
Research Article
Austin Med Sci. 2022; 7(1): 1062.
DHA and EPA are Able to Affect the Development of Stress-Induced Senescence
Janubova M¹*, Gbelcová H², Konarikova K¹, Szentesiova Z¹ and Zitnanova I¹
¹Institute of Medical Chemistry, Biochemistry and Clinical Biochemistry, Faculty of Medicine, Comenius University, Bratislava, Slovakia
²Institute of Medical Biology, Genetics and Clinical Genetics, Faculty of Medicine, Comenius University, Bratislava, Slovakia
*Corresponding author: Janubova M, Institute of Medical Chemistry, Biochemistry and Clinical Biochemistry, Faculty of Medicine, Comenius University, Sasinkova 2, 811 08 Bratislava, Slovakia
Received: February 17, 2022; Accepted: March 12, 2022; Published: March 19, 2022
Abstract
Omega-3 fatty acids are an important part of biological membranes affecting their properties, cell signaling and gene expression. Senescence is an irreversible permanent cell cycle arrest accompanied by changes in cell morphology and physiology. We hypothesized that DHA as well as EPA could suppress the development of stress-induced premature senescence. To induce senescence, MRC-5 human lung fibroblasts were incubated with 100μM hydrogen peroxide/1hour. DHA (10 and 20 μM) or EPA (10, 20, 30 and 40 μM) were added to the cells for 24 hours either before (pretreatment) or after (postreatment) the induction of senescence. Only after postreatment with 10μM DHA or 20μM EPA, we detected slightly improved hallmarks of senescence - decreased percentage of SA-β-galactosidase positive cells, increased cell growth, reduced level of reactive oxygen species, cell cycle progression and decreased p21 protein expression. Based on our results we can conclude that DHA as well as EPA affect the development of peroxide induced senescence.
Keywords: Senescence; Docosahexaenoic acid eikosapentaenoic acid; Omega-3 fatty acids; MRC-5 human lung fibroblasts
Abbreviations
DHA: cis-4,7,10,13,16,19-Docosahexaenoic Acid; EPA: 5,8,11,14,17-Eikosapentaenoic Acid; IL-8: Interleukin-8; MMP-1: Matrix metaloproteinase-1; ROS: Reactive Oxygen Species
Introduction
Senescent cells have been detected in vitro and also in vivo in various types of cells (fibroblasts, endothelial cells, chondrocytes, glial cells, melanocytes, adult stem cells etc.) of different species (mice, rats, primates, humans) [1-6]. Senescent cells are cells that cannot divide anymore. Permanent cell cycle arrest is triggered by replication exhaustion or various types of stressors such as DNA damaging agents, oxidative stress and overexpression of activated oncogenes or mitochondrial dysfunction [7-11]. Senescent cells are characterized by typical changes in morphology and physiology (Table 1) [1,12- 19]. It has been observed that senescent cells accumulate in tissues and organs with age and also occur in the affected tissues and organs of patients with age-related diseases [20]. Accumulation of senescent cells has detrimental effects on organism by contributing to the development of cancer, chronic inflammation and age-related pathologies [21-26]. Moreover, senescent cells can induce senescence in other cells in a paracrine manner [25].
Hallmarks of senescent cells
References
Increased activity of SA-β-galactosidase
[12]
Enlarged and flattened morphology, enlarged nucleus and nucleolus
[1,13]
Increased production of reactive oxygen species
[14]
SDF (senescence associated DNA damage foci)
[15]
SAHF (senescence associated foci of heterochromatin)
[16]
Increased expression of p53, p21 and p16
[16]
SASP (senescence associated secretory phenotype)
[17-19]
Table 1: Typical hallmarks of senescent cells.
Omega-3 fatty acids are an important part of biological membranes influencing membrane elasticity, fluidity, permeability and fusion as well as function of various membrane proteins (enzymes, transporters, receptors) [27]. Amongst the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) have been shown to be the important determinant of cell structure and function [28]. DHA and EPA can act as potent ligands for cellular and nuclear receptors [29], lead to increased expression of antioxidant enzymes [30], decreased mitochondrial reactive oxygen species production [31], promote anti-inflammatory signaling cascades [32,33] and play a role in the protection against development and progression of age-related diseases [29].
We hypothesized that DHA as well as EPA could suppress the development of stress-induced premature senescence. We have investigated the effect of various concentrations of DHA and EPA on peroxide-induced senescence in MRC-5 human lung fibroblasts. Our results showed that DHA as well as EPA were able to suppress the development of peroxide-induced senescence in MRC-5 human lung fibroblasts.
