
Special Article - Urology
Austin Med Sci. 2018; 3(1): 1021.
Bacterial Etiology of Urinary Tract Infection in Delta Medical College and Hospital
Sarker UJ1*, Ahmed MU1, Asna SHZ1, Samad2, Rabbi F3 and Noor R3
1Department of Microbiology, Bangladesh Institute of Health Sciences, Bangladesh
2Department of Microbiology, Delta Medical College, Bangladesh
3Department of Microbiology, Stamford University, Bangladesh
*Corresponding author: Una Jessica Sarker, Department of Microbiology, Bangladesh Institute of Health Sciences, Bangladesh
Received: March 15, 2018; Accepted: April 17, 2018; Published: April 24, 2018
Abstract
The urinary tract is one of the most common sites of bacterial infection, particularly in females; 20-30% of women have recurrent urinary tract infection (UTI) at some time in their life. UTIs in men are less common and primarily occur after 50 years of age. Although the majority of infections are acute and short-lived, they contribute to a significant amount of morbidity in the population. Severe infections result in a loss of renal function and serious long term sequelae. In females, a distinction is made between cystitis, urethritis and vaginitis. The purpose of this study was to look into the prevalence of bacteriuria in Delta Medical College. Specimens of urine were examined from 57 inpatients and out-patients and 53 students and staff. Conventional bacteriological techniques were used. Positive cultures were obtained from 21 samples from inpatients and out-patients and positive cultures were also obtained from 13 samples from students and staff. 22 samples from total positive samples showed symptomatic bacteriuria. The results were as follows: Escherichia coli, 25 cases; 73.53%: Klebsiella species, 9 cases; 26.47%. The most common organism was Escherichia coli, followed by Klebsiella species. Antibiogram of these isolates were also done to identify which antibiotic is effective against theses isolates. In this study I have used six types of antibiotics e.g. Amoxycillin, Gentamicin, Ciprofloxacin, Cephradin, Cotrimoxazole and Nalidixic acid. It was showed that Amoxycillin was most effective against Escherichia coli and Klebsiella species and Ciprofloxacin was most effective against Klebsiella species. Clinical studies were made to correlate the bacteriuria and symptoms. Bacteriological examination should be repeated to confirm the diagnosis if patient does not need immediate treatment. Escherichia coli were the commonest organism. This study was helpful for Clinicians and Microbiologists as a base line study of the bacterial etiology of urinary tract infections in Bangladesh.
Introduction
Urinary tract infection is a bacterial infection that affects any part of the urinary tract. It is a condition where one or more parts of the urinary system (The kidney, ureter, bladder and urethra) become infected [1]. UTIs are the most common of all bacterial infections and can occur at any time in the life of an individual. Almost 95% of cases of UTIs are caused by bacteria that typically multiply at the opening of the urethra and travel up to the bladder. Much less often, bacteria spread to the kidney from the blood stream. The main causal agent is Escherichia coli. Although urine contains a variety of fluids, salts and waste product [2]. It does not usually have bacteria in it. When bacteria get into the bladder or kidney and multiply in the urine, they may cause a UTI. The male and female urinary tracts are relatively same except for the length of the urethra. The most common type of UTI is acute cystitis often referred to as a bladder infection. An infection of the upper urinary tract or kidney is known as pyelonephritis and is potentially more serious. Patients should be hospitalized. Although they cause discomfort, urinary tract infections can usually be easily treated with a short course of antibiotics with no significant difference between the classes of antibiotics commonly used.
Methodology
Working place
All the experiments of this project work were carried out in the laboratories of the department of Microbiology, Delta medical college and Hospital.
Area of collection
All the samples were collected from the respective patients of Delta Medical Hospital. Samples were also collected from students and staff from delta Medical College.
