewing their 16-year experience, which they conclude that an initial treatment with heparin transitioned to 325mg of aspirin resulted in good or stable outcomes [9].
The movement towards aggressive screening and medical management is echoed in more recent studies that have been published reviewing institutional experiences regarding blunt vascular injury [6,7,10,11]. In these publications, although blunt vascular injury remains a source of morbidity, the majority of these studies highlight the safety of medical management of this injury [10,12-15]. In the CADISS trial, 250 patients with cervical artery dissection were randomized to be treated with anticoagulation or antiplatelet therapy. There were 4 strokes that occurred in the combined patient population, with only 1 major hemorrhage occurred in the anticoagulation group [8]. This enforces the safety of antiplatelet therapy as compared to systemic heparinization, which can be associated with an 8-16% of hemorrhagic conversion [3,4,12,13,16]. In another study, Scott et al. review their ten year experience with blunt vascular injury. Intuitively, they report low grade vertebral artery injuries having a mean of 40 day stability on repeat imaging, with a 1.7% stroke rate [17]. In addition, continued vascular studies show resolution or stability of these lesions with good neurologic outcome. For luminal stenos is more than 50% or the presence of a pseudo aneurysm, 95% of injuries remained stable or resolved on repeat imaging [18]. For patients with vessel occlusion, 65% remained persistently occluded, with recanalization in 30% of cases. If neurological injury occurred, it was seen in 7% of patients with diagnosed vessel occlusion, and most occurred in the immediate post-injury period or despite medical management. This was also documented by Morton et al. with Biffl 4 vascular injuries [14,15]. In addition, they separate injury occurring via two mechanisms: hemodynamic failure or embolic events. They also advocated for employing multi-modality monitoring with daily Transcranial Doppler’s (TCD) and/or a screening MRI to stratify patients that may benefit from dual antiplatelet agents. For vertebral artery injuries, there was 9% stroke incidence treated with medical management. Overall, endovascular or surgical intervention was only performed in patients with radiographic progression or neurologic compromise despite maximal medical therapy.
When appropriate, such as absolute contraindication to anticoagulation or failed maximal medical therapy, we performed parent artery occlusion as means of secondary stroke prevention. An example in our case series, a patient presented with a cerebellar ischemic stroke, with an MRA showing occlusion (Figure 1). DSA performed revealed sluggish, but patent vertebral artery (Figure 2). He was treated with a heparin drip. On hospital day two, the patient became progressively lethargic, with repeat imaging revealing bilateral cerebellar hemispheric infarcts. Repeat angiography showed an increasingly patent vertebral artery, potentially increasing embolic phenomenon despite systemic heparinization (Figure 3). After discussion with the stroke and neurosurgery consultants, parent vessel occlusion was performed without long-term neurologic squeal (Figure 4).
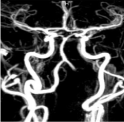
Figure 1: Magnetic resonance angiography depicting the absence of the left
vertebral artery.
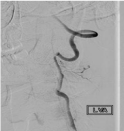
Figure 1: Digital subtraction angiography, AP projection of left vertebral
artery injection depicting presence of dissected vessel with more than 50%
reduction of luminal caliber.
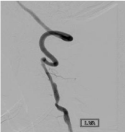
Figure 3: Digital subtraction angiography, ap projection of left vertebral artery
injection depicting recanalization of previously stenotic vessel.
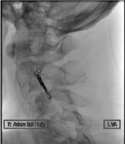
Figure 4: Unsubtracted lateral view of the left vertebral artery demonstrating
the coil mass signifying occlusion of the progressively symptomatic vessel.
We emphasize the superiority of medical management of the vast majority of vessel injury, but in select patients failing initial management, parent vertebral artery occlusion was well tolerated. Although it was our initial practice to aggressively treat high grade injuries given uncertain natural course or long-term follow-up, we have since elected to treat these patients with medical therapy. In the endovascular treatment cohort, it is noteworthy to mention no secondary cerebrovascular insults occurred after treating the diseased vessel. Vertebral artery occlusion may be well tolerated due to the rich collateral supply of the cervical spine [19]. In addition, Biffl IV injuries are less likely to manifest as quadriplegia, but with ischemic symptoms from the posterior circulation. Also, patients that do not manifest with ischemic injury early in the hospital course may be able to tolerate delayed occlusion. Ideally, balloon test occlusion should be performed, but this may not always correlate with a good outcome [20]. Blunt vertebral artery injuries are highly associated with cervical spine fracture dislocations, sublimations, or foramen transversarium injuries [19]. In our series, one patient with jumped facets had the VA dissection change from Biffl 4 on CTA to Biffl 2 after angiogram was performed once surgical correction was achieved. This supports the protective effect in reducing cervical fractures [21]. Repeat vascular imaging after reduction of cervical spine injuries should be considered to help stratify post-surgical anticoagulation.
