
Research Article
Austin Med Sci. 2022; 7(1): 1061.
TEK C2545T Germline Mutation in Blue Rubber Bleb Nevus Syndrome
Wu X, Chen L, Xiao Y, Song Y* and Cheng H*
Department of Dermatology and Venereology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310016, Zhejiang, PR China
*Corresponding author: Hao Cheng, Department of Dermatology and Venereology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310016, Zhejiang, PR China
Yinjing Song, Department of Dermatology and Venereology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou 310016, Zhejiang, PR China
Received: December 20, 2021; Accepted: January 20, 2022; Published: January 27, 2022
Abstract
Objective: Blue rubber bleb nevus (BRBNS) syndrome is characterized by numerous venous malformations, which usually affect the skin, mucous, and gastrointestinal tract. However, the mechanisms of BRBNS syndrome are unclear.
Materials and Methods: Blood samples were obtained from the fivegeneration pedigree and Genomic DNA was extracted from blood samples with a TianGen DNA Extraction Kit. Agilent SureSelect Exon sequencing was applied to capture suspicious mutation sites. The suspected pathogenic gene mutations were amplified with polymerase chain reaction (PCR) and analyzed with Sanger sequencing.
Results: In this study, we found a five-generation pedigree with venous malformations that were suspected to be blue rubber bleb nevi. The cosegregation of disease phenotypes indicated the autosomal dominant mutation phenomenon. Heterozygous and inherited TEK mutations for the c.2545C>T substitution were detected in 3 affected members (II2, II14 and IV2), while the unaffected family members (II6 and IV7) carried the wild-type TEK gene, which cosegregated with the phenotype in this pedigree. Therefore, the novel missense variant c.2545C>T enriched the TEK mutation spectrum and may serve as a valuable genetic marker for the molecular diagnosis and prompt genetic counseling for BRBN.
Conclusion: This study identified a novel inherited germline C2545T mutation in exon 15 of the TEK gene that can contribute to the pathology of pedigree-based venous malformations.
Keywords: Blue rubber bleb nevus syndrome; TEK C2545T mutation; Germline mutation
Introduction
Blue rubber bleb nevus (BRBN), characterized by small multifocal cutaneous and mucosal venous-like lesions, is considered a sporadic and rare syndrome that was first described by Gascoyen in 1860 [1-3]. It is a congenital venous malformation prominent in the skin, soft tissues and gastrointestinal tract and may also occur in other tissues. The cutaneous lesions of BRBN are generally small and blue to purple in color. Patients with BRBN usually exhibit tens to hundreds of lesions at birth, which subsequently grow in size and number. Moreover, gastrointestinal venous malformations are fragile and can cause hemorrhage, intussusception, volvulus, and severe chronic anemia. Surgical resection and repeated sclerotherapy are recommended in these cases [4-6].
TEK mutation has been discovered to mediate a spectrum of venous disorders, including inherited cutaneous venous malformation (VMCM), sporadically occurring unifocal venous malformation (VM), multifocal VM and BRBN. As a member of the endothelial cell tyrosine kinase receptor family, TEK genecoding TIE2 has a unique extracellular region that contains two immunoglobulin (Ig)-like domains, three fibronectin type III repeats, and three epidermal growth factor (EGF)-like domains, which can bind with angiopoietin-1 (ANGPT1) to mediate embryonic vascular development [7, 8]. Pathogenic TEK variants can promote the formation of multiple cutaneous and mucosal venous malformations associated with BRBN [9-11].
BRBN can affect males and females equally without gender bias. However, the condition is found disproportionately among people of various nationalities. The United States has most cases with a reported 20%, followed by of Japan with 15%; a relatively low frequency is reported in other countries (Spain, 9%; Germany, 9%; France, 6%; China, 6%) [12].
In this pedigree-based study, we found an inherited TEK C2545T germline mutation, and a literature review also demonstrated that rare variants of single-nucleotide polymorphisms (SNPs) and somatic double and cis mutations occurred in TEK. All of these findings will enrich the understanding of the TEK-mediated pathogenesis of venous malformation.