Collection of mid-stream (MSU)
The patients were given a sterile, dry wide mouthed, leak –proof container and explained the importance of collecting a specimen with as little contamination as possible. Patients were voluntary agreed to give their sample. Female patients were instructed to clean the area around the urethral opening with clean water, dried the area and collected the urine with the labia held apart. About 20 ml of urine was collected in each container. During collection of samples, Hand gloves, mask, were worn and other precautionary practices were taken for personal safety. Sterile test tubes; sterile, dry, wide mouthed leak proof container, test tube rack, cotton were used to collect the samples aseptically. After collection, the tubes were properly capped, labeled with the date, the name, the identification number and the time of the collection. Then the container with specimen was transported to the Microbiological lab as soon as possible.
Standard procedure of mid-stream urine collection
Washing hands, before urine collection, cleaning the area around penis or vagina. A man should retract the fore skin, if present and cleaning the head of his penis thoroughly with medicated swab. A woman should spread open the fold of the skin around vagina with one hand, then use her other hand to clean the area around her vagina and urethra thoroughly with medicated swab. She should wipe the area from front to back to avoid spreading bacteria to the vagina that is normally found around the anus. After the urine has passed for several seconds, placing the collection container in the stream and collecting about 20 ml of this mid-stream urine without stopping the flow. Should not be touched the rim of the container to the genital area and should not have toilet paper, hair, feces or menstrual blood in the urine sample. If urine is not collected at home and cannot get it to the lab within an hour, the sample should be refrigerated at 4 degree celsius and stored up to 18 hours [3].
The Equipment which were used for culture and sensitivity test in Microbiology laboratory are as follows
Incubator: Bacteria are grown in media usually at 37 degree celcius. So, incubator is used in microbiology laboratory to grow bacteria in media. Incubator is an electrical instrument in which temperature is maintained.
Autoclave: Autoclave is a sterilization instrument by maintaining temperature and pressure. With the help of this instrument the media, petridish, specimen collection bottles, swab stick can be sterilized at 121 degree celsius and 15 pound pressure, keeping for 15 minutes.
Hot air oven: With the help of this instrument conical flask, measuring cylinder, jar, petridish, test tubes are sterilizedat 180 degree celsius for one hour. Temperature can be raised up 24 degree celsius with the aid of Hot air oven.
Sterile petridish: Sterile petridish is used for culture and sensitivity test.
Sterile conical flask: Sterile, dry conical flask is used for taking the powder of media and distilled water to make a media by heating this sterile conical flask.
Sterile measuring cylinder: Sterile, dry measuring cylinder is used for measuring distilled water.
Tripod stand: The sterile, dry conical flask, containing the media is heated with the flame of the spirit lamp by standing on the tripod stand.
Spirit lamp: The conical flask, containing the media is heated with the flame of the spirit lamp. Bacteriological loop is also sterilized with the flame of the spirit lamp.
Bacteriological loop: It is a platinum ring of aluminum rod. With the aid of this loop bacteria are inoculated in media.
Electric balance: It is used to measure the powder of the media.
Reagents were used in this study
Mac-Conkey agra: Base for the isolation and differentiation of Enteric organisms.
Directions: 50.0 gm of the powder was taken in sterile conical flask. 1 L of distilled water was measured by measuring cylinder and poured into the powder. It was mixed thoroughly. Then it was heated with frequent agitation and boiled for one minute to completely dissolve the powder. Auto clave was done at 121 degree celsius for 15 minutes. Overheating was avoided.
Approximate formula per liter:
Pancreatic digest of gelatin: 17g
Paptones: 3g
Lactose: 10g
Bile salt: 1.5 g
Sodium chloride: 5g
Agar: 13.5g
Neutral red: 0.03g
Crystal violet: 0.001g
Mueller Hinton agar: Base for antimicrobial Disk diffusion susceptibility testing:
Direction: 38 g of the powder was taken in sterile conical flask. 1L of Distilled water was measured by measuring cylinder and poured in to the powder. It was mixed thoroughly. Then it was heated with frequent agitation and boiled for one minute to completely dissolve the powder. Autoclave was done at 121 degree celsius for 15 minutes. Overheating was avoided.
Approximate formula per liter:
Beef extract powder: 2.0g
Acid digest of casein: 17.5g
Starch: 1.5g
Agar: 17.0g
Nutrient Agar: Base for the cultivation a wide variety of microorganism.
