
Research Article
Austin Med Sci. 2022; 7(1): 1060.
Antityphoid Activity and Probable Mechanism of Action of 95% Ethanolic Extract of Enantia chlorantha Stem Bark
Djimeli MN¹, Atsafack SS¹, Ndzotang JK¹, Tankeo S¹, Njateng GSS¹, Mendimi Nkodo J-M¹ and Gatsing D¹*
1Faculty of Science, University of Dschang, P.O. Box 67 Dschang, Cameroon
2Faculty of Medicine and Biomedical Sciences, University of Yaounde I, Yaounde, Cameroon
*Corresponding author: Donatien Gatsing, Faculty of Science, University of Dschang, P.O. Box 67 Dschang, Cameroon
Received: December 07, 2021; Accepted: January 19, 2022; Published: January 26, 2022
Abstract
Typhoid fevers are systemic diseases caused by Salmonella Typhi. These bacterial infections come from digestive route. The aim of this study was to evaluate the activity of the 95% ethanolic extract from Enantia chlorantha stem bark and to determine its mechanism of action.
When performed, microdilution in liquid medium, was used for the evaluation of the in vitro antisalmonella activity on Salmonella Typhi ATCC 6539. The phytochemical screening was assessed by the standard methods. The mechanism of action of the extract was performed through biofilms and proton ATPases-H+ Pumps inhibition.
In vitro antisalmonellal activity test reveals that the E. chlorantha extract presented MIC values of 128 μg/mL and MBC of 512 μg/mL on tested microorganism. The MBC/MIC ratio was equal to 4. Phytochemical analysis revealed the presence of alkaloids, anthocyanins, anthraquinons, flavonoids, phenols, and saponins in this extract. The later also inhibited biofilms formation and proton ATPases- H+ bacterial pumps.
The present study demonstrates that ethanolic extract of Enantia chlorantha stem bark possesses an interesting antisalmonella activity and could then be used for the treatment of typhoid fever.
Keywords: Antityphoid Activity; Mechanism of Action; Enantia chlorantha stem bark
Introduction
Typhoid fevers are systemic bacterial infections contracted through digestive route and found especially in developing countries where they are endemic [1]. Patients generally contract them through ingestion of contaminated water or /and foods by excrement of infected peoples or via direct transmission through human being. In 2014, the World Health Organization (WHO) reported that about 216 500 deaths are estimated annually over 22 million cases of typhoid fever [2]. The epidemiologic expansion of this disease could be explained by the adaptation of the responsible microorganism to news drugs, the negligence in the patient control, the non-respect by these patients of prophylaxis and the expensive cost of antibiotics [3]. Thus they continue to emerge and cause public health problem.
Many Plants, including herbs and spices, have many phytochemicals which are a potential source of natural antisalmonella substances, e.g., phenolic diterpens, flavonoids, alkaloids, tannins and phenolic acids [4-6]. It is the case of Enantia chlorantha commonly known as African yellow wood. In Nigeria, its stem bark is commonly used for the treatment of malaria and other ailments of the human body such as cough and wound [7]. The plant has been intensely studied for their antimicrobial activities [8] and antipyretic properties [9]. In Cameroon, stem bark extract is used to treat jaundice and urinary tract infections [10] and typhoid fever [11]. The mechanism of action of this plant has not been tested scientifically in most cases in order to justify its continuous use in traditional folk medicine. This study is therefore aimed at providing scientific evidence to its antibacterial potentials and the mechanism of action of E. chlorantha stem bark extract.
Materials and Methods
Plant material: Collection and identification
The stem bark of Enantia chlorantha was collected in Lekie Division, Central region of Cameroon, in March 2014. Identification of the plant was done at the National Herbarium, in Yaounde- Cameroon, using a voucher specimen registered under the reference N°25918/ SRFCAM. The stem bark was air-dried at room temperature (23±2°C) away from sunlight, and milled to coarse particle at the Biotechnology Centre, University of Yaounde I.
Tested bacteria and culture media
The tested bacterium was a strain of Salmonella Typhi (ATCC 6539), obtained from the American Type Culture Collection (ATCC) coming from the Medical Bacteriology Laboratory of the “Centre Pasteur” of Yaounde, Cameroon. Salmonella Shigella Agar (Italy Liofilchem) was used for activation and maintenance of the strain, and Mueller Hinton Broth (MHB) for the determination of the Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs).