A screening CTA with 3D reconstruction is highly successful in diagnosing blunt vessel injury and is rightly the first line in diagnosing such lesion [22-24]. A meta-analysis demonstrated the sensitivity and specificity of 16-slice CTA approaching the gold standard of Digital Subtraction Angiography (DSA) [2]. Although our case series is limited in numbers, it worthwhile to highlight a discrepancy between conventional angiography and CTA in delineating the severity of dissection. In a select number of patients with high grade injury, angiography may aid in elucidating flow and collateral patterns to aid in risk stratification.
There are several limitations in our paper. First, there is inherent bias in a retrospective analysis of cerebral angiograms. Although the trauma, neurosurgery, vascular, and neuro-interventional services are well integrated, there may be patients who were not identified and a DSA not performed, or because of the initial severity of injuries a DSA was not performed.
In all cases, we had no complications. In patients with Grade III and higher vascular injuries, PAO was well tolerated. This may be due to several factors: low incidence of stroke, well-formed collaterals, timing of treatment. Given the low incidence of stroke, we may have performed the procedure in patients with a well formed circle of Willis with developed collaterals (posterior communicating artery greater than 1mm) [14]. One possibility is that given that most injuries tend to occur within 24 hours of presentation and DSA was usually performed after 24hrs, the patients may inherently be able to tolerate PAO of an already diseased segment without sequel. We have favored treating Grade I or II injuries with single anti-platelet agent (ASA 325), progressing with a second agent (Clopodigrel) if symptoms develop [14]. Only Grade I-II cases refractory to antiplatelet agents are considered for endovascular treatment.
Ideally, patients with blunt cerebrovascular injury should undergo multi-modality monitoring with TCDs, screening MRI, followed with serial CTA [14]. These non-invasive measures may be able to identify and stratify patients at higher risk for embolic events. Once identified, medical management should be initiated under the guidance of a multi-disciplinary team of neurosurgery, trauma surgery, stroke, and neuro-interventionalist to aid in providing the best outcome for these patients.
Conclusion
Post-traumatic carotid or vertebral vessel injury is an increasingly recognized pathology. Given the complexity of polytrauma patients with multi-organ involvement, patients with high grade (Biffl 3 and higher) may benefit from digital subtraction angiography. In select cases with failed or contraindication to medical therapy, use of modern neuro-endovascular techniques may be utilized to safely treat diseased vessel segments to provide definite therapy to prevent future stroke occurrence.
References
- Biffl WL, Ray CE, Moore EE, Franciose RJ, Aly S, Heyrosa MG, et al. Treatment-related outcomes from blunt cerebrovascular injuries: importance of routine follow-up arteriography. Ann Surg. 2002; 235: 699-706; discussion 706-707.
- Bromberg WJ, Collier BC, Diebel LN, Dwyer KM, Holevar MR, Jacobs DG, et al. Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma. 2010; 68: 471-477.
- Fabian TC, Patton JH Jr, Croce MA, Minard G, Kudsk KA, Pritchard FE. Blunt carotid injury. Importance of early diagnosis and anticoagulant therapy. Ann Surg. 1996; 223: 513-522.
- Miller PR, Fabian TC, Bee TK, Timmons S, Chamsuddin A, Finkle R, et al. Blunt cerebrovascular injuries: diagnosis and treatment. J Trauma. 2001; 51: 279-285.
- Harrigan MR, Hadley MN, Dhall SS, Walters BC, Aarabi B, Gelb DE, et al. Management of vertebral artery injuries following non-penetrating cervical trauma. Neurosurgery. 2013; 72: 234-243.