Direction: 28 g of the powder was taken in sterile conical flask. 1 L of Distilled water was measured by measuring cylinder and poured in to the powder. It was mixed thoroughly. Then it was heated with frequent agitation and boiled for one minute to completely dissolve the powder. Autoclave was done at 121 degree celsius for 15 minutes. Overheating was avoided.
Approximate formula per liter:
Agar: 15.0 g
Veg. peptone: 5.0 g
Sodium chloride: 5.0 g
Veg. Extract: 1.5 g
Yeast Extract: 1.5 g
Triple Sugar iron
Triple Sugar Iron is only a screening device. The important components of this medium are ferrous sulfate and the three sugars glucose, lactose and sucrose. The glucose is present in one –tenth the concentration of the other two sugars. The medium in the tube has a solid, poorly oxygenated area on the bottom, called the butt and an angled, well –oxygenated area on top, called the slant. The organism was inoculated into the butt and across the surface of the slant.
Examination of urine
First day: Physical, chemical and microscopic examinations were carried out in doing this study.
Physical examination includes: Color, appearance, specific gravity
Chemical examination includes: Reaction, the presence of proteins, the presence of reducing substance, the presence of ketone body, bilirubin, bile salts, and blood.
Microscopic examination of urine: In Microbiology laboratory microscopic examination is done to find out presence of organized and unorganized deposits, epithelial cells, pus cells, red blood cells, pus cells and epithelial cells. In severe condition calcium oxalate, crystals, triple phosphate crystals are also examined by microscopic examination. But in my study I did microscopic examination to find out pus cells, red blood cells and epithelial cells. Urine was centrifuged at 3000 rotation per minute for five minutes in centrifuged machine. Slide was cleared with cotton and then numbered according to the identification number of the patient. Then one drop of urine was poured on to the slide from the test tube and covered this drop by cover slip. Then slide was examined under 40x objective of Microscope.
Inoculation and incubation
For urine sample: First of all equipment was autoclaved which were needed in doing this study the previous day. Then a bacteriological loop was sterilized, which held about 0.001ml of urine. According to conventional method streak plate technique was used to inoculate the samples on Mac-Conkey media and Blood agar for isolation of microorganisms and all the plates were incubated at 37oC for 24 hours.
Counting and recording: Plates containing less than 104 colonies were counted as not significant bacteriuria and also pus cells were not significantly present. Plates containing more than 105 colonies were counted as significant bacteriuria with significantly presence of pus cells.The counts were recorded as colony forming unit (cfu)/per g. The number of bacteria per ml of original culture was calculated by multiplying the number of colonies counted by the dilution factors divided by the volume of sample used for spreading. That is, Number of cells per ml = number of colonies x dilution factor/volume of sample used =cfu/g.
Isolation of microorganism
Following microbiological media were used for isolation of different pathogenic bacteria from the samples:
MacConkey agar (MAC): MacConkey agar was used for the isolation of gram-negative bacteria and used for differentiate between lactose fermented & non-lactose fermenter.
Nutrient agar (NA): contain all the elements that most bacteria need for growth and are non-selective, so they are used for the general cultivation and maintenance of bacteria kept in laboratory culture collections.
Motility test: Motility test was used for differentiate between motile and non-motile bacteria.
Storage of the isolated microorganisms
After inoculation, the isolated microorganisms were refrigerated at 4 degree celsius for further testing.
Identification of microorganisms
Identification of bacteria: Both primary and confirmative identification of bacteria was performed.
Primary identification of bacteria: Primary identification of bacteria was performed based on the selectivity of media, change of media after growth, morphological characteristics of the colonies and microscopic characteristics.
Colony morphology: Morphological characteristics including size, shape, surface, texture, edge, elevation, color, opacity etc from different types of colonies in different culture media were observed and recorded.
Confirmative identification of bacteria: Confirmative identification was accomplished based on biochemical identification.
Several biochemical tests were performed according to the manual of methods for general Bacteriology by American Society of Microbiology (ASM, 1981) to identify the bacteria. The following biochemical tests were included.
Triple sugar iron (TSI) test: This media was used for initial identification of gram-negative bacilli. Three primary characteristics of a bacterium detected by this media ability to produce gas.