Methods
Extract preparation
Hundred grams (100g) of Enantia chlorantha Stem bark powdered were macerated at room temperature (23 ± 2°C) in 1000 ml absolute ethanolic solvent (EtOH 95%) for 48 hours, and then filtered with Whatman paper (N°1). The filtrate was concentrated at 45°C using a rotary evaporator (Buchi R200) and the obtained volume was later dried at 40°C. The plant extracts were stored in sterilized bottles at room temperature till further usage.
Inocula preparation
Bacterial cell suspensions were prepared at 1.5×108 Colony- Forming Unit/mL (CFU/mL) following 0.5 McFarland turbidity. For this purpose, 18 hours old bacterial cultures were prepared in Salmonella Shigella Agar (SSA). From this culture, few colonies of bacteria were collected aseptically with a sterile wire loop and introduced into 10 mL of sterile 0.9% saline water. These suspensions were diluted 100 times with MHB to yield about 1.5×106 CFU/mL before use.
In vitro antisalmonellal test
The susceptibility of Salmonella species was tested by broth micro-dilution method. They were used against the Salmonella specie listed. As described by Newton and co-workers [12], the E. chlorantha extract was dissolved in 2.5% dimethylsulfoxide (DMSO) solution and two-fold serial dilutions of the test substances were made with Mueller Hinton Broth to yield a volume of 100 μL per well. One hundred microliters (100 μL) of 1.5×106 CFU/mL bacterial suspensions were added to respective wells containing test samples (except extract and medium sterility control wells) and mixed throughly to give final concentrations ranging from 8 to 1024 μg/ mL for extracts. Oxytetracyclin and ciprofloxacin were used as standard antibiotics at concentrations ranging from 1 to 128 μg/mL and 0.5 to 64 μg/mL respectively, followed by 18 hours incubation at 37°C of these preparations. The inhibitory concentration of the extract was determined after addition of 40 μL of 0.2 mg/mL of p-iodonitrotetrazolium chloride (INT) (Sigma Aldrich, South Africa) and incubation at 37 °C for 30 minutes. Viable bacteria change the yellow dye (INT) to pink color. The lowest concentrations at which there was no visible color change were considered as MICs. The MBC values were determined by adding 50 μL aliquots of the preparations (without INT), which did not show any visible color change after incubation during MIC determination, into 150 μL of fresh broth. These preparations were further incubated at 37 °C for 48 hours and MBCs were revealed by the addition of INT as above. All extract concentrations at which no color change were considered as bactericidal concentrations, and the smallest of these concentrations was considered as the MBC. These tests were carried out in triplicates.
Identification of the chemical compounds found in the 95% ethanolic extract of E. chlorantha stem bark
The chemical compounds contained in the E. chlorantha extract were detected by qualitative phytochemical analyzes. For this, the various extracts were subjected to a phytochemical screening according to the methods described by Harbone (1998) [13].
Study of the mechanism of action of the 95% ethanolic extract from E. chlorantha stem bark on Salmonella Typhi (ATCC6539)
Two mechanisms of antibacterial action of the ethanolic extract of E. chlorantha stem bark were studied using the strain Salmonella Typhi (ATCC6539). The illustrative curves or graphs of these mechanisms were obtained using the Microsoft Excel software.
Evaluation of the effect 95% ethanolic extract of E. chlorantha stem bark on ATPases-H+ proton pumps
The ability of the ethanolic extract to inhibit Salmonella Typhi (ATCC6539) H+, an ATPase pump was evaluated by monitoring the acidification of the external medium through pH measurement using a pH-meter (Hanna INSTRUMENTS: HI 2211, Woonsocket Rhode Island, USA).
For this purpose, a bacterial culture was previously prepared in a liquid medium. Conserved bacterial cells (in a mixture of MHBglycerol) were streaked in 90 mm petri dishes, and the culture was incubated at 37°C. For 18 hours. A bacterial colony was then removed and introduced into 20 ml of MHB contained in an Erlenmeyer flask. The mixture was incubated at 37°C with stirring. After 18 hours of incubation, the bacteria culture called pre-culture (OD600 = 1) was used to prepare the bacterial culture for subsequent experiments. Aliquots of bacterial pre-culture were removed and introduced into Erlenmeyer flasks containing MHB at the rate of 1 mL of preculture for a final volume of 100 mL (1/100 v/v dilution). After 18 hours of incubation with shaking at 37°C, a volume of 100 mL of bacterial culture was centrifuged at 4000 rpm for 30 minutes under cold conditions. The pellet obtained was washed with distilled water, then 50 mM KCl and re-suspended in 50 mL of KCl. The suspension was kept at 4 °C for 18 hours. A volume of 0.5 mL from the sample solution (at the MIC) dissolved in DMSO/MHB was added to 4 mL taken from this suspension. After 10 minutes of pre-incubation at 37°C, the acidification of the medium was started by adding 0.5 ml of a 20% (w/v) glucose solution.