Material and Methods
Chemicals
Minimum Essential Medium (MEM), 1% non-essential amino acids, 1% L-glutamine and penicilin-streptomycin mixture, 10% fetal bovine serum, 30% w/w hydrogen peroxide, cis-5,8,11,14,17- eikosapentaenoic acid, cis-4,7,10,13,16,19-docosahexaenoic acid, Thiazolyl Blue Tetrazolium Bromide (MTT), Senescence Cells Histochemical Staining Kit, Protease Inhibitor Cocktail Set III, EDTA-Free, Triton X-100 were purchased from Sigma (Merck), Germany. DC protein assay kit and Clarify Western ECL Substrate kit were obtained from Bio-Rad, USA. Muse™ Cell Cycle Kit and Muse® Oxidative Stress Kit were obtained from Merck, Germany. Anti-p21 mouse monoclonal primary antibody was obtained from Sigma (Merck), Germany. a-tubulin mouse monoclonal primary antibody, anti-mouse IgG secondary antibody conjugated to HRP were obtained from Santa-Cruz, Germany.
Cell culture
MRC-5 human lung fibroblasts (ECACC, England) were cultured in MEM containing 10% fetal bovine serum, 1% non-essential amino acids, 1% L-glutamine and penicilin-streptomycin mixture. Cells were incubated at 37°C in a 5% CO2 atmosphere in an incubator. Cells at passage number 12 to 13 were used.
Induction of stress-induced premature senescence in MRC-5 human lung fibroblasts
We used the MRC-5 model of peroxide-induced senescence previously established in our laboratory [35].
Cells were cultured for 24 hours and the senescence was induced by their treatment with 100μM hydrogen peroxide for 0.5 hours. Markers of senescence were determined on the 4th day after the induction of senescence. Just before use hydrogen peroxide was dissolved in 1xPBS to prepare 100mM stock solution.
Treatment of MRC-5 human lung fibroblasts with DHA and EPA
DHA and EPA were dissolved in dimethyl sulfoxide to prepare 50μM and 100μM stock solution, respectively and stored at -20°C.
Pretreatment: DHA or EPA was added to the cells for 24 hours. The medium was then replaced and senescence was induced by the treatment of the cells with 100μM hydrogen peroxide for 0.5 hours. Cell growth was determined on the 4th day after the induction of senescence.
Postreatment: DHA or EPA was added to the cells immediately after removing culture medium containing 100μM hydrogen peroxide. Postreatment with DHA or EPA lasted for 24 hours. The culture medium was then replaced and markers of senescence were determined on the 4th day after the induction of senescence.
Cells treated with dimethyl sulfoxide 5000x diluted in the medium but not treated with DHA or EPA were used as an untreated control.
MTT viability/proliferation assay
Cells were seeded on 96-well plates at a density of 3,200 cells/well and treated as described above. On the 4th day after the senescence induction thiazolyl blue tetrazolium bromide (MTT) was added to the culture medium. After 4-hour incubation at 37°C in a 5% CO2 atmosphere, the culture medium containing MTT was removed and 200μl of DMSO was added to each well. The absorbance was measured at 490nm with a spectrophotometer BioTek EL808, USA.
SA-β-galactosidase assay
Cells were seeded on 24-well plates at a density of 5,000 cells/well (control cells) or 38,000 cells/well (cells with the induced senescence). Cells were treated as described above.
Cellular senescence was determined by SA-β-galactosidase staining. SA-β-galactosidase staining was performed using the Senescence Cells Histochemical Staining Kit (Sigma/Merck, Germany) according to the manufacturer’s guidelines. The staining was evaluated after 16-18h incubation at 37°C in a CO2 - free atmosphere. Cells from 18 different fields were counted. A percentage of blue stained cells (SA-β-galactosidase positive cells) were presented as the percentage of senescent cells.
Detection of reactive oxygen species
Cells were seeded on culture plates at a density of 190,000/30mm dish and treated as described above. On the 4th day after the induction of senescence, cells were processed and reactive oxygen species were detected according to MUSE® Oxidative Stress Kit (Merck).
Detection of cell cycle phases
Cells were seeded on culture plates at a density of 600,000 cells/60mm dish and treated as described above. On the 4th day after the induction of senescence, cells were processed and cell cycle phases were detected according to MUSE® Cell Cycle Assay kit (Merck) protocol.