Materials and Methods
Patients
The 69-year-old female proband presented to our department with multiple blue-violet papules and nodules all over the body since birth. Some of her family members share the same clinical features. Information on five generations of the family was collected via interviews, including gender, symptoms, age at symptom onset, etc. Blood samples from the proband’s aunt (II2), brother (III14), daughter (IV2), and other unaffected family members (III16 and IV7) were obtained to mine the possible pathogenic gene mutations. Informed consent was obtained from all participants.
Genetic sequencing
Genomic DNA was extracted from blood samples with a TianGen DNA Extraction Kit. Agilent SureSelect Exon sequencing was applied to capture suspicious mutation sites. FastQC was utilized to perform quality control of the original sequencing data, which was further screened with the human genetic variation database (HGVD), the relevant guidelines of the American College of Medical Genetics and Genomics (ACMG), literature reports, functional tests, genetic models, and genotype-phenotype correlation analysis. The suspected pathogenic gene mutations were amplified with polymerase chain reaction (PCR) and analyzed with Sanger sequencing.
Search strategy
To comprehensively review BRBN cases with TEK mutations, literature retrieval was performed on PubMed, MEDLINE, and the Chinese Biomedical Database with mesh terms ‘blue rubber bleb nevus’ and ‘TEK Receptor Tyrosine Kinase’. Articles published in the English and Chinese languages were independently screened. Available clinical data, including sporadic or inherited distribution and types of TEK variants, were sorted out.
Results
Clinical features of a BRBN family
Proband: Inpatient medical examination found that there were multifocal rubbery and blue-violet cutaneous venous malformations on the finger, tongue and lip (Figure 1) and skin-colored subcunaeous VMs on the limbs and face, all of which could be traced back to the time of birth. The size for most lesions was between 0.5 and 1 cm. Pelvic enhanced MRI images showed masses in the bilateral common iliac artery bifurcation that were isointense on T1-weighted images and gave high signal on T2-weighed images, which were considered hemangiomas (Figure 1d). In addition to the patient’s hemangiomas, adenocarcinoma had been diagnosed in the left colon requiring surgical resection 1 year earlier. During the laparoscopic surgery, multiple hemangiomas were found in the intestinal mesentery and beside the iliac vessels, which was consistent with the pelvic MRI results.
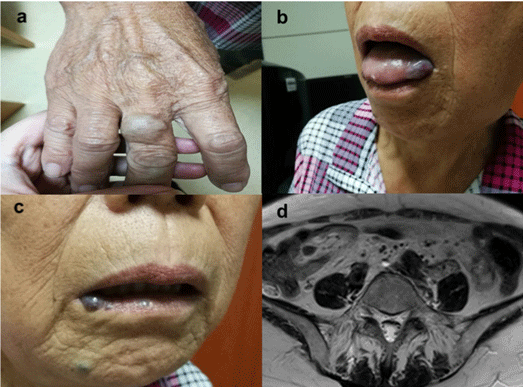
Figure 1: Skin and mucous lesions of the pedigree in the right finger (a),
tongue mucosa (b), and lips (c), and hemangiomas can be observed in the
right common iliac artery bifurcation inside and the left common iliac artery
bifurcation outside the upper group with MRI enhancement (d).
Other affected family members: The five-generation Chinese family with venous malformations was traced. A total of 8 patients are shown in the pedigree chart (Figure 2). The proband’s grandfather had the earliest onset (I1), and her grandmother showed no abnormal changes. The proband’s mother had 3 sisters and 1 younger brother, among whom the proband’s mother and 1 aunt were affected. Her aunt (II2) showed VMs on the lips and face (Figure 3a). Her mother who had already passed away was recalled with similar skin lesions. In addition, 1 sister and 1 brother of 5 siblings of the proband were affected, and the other 3 sisters were considered healthy. The proband’s sister (III12) had VMs on the lips and fingers (Figure 3d). The proband’s daughter (IV2) has a blue-violet VM on the ear, lips, neck, and hands (Figure 3b and 3c). All patients had similar skin lesions at birth, which grew gradually, and some of them were painful.