Subsequently, the pH of the medium was measured every 15 minutes for 90 minutes. The negative control was represented by DMSO 2.5%. The pH values noted allowed the plotting of the pH progression curves as a function of time [pH = f (time)]. Any significant increase in pH in the presence of an antibacterial was attributed to an inhibitory effect of the H+ /ATPases pumps activity. This inhibition may be detrimental to the survival of the bacterium in that the proton pumps provide the bacteria the necessary energy for its metabolism and thus its development [14].
Evaluation of the anti-biofilm activity of the 95% ethanolic extract of E. chlorantha stem bark
The anti-biofilm potential of the ethanolic extract was determined according to the method described by Nikolic et al. (2014) [15]. Briefly, a volume of 100 μL of Mueller Hinton Broth was introduced into a 96-well microplate. The substances to be tested were introduced into the upper wells and diluted in a geometrical progression of order 2. Thereafter, 100 μL of a bacterial suspension of the Mac Farland 0.5 scale was introduced into the wells. The positive control consisted of culture medium, the microorganism and the reference antibiotic (ciprofloxacin); the neutral control of the culture medium alone; and the negative control consisted of the medium and the microorganism. The plates were incubated with shaking (130 rpm) for 24 h at 37°C. At the end of the incubation, the planktonic cells (which did not form biofilms) were removed from the microplates trough washing in 1N phosphate buffered saline pH 7.24. The plates were oven-dried at 65 °C for 10 minutes and the biofilm formed by the adherent cells was fixed with 3- [4,5-dimethyl-2-thiazolyl] -2,5-diphenyl. -2H-tetrazoliumbromide (MTT) for 30 minutes at laboratory temperature, and then rinsed with sterile distilled water and dried again. Subsequently, 200 μL of 30% acetic acid was added to the microplates. The optical densities were measured using an Elisa microplate reader (Dialab, France) at 570 nm. The inhibition percentage of the biofilm was calculated according to the following formula:
% I = [(OD negative control – OD test)/ OD negative control] × 100.
Results
In vitro antibacterial test
The ethanolic extract of the E. chlorantha stem bark presented the MIC value of 128 μg/ml and the MBC value of 512 (Table 1). Those values were more than those obtained with the reference antibiotics. The rate MBC/ MIC was equal to 4.
95% Ethanolic extract
Oxytetracyclin
Ciprofloxacin
MIC (μg/mL)
128
2
0.5
MBC (μg/mL)
512
4
2
MBC/MIC
4
2
4
MIC: Minimal Inhibitory Concentration; MBC: Minimal Bactericidal Concentration.
Table 1: MIC, MBC and MBC/MIC of the ethanolic extracts of E. chlorantha stem bark on Salmonella Typhi (ATCC6539).
The chemical compounds found in the 95% ethanolic extract of E. Chlorantha stem bark
Table 2 shows the qualitative distribution of the main groups of compounds in E. chlorantha bark extracts. It reveals the total absence of tannins and the presence of some secondary metabolites including alkaloids, anthocyanins, anthraquinons, flavonoids, phenols, steroids, saponins and triterpens.
Compounds
Alkaloids
Anthocyanins
Anthraquinons
Flavonoids
Phenols
Saponins
Steroids
Tannins
Triterpens
+
+
+
+
+
+
+
-
-
(-) Absent, (+) Present.
Table 2: Groups of compounds found in the 95% E. chlorantha stem bark extracts.
Mechanism of action of the ethanolic extract of E. chlorantha stem bark on Salmonella Typhi (ATCC6539)
Action of the extract on the membrane integrity
Figure 1 shows pH variation curves of culture media inoculated with the strain of Salmonella Typhi (ATCC6539) as a function of time, in the presence of the extract of E. chlorantha (EC) stem bark and DMSO (control). It appears that in the presence of the extract, the pH of 6.5 culture medium decreases very slightly; it reached the value of 5.95 only after 90 minutes. However, the pH obtained in the presence of the negative control (DMSO at 2.5%) decreases significantly over time and reached the value of 3.68 after 90 minutes, thus causing a strong acidification of the medium.