Western blot analysis
Cells were seeded on culture plates at a density of 2,000,000 cells/100mm dish and treated as described above. Proteins were isolated from the cells on the 4th day after the induction of senescence. Cells were washed with warm PBS, trypsinized and centrifuged at 700g. Pellets were washed with a cold PBS and centrifuged three times at 700g and then stored at -20°C. Cell lysis was performed within 14 days since preparation. Cells were lysed for 40 minutes at 4°C in lysis buffer consisting of 1% SDS, 1% Triton X-100, 100mM NaCl, 1mM EDTA, pH 6.9, 50mM Tris pH 8.0 and inhibitors of proteases (1:200), then centrifuged for 20 minutes at 21000g.
Protein concentrations were determined by the DC protein assay kit (Bio-Rad, USA) according to the manufacturer´s protocol.
5μg or 10μg of proteins were separated by 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis for 50 minutes at 150V and then transferred to nitrocellulose membranes for 1hour at 80V. Membranes were blocked with 5% skimmed milk for 1hour and then incubated with primary antibodies. The mouse primary antibodies against p21 (1:1000) and a-tubulin (1:4000) were used in this study. Finally, the membranes were treated with an anti-mouse IgG secondary antibody (1:5000). The blots were developed using Clarify Western ECL Substrate kit (Bio-Rad, USA) according to the manufacturer´s protocol. The quantification of relative protein expression was done using the software Image Lab 5.0.
Statistics
The results are presented as the mean ± SD of a minimum of three independent experiments. Statistical significance was determined by the One-way ANOVA with a Bonferroni correction or by the Student´s t-test when appropriate. A value of p <0.05 was considered significant.
Results
Effect of DHA and EPA treatments on the cell growth in stress-induced premature senescence in MRC-5 human lung fibroblasts
At first we investigated effects of DHA (10 and 20 μM) and EPA (10, 20, 30 and 40 μM) on the cell growth in MRC-5 human lung fibroblasts in which the senescence was not induced. Cells were treated with DHA or EPA for 24 hours. We did not observe a large decrease in the cell growth in either DHA-treated cells or EPA-treated cells compared to DMSO control (Figure 1A).
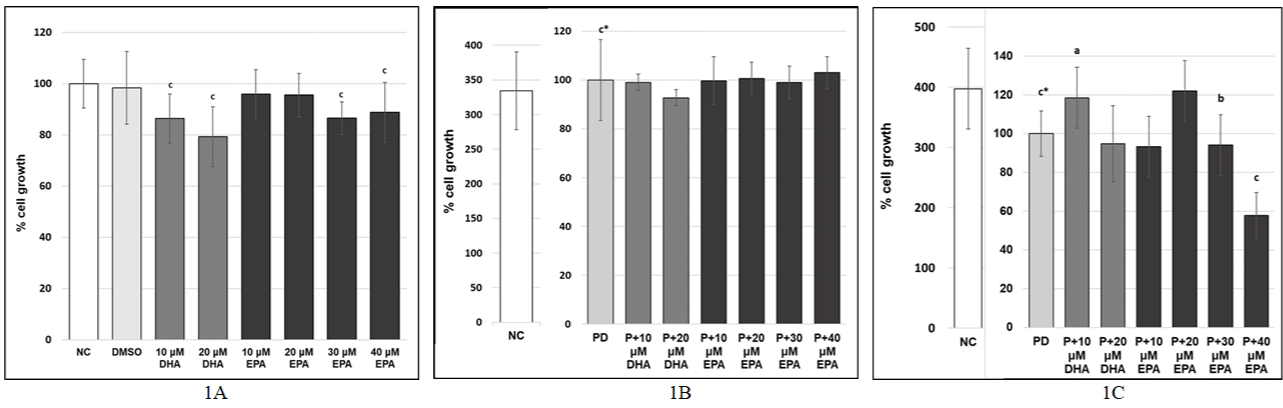
Figure 1: A) Effect of DHA and EPA on cell growth in MRC-5 human lung fibroblasts NC - normal cells (without DHA, EPA or dimethyl sulfoxide); DMSO control
- cells treated with dimethyl sulfoxide 2000x/24h diluted in the medium but not treated with DHA or EPA; B, C) Effect of DHA and EPA on cell growth under stress
inducing premature senescence in MRC-5 human lung fibroblasts; NC: Normal cells treated with 1xPBS 1000x diluted in the medium for 0.5 hours; PD - cells
treated with 100μM hydrogen peroxide for 0.5 hours and then with DMSO 2000x/24h (postreatment) diluted in the medium; PE cells treated with 100μM hydrogen
peroxide for 0.5 hours and then with 20μM EPA/24h (postreatment) diluted in the medium; PH - cells treated with 100μM hydrogen peroxide for 0.5 hours and then
with 10μM DHA/24h (postreatment) diluted in the medium; The values are means ± SD of three independent experiments (n=8-24). Statistical significance was
determined by the One-way ANOVA with the Bonferroni correction. NC vs. DMSO; DMSO vs. DHA; DMSO vs. EPA; NC vs. PD; PD vs. PH; PD vs. E; a-p <0.05;
b-p <0.01; c-p <0.001; c*-p <0.001.