Eosine-methylene blue (EMB) test: this media was used for E.coli bacteria by green metalic sheen.
Antibiotic susceptibility test
Antibiotic susceptibility test of the identified organisms was determined by Kirby- Bauer method using Mueller-Hinton agar medium (Barry and Thornberry, 1985). Antibiotic potency of antibiotic discs used was given.
Potency of antibiotic discs used (Oxoid, UK) (Table 1).
Antibiotic used
Potency (µg/disc)
Cephradine (CV)
30
Nalidixic acid (NA)
30
Ciprofloxacin (CIP)
5
Gentamicin (CN)
10
Amoxycillin (AM)
10
Cotrimoxazole (CO)
23.75
Table 1: Potency of antibiotic discs used (Oxoid, UK).
Single isolated colony was touched by sterile loop and emulsified in 1 ml of sterile normal saline followed by vortex. Turbidity of the suspension was compared with 0.5 McFarland standard. Sterile swab was inserted into the tube containing bacterial suspension. Swab was merged and stirred to mix well. Extra suspension was squeezed by rubbing on the wall. Bacterial suspension was inoculated on Mueller- Hinton agar medium by swab. Swab was streaked evenly over the surface of the medium in three directions; rotating the plate approximately 60 degree to ensure even distribution. Plate was closed by lid and kept for 5 minutes to dry. Antimicrobial discs were evenly distributed on the inoculated plate by multi disc dispenser. The discs were placed about 15mm apart from the edge of the plate and not closer than about 25mm from disc to disc. Six discs were applied at each plate (90mm disc). The plate was inverted and incubated aerobically at 37oC for 16-18 hours. After overnight incubation, the plates were examined to ensure the confluent or near confluent growth. A ruler was used on the underside of the plate to measure the diameter of each zone of inhibition in mm. The endpoint of inhibition was determined from the beginning of growth. The zone diameter for individual antibiotics was translated into susceptible, intermediate and resistant categories by referring to an interpreting table.
Results
Physical and chemical analysis of urine sample collected from inpatient, outpatient and student-staff of Delta Medical College
Quantity: Most of the patients’ normal urine output is 700ml to 2500ml in 24 hours depending mainly on fluid intake.
Color: Most of the patients’ urine color is straw color due to urochrome.
Appearance: Most of the urine sample is clear and transparent. Very few urine sample appear as smoky due to presence of pus and blood cells.
Specific gravity: Normal specific gravity is between 1010 and 1020.
Reaction: All urine samples are usually acidic. No urine sample contains protein, reducing substance and organized, unorganized deposit.
Microscopical analysis of urine sample: Microscopic examination was done to find out presence of red blood cells, pus cells and epithelial cells in urine sample. Pus cells and epithelial cells are commonly found in urine sample. In my laboratory work about 34 positive urine sample contains more than 6 pus cells which are indicated. Epithelial cells were present, which were not indicated (Tables 2 & 3).
Rectovaginal fistula with Artesia of rectum and colostomy
1
Incontinence due to manipulation
2
Fever
6
Vomiting
4
Recurrent UTI
5
Dysuria
6
Retinoblastoma
4
Chronic renal failure
3
Abdominal pain
2
Loin pain
1
Table 2: The main presenting features of patients are as follows.
Age
Patients
Staff’s & Students
Total Number Tested
Number Tested
Number Positive
Number Tested
Number Positive
10-20 years
7
2
5
Nill
12
21-30 years
17
9
35
12
52
31-40 years
8
3
3
Nill
11
41-50 years
11
5
3
1
14
51-60 years
7
2
3
Nill
10
61-70 years
3
Nill
2
Nill
5
71-80 years
4
Nill
2
Nill
6
Total
57
21
53
13
110
Table 3: Age distribution of patients, staffs and students under study.
Microbiological analysis
Antibiotic susceptibility test: After 24 hours of incubation, Mueller-Hinton agar (MHA) plates carried out by Kirby-Bauer method using cephradine, nalidixic acid, gentamicin, ciprofloxacine, amoxicillin and cotrimoxazole. The drug resistance pattern varies considerably with different drugs (Tables 3-11 and Figures 1-8).