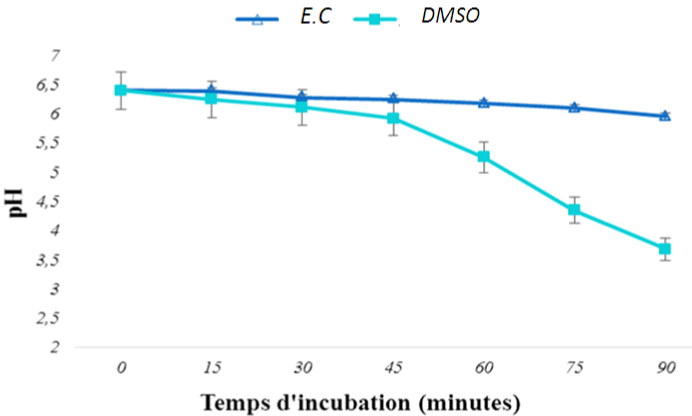
Figure 1: Variation of the pH of the culture medium inoculated with Salmonella
Typhi strain as function of time.
Anti-biofilm activity of the E. Chlorantha extract
The histograms in Figure 2 represent inhibition percentage of biofilm formation of 95% ethanolic extract of the E. chlorantha stem bark against S. Typhi strain. The analysis of this figure shows that, this extract presented an inhibition percentage of 89.12% which is slightly lower than that of ciprofloxacin (90.12%) used as a positive control.
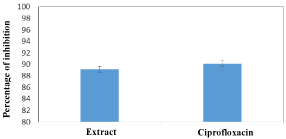
Figure 2: Effect of the 95 % ethanolic extract of E. Chlorantha stem barks on
the inhibition biofilm formations.
Discussion
The search for new antimicrobial substances is an important challenge to overcome the inadequacies of those already existing. One way to do this is by evaluating their activities [16]. The minimal inhibitory concentration obtained with 95% ethanolic extract of E. chlorantha stem bark (128 μg/mL) showed that it activity is moderate because according to Kuete and co-workers’ scale, an extract has significant activity when MIC ≤ 100 μg/mL; moderate when 100<MIC≤625 μg/mL and weak if MIC > 625) [17]. This activity could be due to the presence of secondary metabolites such as alkaloids, anthocyanins, anthraquinons, flavonoids, phenols, and saponins in these extract as observed during the experimentation. This extract is bactericidal on this tested Salmonella because of the fact that MBC/ MIC is equal to 4 and it is well known that the relation MBC/MIC≤ 4 means that the extract is bactericidal. This result could be explained by the presence of secondary compounds which are proven to have antibacterial properties [18-20].
The study of the possible mechanisms of action of the extract (inhibition percentages of biofilm formation of E. chlorantha stem bark extract), demonstrated its ability to inhibit Salmonella Typhi -induced H+ / ATPase proton pumps. Indeed, the energy required for the development of the metabolic reactions of the bacteria depends on the proper functioning of these pumps. The inhibition of the latter by a substance maintains H+ protons pump in its inactive form, and thus compromising the survival of the bacteria that will die from lack of energy. It is well known that bacteria are viable in a wide range of pH (1-11) and bacterial cytoplasmic pH is kept close to neutral [21]. It is also generally accepted that bacterial cytoplasmic pH is regulated by various cation transport systems [21]. In this experiment, the extract of E. chlorantha stem bark compared to the control induced the inhibition of proton pumps in S. Typhi, suggesting that S. Typhi is one of the targets of action of certain active compounds present in this extract.
A biofilm is a sessile form of bacterial existence on solid surfaces or air-liquid interfaces, in which bacteria multiply [22]. Its eradication is difficult to achieve because of the host defenses and the inherent resistance of antibiotics and biocides. In addition, several mechanisms are used to explain the resistance of biofilms to antimicrobials, making it difficult to predict the behavior of biofilm cells. The anti-biofilm activity of the ethanolic extract of E. chlorantha stem bark evaluated after 24 hours gave a percentage of biofilm inhibition of 89.12%, slightly lower than that of ciprofloxacin (90.12%) which was used as a positive control. This extract would significantly affect the elimination and / or inactivation of biofilms. In fact, the extracts of this medicinal plant can influence the formation of the biofilm by damaging the structures of the microbial membrane [23], by inhibiting the synthesis of peptidoglycan and/or modulating the detection of quorum [24]. Regarding the latter case, several approaches involving quorum interference have been studied and they represent the most recent strategies to counter gastroenteric infections including salmonellosis.
Conclusion
This study showed that although the activity of this plant appears moderate as compare with the standard antibiotics, it exerts significant antisalmonellal effects on the strain investigated in this study. Studies on the ability of E. chlorantha to inhibit biofilm formation and salmonella proton pumps were interesting and might lead to new chemical drugs with antisalmonellal potential. This plant constitutes a good source of natural substance and can be useful in the treatment of typhoid fever.