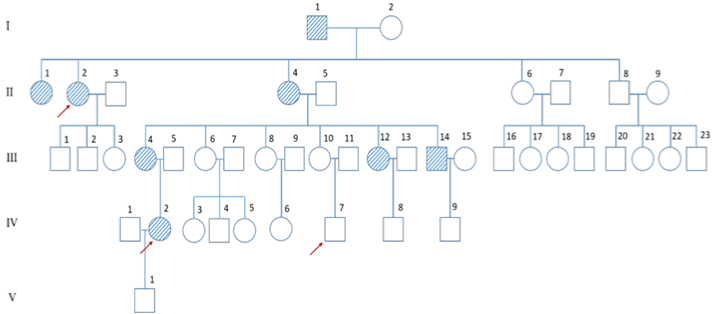
Figure 2: Pedigree diagram depicting the proband (III4), other affected and unaffected members. Samples for full exome detection were II2, IV2 and IV7 (marked
by arrows in the figure). Solid symbol, pedigree members with BRBN; blank symbols, members without BRBN.
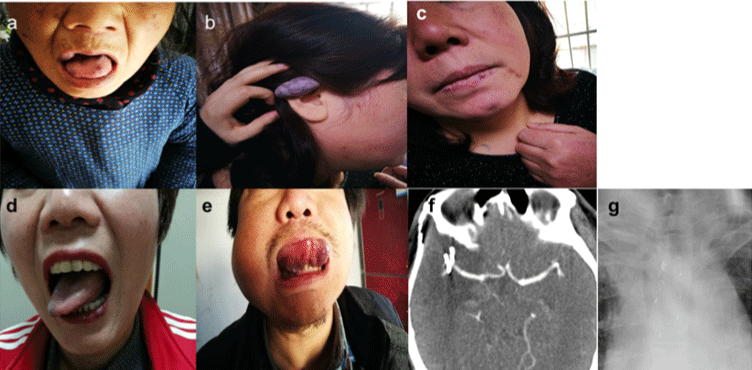
Figure 3: Skin and mucous lesions and image detection in the family members of the pedigree. The lip rash in the proband’s paternal aunt (a), ear rash in the
proband’s daughter (b), neck rash (c) and lip rash (d) in the proband’s sister, and lips and tongue mucosal rash in the proband’s brother (e). Cerebral image of the
proband’s brother, who underwent a ruptured cerebral aneurysm 3 years ago (f). Chest radiographs detected multiple mass-like high-density shadows, suggesting
the occurrence of hemangioma (g).
However, the proband’s younger brother (III14) was affected in a more aggravated way. He not only had blue-violet nodules (Figure 3e) on the sublingual mucosa, hands, arms, and back but was also had hemangiomas in internal organs, including the brain, glottis and esophagus. He underwent brain aneurysm surgery because of a rupture (Figure 3f). A hemangioma of 2cm diameter was found in front of the glottis under laryngoscopy. The posterior chest radiograph showed multiple hemangiomas in the esophagus (Figure 3g).
Mutation in the TEK gene
Whole-exome sequencing identified that affected members, including the proband’s aunt (II2) and daughter (IV2), were heterozygous for c.2545C>T (p. R849 W) in the TEK gene. Other members were subjected to TEK genetic analysis, and it was revealed that the proband’s younger brother (III14), who suffered from more severe BRBN, also carried TEK variants, while the unaffected family members (III16 and IV7) carried the wild-type gene (Figure 4). The cosegregation of disease phenotypes in this affected family indicated the autosomal dominant mutation phenomenon (Figure 2).