Source
Source description
Total count (No of colonies)
Section-1
Outpatient department
A small room, where patients’ sample such as: blood, urine was given. .
TFTC
Section-2
Admitted patient in hospital
3rd floor of the Hospital, where severe kidney infection patients were present such as: bladder infection, pyerlonephritis etc.
TNTC
Section-3
College environment
Environment, where possibility of bacterial contamination was frequently less occurred.
TFTC
TFTC: Too Few To Count; TNTC: Too Numerous To Count.
Table 4: Total bacterial count of hospital environment and college environment on nutrient agar.
Isolate no
Colony characteristics
Size
Form
Colour
Margin
Elevation
01
Medium
Circular
Pink
Entire
Flat
02
Medium
Circular
Pink
Entire
Flat
03
Medium
Circular
Pink
Entire
Flat
04
Small
Irregular
Slight pink
Entire
Raised
05
Small
Irregular
Mucoid
Entire
Slight raised
06
Medium
Circular
Pink
Entire
Flat
07
Medium
Circular
Pink
Entire
Flat
08
Small
Irregular
Mucoid
Entire
Raised
09
Small
Irregular
Mucoid
Entire
Slight raised
10
Medium
Circular
Pink
Entire
Flat
11
Medium
Circular
Pink
Entire
Flat
12
Small
Irregular
Mucoid
Entire
Raised
13
Small
Irregular
Mucoid
Entire
Raised
14
Small
Irregular
Mucoid
Entire
Raised
15
Medium
Circular
Pink
Entire
Flat
16
Medium
Circular
Pink
Entire
Flat
17
Medium
Irregular
Slight pink
Entire
Raised
18
Medium
Irregular
Slight pink
Entire
Raised
19
Medium
Irregular
Slight pink
Entire
Raised
20
Medium
Circular
Pink
Entire
Flat
21
Medium
Circular
Pink
Entire
Flat
22
Small
Irregular
Mucoid
Entire
Slight raised
23
Small
Irregular
Mucoid
Entire
Slight raised
24
Small
Irregular
Mucoid
Entire
Slight raised
Isolate no
Colony characteristics
Size
Form
Colour
Margin
Elevation
25
Medium
Circular
Pink
Entire
Flat
26
Medium
Circular
Slight pink
Entire
Flat
27
Medium
Circular
Pink
Entire
Flat
28
Small
Circular
Pink
Entire
Flat
29
Small
Irregular
Slight pink
Entire
Raised
30
Small
Irregular
Slight pink
Entire
Raised
31
Small
Irregular
Slight pink
Entire
Raised
32
Small
Irregular
Pink
Entire
Flat
33
Small
Irregular
Pink
Entire
Flat
`34
Small
Irregular
Pink
Entire
Flat
Table 5: Isolation and identification of pathogens on Mac-Conkey agar from Urine sample.
Isolate
No.