- Biffl WL, Moore EE, Elliott JP, Ray C, Offner PJ, Franciose RJ, et al. The devastating potential of blunt vertebral arterial injuries. Ann Surg. 2000; 231: 672-681.
- Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Burch JM. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999; 47: 845-853.
- TC. Trial Investigators. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomized trial. Lancet Neurol. 2015; 14: 361-367.
- Wagenaar AE, Burlew CC, Biffl WL, Beauchamp KM, Pieracci FM, Stovall RT, et al. Early repeat imaging is not warranted for high-grade blunt cerebrovascular injuries. J Trauma Acute Care Surg. 2014; 77: 540-545.
- Stein DM, Boswell S, Sliker CW, Lui FY, Scalea TM. Blunt cerebrovascular injuries: does treatment always matter? J Trauma. 2009; 66: 132-143.
- Young RM, Sherman JH, Wind JJ, Litvack Z, O’Brien J. Treatment of craniocervical instability using a posterior-only approach: report of 3 cases. J Neurosurg Spine. 2014; 21: 239-248.
- Cothren CC, Moore EE, Biffl WL, Ciesla DJ, Ray CE, Johnson JL, et al. Anticoagulation is the gold standard therapy for blunt carotid injuries to reduce stroke rate. Arch Surg. 2004; 139: 540-545; discussion 545-546.
- Fusco MR, Harrigan MR. Cerebrovascular dissections: a review. Part II: blunt cerebrovascular injury. Neurosurgery. 2011; 68: 517-530.
- Morton RP, Hanak BW, Levitt MR, Fink KR, Peterson EC, Vilela MD, et al. Blunt traumatic occlusion of the internal carotid and vertebral arteries. J Neurosurg. 2014; 120: 1446-1450.
- Morton RP, Levitt MR, Emerson S, Ghodke BV, Hallam DK, Sekhar LN, et al. Natural History and Management of Blunt Traumatic Pseudoaneurysms of the Internal Carotid Artery: The Harborview Algorithm Based Off a 10-Year Experience. Ann Surg. 2015.
- Eachempati SR, Vaslef SN, Sebastian MW. Reed RL 2nd. Blunt vascular injuries of the head and neck: is heparinization necessary? J Trauma. 1998; 45: 997-1004.
- Scott WW, Sharp S, Figueroa SA, Madden CJ, Rickert KL. Clinical and radiological outcomes following traumatic Grade 1 and 2 vertebral artery injuries: a 10-year retrospective analysis from a Level 1 trauma center. J Neurosurg. 2014; 121: 450-456.
- Scott WW, Sharp S, Figueroa SA, Eastman AL, Hatchette CV, Madden CJ, et al. Clinical and radiological outcomes following traumatic Grade 3 and 4 vertebral artery injuries: A 10-year retrospective analysis from a Level I trauma center. The Parkland Carotid and Vertebral Artery Injury Survey. J Neurosurg. 2015; 122: 1202-1207.
- Inamasu J, Guiot BH. Vertebral artery injury after blunt cervical trauma: an update. Surg Neurol. 2006; 65: 238-245.
- Sekhar LN, Natarajan SK, Ellenbogen RG, Ghodke B. Cerebral revascularization for ischemia, aneurysms, and cranial base tumors. Neurosurgery. 2008; 62: 1373-1408.
- Foreman PM, Griessenauer CJ, Chua M, Hadley MN, Harrigan MR. Corrective spinal surgery may be protective against stroke in patients with blunt traumatic vertebral artery occlusion. J Neurosurg Spine. 2015; 1-6.
- Berne JD, Reuland KS, Villarreal DH, McGovern TM, Rowe SA, Norwood SH. Sixteen-slice multi-detector computed tomographic angiography improves the accuracy of screening for blunt cerebrovascular injury. J Trauma. 2006; 60: 1204-1209; discussion 1209-1210.
- Biffl WL, Egglin T, Benedetto B, Gibbs F, Cioffi WG. Sixteen-slice computed tomographic angiography is a reliable noninvasive screening test for clinically significant blunt cerebrovascular injuries. J Trauma. 2006; 60: 745-751; discussion 751-752.
- Bub LD, Hollingworth W, Jarvik JG, Hallam DK. Screening for blunt cerebrovascular injury: evaluating the accuracy of multidetector computed tomographic angiography. J Trauma. 2005; 59: 691-697.