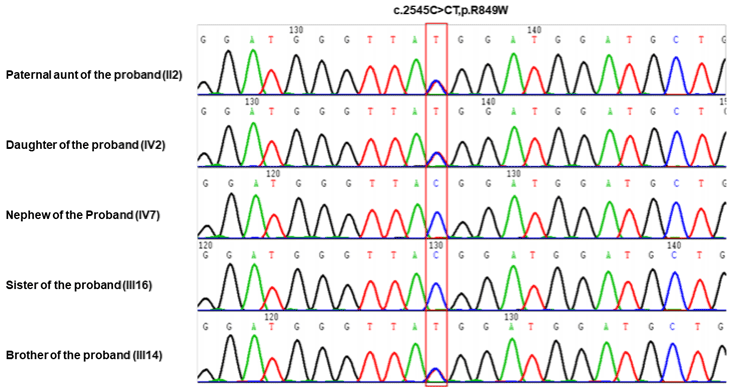
Figure 4: TEK gene mutation (c.2545C>T) detection results in family members. Genomic DNA was extracted from blood samples from the five-generation pedigree
with a TianGen DNA Extraction Kit. Agilent SureSelect Exon sequencing was applied to capture suspicious mutation sites. The suspected pathogenic gene
mutations were amplified with polymerase chain reaction (PCR) and analyzed with Sanger sequencing.
Review of TEK alteration reported in BRBN
A comprehensive review of TEK alterations detected in BRBN was implemented. Data from three previous studies along with the present study were incorporated (Table 1). The overall population frequency was very low (1/100000), and 3 of 4 cases with TEK mutations were reported in China. Both C591T SNPs [13] and C2545T mutations [14] have been found in sporadic cases. In our pedigree-based study, the C2545T germline mutation was detected in an inherited form for the first time. No double and cis mutations (T1105N-T1106P) were found on the same allele of TEK to induce ligand-independent TEK activation, as in the study in Belgium [9].
Country
Reporter
Distribution
Locus
Variants
China
Ji-Rong Huo [13]
Sporadical
C591T
Single nucleotide polymorphism
Julie Soblet [9]
Sporadical
T1105N-T1106P
Double and cis somatic mutations
China
Ke-Ling Wang [14]
Sporadical
C2545T
Somatic mutations
China
Xia Wu
Pedigree
C2545T
Germline mutation
Table 1: TEK variants reported in blue rubber bleb nevus (BRBN) syndrome patients.
Discussion
The genetic characteristics of BRBN are generally considered sporadic. The autosomal dominant TEK somatic mutation C2545T is the most common sporadic mutation detected in exon 15 of the TEK receptor (written as Arg849Trp or R849 W). The mutation, which can replace the amino acid arginine with the amino acid tryptophan at position 849, was noted in a sporicidal report [14]. In our pedigreebased investigation, an inherited C2545T germline mutation was identified. It is assumed that the genotype of the proband’s grandfather is a heterozygous Aa (A is a dominant pathogenic gene for this disease), and family members’ mates are healthy individuals aa. The heritability of the disease is 50%, but why the incidence rate is higher in females remains unknown.
A previous study indicated that 1 of 3 sporadic BRBN cases and 9 of 50 normal controls carried a heterozygous same-sense mutation (C591T) in the TEK gene, which suggested that the mutation was just an SNP and had no relationship with the pathogenesis of BRBN [13]. It is interesting to note that double (cis) mutations, that is, two somatic mutations (T1105N-T1106P) on the same allele of the TEK gene encoding the intracellular domains of TIE2, are observed in Belgian sporicidal patients with BRBN, while their blood DNA does not exhibit any evidence of such mutations [9]. All of these variants along with the C2545T mutation can lead to an increase in the autophosphorylation level of TIE, followed by inhibited endothelial cell apoptosis and continuous promotion of endothelial cell growth. Dysregulated vascular endothelial cells and perivascular smooth muscle cells eventually result in venous malformations.