Biochemical test
Growthon EMB
agar
Suspected
micro-
organisms
TSI
Citrate
Utilization test
Slant
Butt
Gas
01
Y
Y
+
+
Green Metallic sheen
E.Coli
02
Y
Y
+
-
Green Metallic sheen
E.coli
03
Y
Y
+
+
ND
E.coli
04
Y
Y
+
+
Green Metallic Sheen
E.coli
05
Y
Y
+
+
Glossy pink
Klebsiella
06
Y
Y
+
-
Green Metallic sheen
E.coli
07
Y
Y
+
+
Green Metallic Sheen
E.coli
08
Y
Y
+
+
Glossy pink
Klebsiella
09
Y
Y
+
+
Glossy pink
Klebsiella
10
Y
Y
+
+
Green Metallic Sheen
E.coli
11
Y
Y
+
+
Green Metallic Sheen
E.coli
12
Y
Y
+
+
Glossy pink
Klebsiella
13
Y
Y
+
+
Glossy pink
Klebsiella
14
Y
Y
+
+
Glossy pink
Klebsiella
15
Y
Y
+
+
Green Metallic Sheen
E.coli
16
Y
Y
+
+
ND
E.coli
17
Y
Y
+
+
Green Metallic Sheen
E.coli
18
Y
Y
+
+
Green Metallic Sheen
E.coli
19
Y
Y
+
+
Green Metallic Sheen
E.coli
20
Y
Y
+
+
Green Metallic Sheen
E.coli
21
Y
Y
+
+
Green Metallic Sheen
E.coli
22
Y
Y
+
+
Glossy pink
Klebsiella
23
Y
Y
+
+
Glossy pink
Klebsiella
24
Y
Y
+
+
Glossy pink
Klebsiella
25
Y
Y
+
+
Green Metallic Sheen
E.coli
26
Y
Y
+
+
Green Metallic Sheen
E.coli
27
Y
Y
+
+
Green Metallic Sheen
E.coli
28
Y
Y
+
+
Green Metallic Sheen
E.coli
29
Y
Y
+
+
Green Metallic Sheen
E.coli
30
Y
Y
+
+
Green Metallic Sheen
E.coli
31
Y
Y
+
+
Green Metallic Sheen
E.coli
32
Y
Y
+
+
Green Metallic Sheen
E.coli
33
Y
Y
+
+
Green Metallic Sheen
E.coli
34
Y
Y
+
+
Green Metallic Sheen
E.coli
Table 6: Results of biochemical tests of the Gram negative isolates of Positive urine sample.
Isolate no
Total count
No of pus cells
Symptomatic Bacteriuria
Asymptomatic Bacteriuria
01
105 cfu/ml
6-9/HPF
+
_
02
105 cfu/ml
5-7/HPF
+
_
03
105 cfu/ml
6-8/HPF
+
_
04
105 cfu/ml
5-8/HPF
+
_
05
105 cfu/ml
6-9/HPF
+
_
06
105 cfu/ml
6-9/HPF
+
_
07
105 cfu/ml
2-3/HPF
_
+
08
105 cfu/ml
5-8/HPF
+
_
09
105 cfu/ml
2-3/HPF
_
+
10
105 cfu/ml
2-3/HPF
_
+
11
105 cfu/ml
2-3/HPF
_
+
12
105 cfu/ml
6-9/HPF
+
_
13
105 cfu/ml
5-7/HPF
+
_
14
105 cfu/ml
6-8/HPF
+
_
15
105 cfu/ml
2-3/HPF
_
+
16
105 cfu/ml
2-3/HPF
_
+
17
105 cfu/ml
2-3/HPF
_
+
18
105 cfu/ml
6-9/HPF
+
_
19
105 cfu/ml
5-7/HPF
+
_
20
105 cfu/ml
6-8/HPF
+
_
21
105 cfu/ml
6-8/HPF
+
_
22
105 cfu/ml
5-8/HPF
+
_
23
105 cfu/ml
6-9/HPF
+
_
24
105 cfu/ml
6-9/HPF
+
_
25
105 cfu/ml
6-8/HPF
+
_
26
105 cfu/ml
5-8/HPF
+
_
27
105 cfu/ml
6-9/HPF
+
_
28
105 cfu/ml
6-9/HPF
+
_
29
105 cfu/ml
6-8/HPF
+
_
30
105 cfu/ml
2-3/HPF
_
+
31
105 cfu/ml
2-3/HPF
_
+
32
105 cfu/ml
2-3/HPF
_
+
33
105 cfu/ml
2-3/HPF
_
+
34
105 cfu/ml
2-3/HPF
_
+
+=positive; - = negative
Table 7: Presence of pus cells, symptomatic and asymptomatic bacteriuria in culture positive urine samples.
Isolate no.