References
- Ansart S, Garré M. Maladies infectieuses: Fièvre typhoïde. Elsevier Masson SAS paris. 2008; 8: 19-20.
- WHO. Weekly epidemiological record. Typhoid fever surveillance and vaccine use, South-East Asia and Western Pacific Regions. 2004; 89: 429-440.
- Barré-Sinoussi F. Mondialisation et pathologies infectieuses: un défi annoncé par l’émergence du VIH/SIDA. Séance solennelle de l’académie des sciences. Réception des nouveaux Membres sous la coupole de l’Institut de France. 2009: 1-5.
- Amro B, Aburjai T, Al-Khalil S. Antioxidative and radical scavenging effects of olive cake extract. Fitoterapia. 2002; 73: 456-461.
- Cai YZ, Luo Q, Sun M, Corke H. Anti- oxidant activity and phenolic compounds of 112 Chinese medicinal plants associated with anticancer. Life Sciences. 2004; 74: 2157-2184.
- Moure A, Cruz JM, Franco D, Dominguez JM, Sineiro J, Dominguez H, et al. Natural antioxidants from residual sources. Food Chemistry. 2001; 72: 145-171.
- Handbook of African Medicinal Plants. Library of Congress cataloguing in Publication Data, New York. 2008.
- Gill LS, Akinwumi C. Nigerian folk medicine: Practices and beliefs of the Ondo people. Journal of Ethnopharmacology. 1986; 18: 259-266.
- Adesokan AA, Akanji MA, Yakubu MT. Antibacterial potential of aqueous extracts of E. chlorantha stem bark. African Journal of Biotechnology. 2007; 6: 2502- 2505.
- Adjanohoun JE, Aboobakar N, Dramane K. Traditional Medicine and Pharmacopoeia: Contribution to Ethnobotanical and Floristic Studies in Cameroon, Porto-Novo, Benin. Organisation of African Unity Scientific Technical and Research Commission. Centre National de Production de Mannels Scolaries. 1996.
- Djimeli MN, Mendimi JM, Njateng GS, Fokunang C, Kodjio N, Atsafack SS, et al. In vitro Antioxidant and Anti-salmonellal activities of Stem Bark extracts of Enantia chlorantha (Annonaceae). Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2017: 0975-8585.
- Newton SM, Lau C, Gurcha SS, Besra GS, Wright CW. The evaluation offorty-three plant species for in vitro antimycobacterial activities; isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J Ethnopharmacol. 2002; 79: 57-67.
- Harbone JB. Phytochemical methods: A guide to modern techniques of plants analysis. London: Chapman and Hall Ltd. 1998: 50-116.
- Kobayashi H. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. Journal of Biological Chemistry. 1985; 260: 72-76.
- Nikolic M, Vasic S, Ðurdevic J, Stefanovic O, Comic L. Antibacterial and anti-biofilm activity of ginger (zingiber officinale (roscoe)) ethanolic extract. Kragujevac Journal of Science. 2014; 36: 129-136.
- Nunes APF, Teixeira LM, Bastes CCR, Silva MG, Ferreira RB, Fonseca LS, et al. Genomic characterization of oxacillin-resistant Staphylococcus epidermidis and Staphylococcus haemolyticus isolated from Brazilian medical centers. Journal of Hospital Infection. 2005; 59: 19-26.
- Kuete V. Potential of Cameroonian plants and derived products against microbial infections. Planta Med. 2010; 76: 1-13.
- Ajaiyeoba EO. Phytochemical and antibacterial of Parkia biglobosa and Parkia bicolor leafs extracts. Afr. J. Biochem. Res. 2002; 5: 125-129.
- Cowan MM. Plant products as antimicrobial agent. Cli. Micr.Rev. 1999; 4: 564-582.
- Ogunnusi TA, Oso BA, Dosumu OO. Isolation and antibacterial activity of triterpenes from Euphorbia kamerunicaPax. Int. J. Biol. Chem. Sci. 2010; 4: 158-167.
- Padan E, Zilbersteidn D, Schuldinesr S. pH homeostasis in bacteria. Biochimica et Biophysica Acta. 1981; 650: 151-166.
- Slobodníková L, Fialová S, Rendeková K, Kovác J, Mucaji P. Antibiofilm activity of plant polyphenols. Molecules. 2016; 21: 1717.
- Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmingto JR, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). Journal of Applied Microbiology. 2000; 88: 170-175.
- Hartmann A, Rothballer M, Hense BA, Schröder P. Bacterial quorum sensing compound is important modulators of microbe-plant interactions. Frontiers in Plant Science. 2014; 5: 131.