At present, venous malformations caused by TEK gene mutations also include inherited mucocutaneous venous malformations (VMCMs), common focal venous malformations, and multiple focal venous malformations [15,16]. VMCMs and BRBN have similar manifestations, which are difficult to distinguish in most cases. However, we could identify subtle differences in clinical manifestations to discriminate these 2 diseases. VMCMs are light, small and asymptomatic lesions that are more frequently found in the neck, face or limbs. Lesions on the body and trunk are rare. Mucosal lesions of VMCM are common in the lips, tongue, and oral mucosa, with viscera hardly affected. The hereditary pattern of VMCMs is similar to BRBN, autosomal dominant with the same mutation gene [17-19]. Inherited germline C2545T mutation of TEK in venous malformation will further enrich our understanding of how these venous malformations are generated.
Although a variety of treatment methods are used for BRBN, such as laser sclerotherapy, interferon administration, and combined surgical and endoscopic cryoablation, there is no effective radical treatment [20,21]. In addition, mutated TEK can bind to angiopoietins and then regulate cell proliferation, adhesion, migration, angiogenesis, and vascular quiescence via the TIE2-PI3K-AKT-mTOR pathway to play a crucial role in diseases caused by abnormal proliferation of endothelial cells [22,23]. Therefore, blocking the PI3K-AKT-mTOR pathway may benefit patients. For example, rapamycin, an inhibitor of mTOR, can effectively reduce AKT pathway phosphorylation in vivo and in vitro to control the progression of skin lesions [20,24,25]. Although rapamycin and sirolimus have been used in a few clinical trials, multiple-center investigations are recommended to obtain clear follow-up results.
One limitation of our study is that pedigrees are extremely unusual, which makes it difficult to find subjects, investigate and compare the genetic mechanisms. A shortage of publications about TEK variant detection is another limitation. We have tried our best to present an overall picture of TEK variants in venous malformations based on available data. The clinical case summarization and relevant variants identified in the TEK gene may provide more evidence to better understand the pathologic mechanism.
Conclusion
This study identified a novel inherited germline C2545T mutation in exon 15 of the TEK gene that can contribute to the pathology of pedigree-based venous malformations. A literature review summarizes the relevant TEK variants in BRBN and stresses the importance of further detailed mechanism detection.
Declartion
Data Availability Statement: The data sets generated during the current study are available from the corresponding author upon request.
Acknowledgments: We would like to thank Mu Sha for technical assistants and Qiang Zhou for writing assistance.
Funding information: This work was supported by grants from the Medical Scientific Research Foundation of Zhejiang Province, China (2019330777).
Statement of Licensing Committee and Institution: This study was approved by the Ethics Committee of Sir Run Run Shaw Hospital of ZheJiang University School of Medicine (approval no. 20180228- 2).
Author contributions: Xia Wu, Hao Cheng conceived and designed the study. Xia Wu and Luxia Chen performed the experiments. Luxia Chen, Ying Xiao analyzed the data. Xia Wu and Hao Cheng wrote and reviewed the manuscript. Xia Wu and Hao Cheng have overall responsibility for this manuscript. Hao Cheng was responsible for supervision.
References
- Bazzell A, Williams S, Turner J, et al. Blue Rubber Bleb Nevus Syndrome: A Surgical Dilemma [J]. Am Surg. 2019; 85: e492-e493.
- Mayba JN, Cullingham K. Blue rubber bleb nevus syndrome [J]. CMAJ. 2019; 191: E841.
- Sullivan CA. Blue Rubber Bleb Nevus Syndrome [J]. Anesthesiology. 2018; 129: 1169.
- Ballieux F, Boon LM, Vikkula M. Blue bleb rubber nevus syndrome [J]. Handb Clin Neurol. 2015; 132: 223-230.
- Moser CM, Hamsch C. Successful treatment of cutaneous venous malformations in a patient with blue rubber bleb naevus syndrome by Nd:YAG laser [J]. Br J Dermatol. 2012; 166: 1143-1145.
- Petek B, Jones RL. The management of ophthalmic involvement in blue rubber bleb nevus syndrome [J]. GMS Ophthalmol Cases. 2014; 4: Doc04.