Suspected Organism
Antibiogram
Resistant (R)
Intermediate (I)
Sensitive (S)
01
E.coli
CV,NA,CO,AM
-
CIP,CN
02
E.coli
CV,NA,AM
-
CO,CN.CIP
03
E.coli
AM, CN
CO,NA
CIP, CV
04
E.coli
AM, CN, CO,NA
-
CIP , CV
05
Klebsiella
AM, ,CV,CIP
-
NA, CN, CO
06
E.coli
CIP, CN, ,AM
CO,NA
CV
07
E.coli
CIP,CN
AM,CV,CO
NA
08
Klebsiella
CIP,CN,AM
CO,NA
CV
09
Klebsiella
CIP,CN,AM
CO,NA
CV
10
E.coli
CV,NA,AM
-
CO,CN,CIP
11
E.coli
CV,NA,AM
-
CO,CN,CIP
12
Klebsiella
CIP,CN,AM
CO,NA
CV
13
Klebsiella
CIP,CN,AM
CO,NA
CV
14
Klebsiella
CIP,CN,AM
CO,NA
CV
15
E.coli
CV,NA,AM
-
CO,CN,CIP
16
E.coli
AM,CN,CV
CO,NA
CIP
17
E.coli
CIP,CN,CV
CO,NA
AM
18
E.coli
CV,NA,AM
-
CO,CN,CV
19
E.coli
AM,CN,CO,NA
-
CIP,CV
20
E.coli
CIP,CN
CO,NA
AM,CV
21
E.coli
CIP,CN,CO,NA
-
AM,CV
22
Klebsiella
CIP,CN,AM
-
CV
23
Klebsiella
CIP,CN,AM
-
CV
24
Klebsiella
CIP,CN,AM
-
CV
25
E.coli
AM,CN,CO,NA
-
CIP,CV
26
E.coli
CIP,CN
CO,NA
AM,CV
27
E.coli
CV,NA,AM
-
CO,CN,CIP
28
E.coli
CV,NA,AM
-
CO,CN,CIP
29
E.coli
AM,CN,CO,NA
-
CIP,CV
30
E.coli
AM,CN
CO,NA
CIP,CV
31
E.coli
AM,CN,CO,NA
-
CIP,CV
32
E.coli
AM,CN
CO,NA
CIIP,CV
33
E.coli
AM,CN,CO,NA
-
CIP,CV
34
E.coli
AM,CN,CO,NA
-
CIP,CV
AM: Amoxycillin (10µg); NA: Nalidixic acid (30µg); CIP: Ciprofloxacin (5µg); CN: Gentamicin (10µg); CV: Cephradin (30µg); CO: Cotrimoxazole (23.75µg)
Table 8: Antibiogram patterns of different Gram negative isolates.
Antimicrobials
Escherichia coli tested
No. Sensitive (%)
Amoxycillin
25
20(80%)
Nalidixic Acid
25
16(64%)
Gentamicin
25
17 (68%)
Ciprofloxacin
25
6 (24%)
Cephradine
25
10 (40%)
Cotrimoxazole
25
9 (36%)
Table 9: Sensitivity Test result of Escherichia coli to 6 Antimicrobials.
Antimicrobials
Klebsiella Tested
No. Sensitive (%)
Amoxycillin
9
9 (100%)
Nalidixic acid
9
Nil
Gentamicin
9
8(89%)
Ciprofloxacin
9
9(100%)
Cephradine
9
1(11%)
Cotrimoxazole
9
Nil
Table 10: Sensitivity Test result of Klebsiella to 6 Antimicrobials.
Microorganism
Number of microorganism
Percentage (%)
E. coli
25
73.53 %
Klebsiella
9
26.47 %
Total
34
Table 11: Percentage of microorganisms isolated from urine samples.
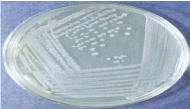
Figure 1: Bacterial Colonies on Nutrient agar.

Figure 2: Urine sample of patients & students-staff.

Figure 3: Bacterial colonies on Mac-conkey agar media shows pink colony
& Mucoid colony.

Figure 4: Acid slant, Acid Butt and able to produce gas.

Figure 5: Citrate utilization test.
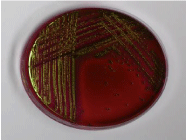
Figure 6: Green metallic colonies on EMB agar indicates E.coli.
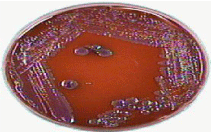
Figure 7: Glossy pink colonies on EMB agar indicates Kliebsiella spp.