- Jayson GC, Zhou C, Backen A, et al. Plasma Tie2 is a tumor vascular response biomarker for VEGF inhibitors in metastatic colorectal cancer [J]. Nature communications. 2018; 9: 4672.
- Yun JH, Lee HM, Lee EH, et al. Hypoxia reduces endothelial Ang1-induced Tie2 activity in a Tie1-dependent manner [J]. Biochemical and biophysical research communications. 2013; 436: 691-697.
- Soblet J, Kangas J, Natynki M, et al. Blue Rubber Bleb Nevus (BRBN) Syndrome Is Caused by Somatic TEK (TIE2) Mutations [J]. J Invest Dermatol. 2017; 137: 207-216.
- Ten Broek RW, Eijkelenboom A, Van Der Vleuten CJM, et al. Comprehensive molecular and clinicopathological analysis of vascular malformations: A study of 319 cases [J]. 2019; 58: 541-550.
- Kangas J, Natynki M, Eklund L. Development of Molecular Therapies for Venous Malformations [J]. Basic & clinical pharmacology & toxicology. 2018; 123: 6-19.
- Jin XL, Wang ZH, Xiao XB, et al. Blue rubber bleb nevus syndrome: a case report and literature review [J]. World J Gastroenterol. 2014; 20: 17254- 17259.
- Huo J, Wang S, Xia K, et al. Relation Between Blue Rubber Bleb Nevus Syndrome (BRBNS) and TIE2 Gene Mutation [J]. Medical clinical research. 2009; 26: 1577-1579.
- Wang KL, Ma SF, Pang LY, et al. Sirolimus alternative to blood transfusion as a life saver in blue rubber bleb nevus syndrome: A case report [J]. Medicine (Baltimore). 2018; 97: e9453.
- Blum L, Gallas S, Cottier JP, et al. [Percutaneous sclerotherapy for the treatment of soft-tissue venous malformations: a retrospective study of 68 patients] [J]. J Radiol. 2004; 85: 107-116.
- Piraino D, Andolina G, Messina A. Truncular Venous Malformations in Meniere Disease Patients: The Key for a New Treatment Perspective? J Vasc Interv Radiol. 2016; 27: 766-768e1.
- Zuniga-Castillo M, Teng CL, Teng JMC. Genetics of vascular malformation and therapeutic implications [J]. Current opinion in pediatrics. 2019; 31: 498- 508.
- Fereydooni A, Dardik A, Nassiri N. Molecular changes associated with vascular malformations [J]. Journal of vascular surgery. 2019; 70: 314-326. e1.
- Greene AK, Goss JA. Vascular Anomalies: From a Clinicohistologic to a Genetic Framework [J]. Plastic and reconstructive surgery. 2018; 141: 709e-717e.
- Yuksekkay AH, Ozbek O, Keser M, et al. Blue rubber bleb nevus syndrome: successful treatment with sirolimus [J]. Pediatrics. 2012; 129: e1080-1084.
- Arena M, Virdis M, Morandi E, et al. Blue rubber bleb nevus syndrome: combined surgical and endoscopic treatment [J]. Endoscopy. 2015; 47: E372-373.
- Natynki M, Kangas J, Miinalainen I, et al. Common and specific effects of TIE2 mutations causing venous malformations [J]. Human molecular genetics. 2015; 24: 6374-6389.
- Ji Y, Chen S, Li K, et al. Signaling pathways in the development of infantile hemangioma [J]. Journal of hematology & oncology. 2014; 7: 13.
- Ferres-Ramis L, Knopfel N, Salinas-Sanz JA, et al. Rapamycin in the treatment of blue rubber bleb nevus syndrome [J]. Actas Dermosifiliogr. 2015; 106: 137-138.
- Boscolo E, Limaye N, Huang L, et al. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects [J]. The Journal of clinical investigation. 2015; 125: 3491-3504.