Figure 8: Antibiotic susceptibility test. Clear zone around any antibiotic disk
indicates bacterial growth inhibition and susceptibility to that antibiotic.
Discussion
The incidence of urinary tract infection is frequently occurred in Bangladesh especially in female because of lacking knowledge of urinary tract infection, unhygienic environment and financial environment. Urine must be examined fresh and the test can be repeated before starting treatment unless the individual is suffering from fever. Only presence of symptoms such as urethral syndrome is not urinary tract infection and growth of bacteria in urine may be due to asymptomatic bacteriuria or contamination in absence of symptoms [4]. The present study was limited in Delta Medical College Hospital, which is a non-government hospital. A total of 110 urine samples, 57 were from out and admitted patients and 53 were from healthy staff and students. Among the 57 urine samples that were collected from patients, 21 samples showed growth on MacConkey media and the prevalence rate was 24.52%. This percentage varies according to nature and type of infection whether acute or chronic. Most of the patients were in 21-30, 31-40 and 41- 50 years og age groups. Two cases in 10-20 years age group and 2 cases in 51-60 years age group showed to positive urine culture. In cases of staffs and students, most of them were in the group of 21-30 years. Only one case was found to have positive urine culture in 41-50 years of age. These results may be used as base line information for building up wealth of knowledge of urinary tract infection. It is very important to know the etiology of infection in any system particularly in urinary system, where infection must be microbiologically cured. In this study, is the most common organism causing urinary tract infection was Escherichia coli (74%). This organism is the single bacteria reported by many workers as commonest cause of urinary tract infection. Second common organism was Klebsiell species (26%). In this study, 22 urine samples from the total 110 samples had pyuria which may indicate UTI. White blood cells and red blood cells were nil in all urine of patients and staff-students. Organized and unorganized deposits were also nil in all urine of patients and staff-students. The organisms which are commonly isolated from clinical specimens were tested against appropriate antimicrobials. This information will help clinicians and microbiologists to select the appropriate antibiotic for treatment of infection caused by the organism. Current knowledge of antibiogram also provides basic information on the status of the strains in the environment. In this study six types of antibiotics were used for doing sensitivity test against two types of identified organism. Antibiotic susceptibility test was performed with isolated bacteria by Kirby-Bauer method using commonly prescribe antibiotics e.g. Cephradin (30µg), Nalidixic acid (30µg), Ciprofloxacin (5µg), Gentamycin (10µg), Amoxycillin (10µg) and Cotrimoxazole (23.75µg). The drug resistance patterns varied considerably with different antibiotics. E. coli was found sensitive to Ciprofloxacin, Gentamycin and Nalidixic acid. The second common organism Klebsiella showed sensitivity to Amoxycillin, Gentamycin and Ciprofloxacin. It is reported that antibiotics should be wisely used but facilities of testing and reliability of testing are limited. It is rightly pointed out that in urgent cases where antimicrobial treatment should be started immediately empirical basis as bacteriological test takes 24 to 48 hours, this decision must also be based on the previous knowledge of sensitivity pattern collected over a period of time.
Conclusion
The locally isolated organism and their antibiograms allow clinicians and microbiologist to decide on treatment of urinary tract infection. Further studies are necessary for different groups of patients i.e. patients in hospital and community, acute or chronic infection and compare them with patients with anatomical abnormalities of the urinary tract, who tend to suffer for longer time and needs follow up for a longer period. This study shows more or less similarity to the other studies which were done by other researchers.
References
- Allasio D, Fischer H. Maintaining a sterile urinary tract: the role of antimicrobial peptides. J Urol. 2005; 182: 21-28.
- Berkman J, Rifkin H. Unilateral nodular diabetic glomerulosclerosis (Kimmelstiel-Wilson): Report of a case. Metabolism (Elsevier Inc.). 1973; 22: 715-772.
- Mittendorf SA, Mims AM. Prevention of preterm delivery and low birth weight associated with asymptomatic bacteriuria. Clin Infect Dis. 1992; 14: 927-932.
- Harding SA. How long should catheter-acquired urinary tract infection in women be treated? A randomized controlled study. Ann Intern Med. 1991; 114: 713-719.