Pretreatment with DHA or EPA
We found that pretreatment with DHA (10 and 20 μM) as well as pretreatment with EPA (10, 20, 30 and 40 μM) did not significantly affect the cell growth (all treatments p >0.12) (Figure 1B).
Postreatment with DHA or EPA
We observed that postreatment with 10μM DHA was associated with a significant increase in the cell growth (to 118.5 ± 15.75%) (Figure 1C) compared to P+DMSO control. Moreover, postreatment with 20μM EPA also led to significantly increased cell growth (to 122.05 ± 15.75%) compared to P+DMSO control (Figure 1C). On the other hand, postreatment with 40μM EPA significantly reduced the cell growth (to 57.57 ± 11.81%) compared to P+DMSO control (Figure 1C). Based on our results, we decided to investigate effects of 10μM DHA and 20μM EPA postreatment on the senescence in our next experiments since increased cell growth could be a sign of the reduced senescence.
Effect of DHA and EPA postreatment on SA-β-galactosidase activity in stress-induced premature senescence in MRC- 5 human lung fibroblasts
We investigated the effects of 10μM DHA or 20μM EPA postreatment on SA-β-galactosidase activity in stress-induced premature senescence in MRC-5 human lung fibroblasts since increased activity of SA-β-galactosidase is one of the typical hallmarks of senescence (12). We observed that postreatment with 10μM DHA as well as postreatment with 20μM EPA led to significant decrease of the percentage of SA-β-gal positive cells (69.86 ± 8.07% and 65.93 ± 8.94% respectively) compared to the percentage of SA-β-gal positive cells in PD control (83.73 ± 5.05%) (Figure 2A and 2B).
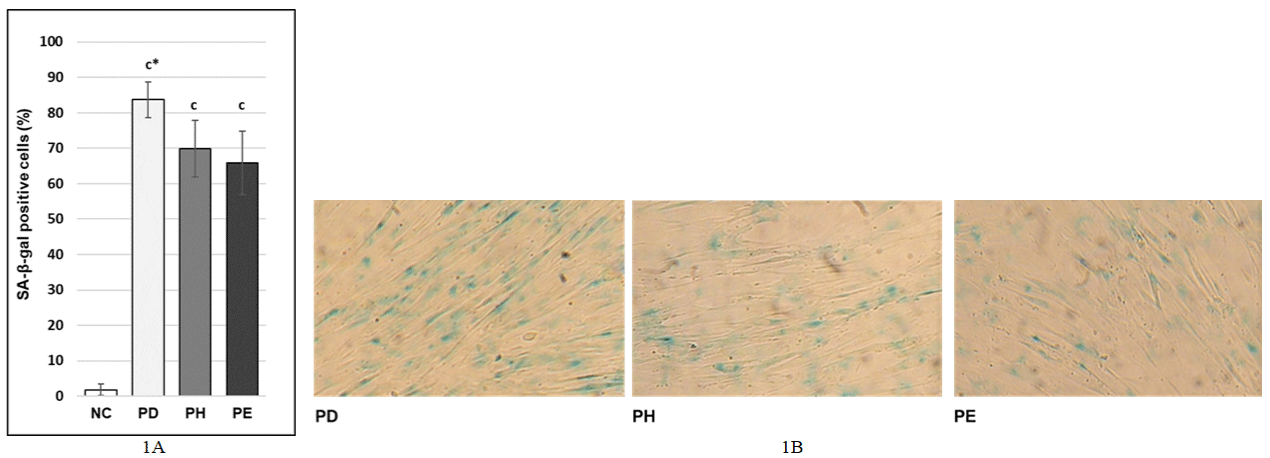
Figure 2: A, B) Effect of DHA and EPA postreatment on activity of SA-β-galactosidase under stress inducing premature senescence in MRC-5 human lung
fibroblasts; NC: Normal cells treated with 1xPBS 1000x diluted in the medium for 0.5 hours; PD - cells treated with 100μM hydrogen peroxide for 0.5 hours and
then with DMSO 2000x/24h (postreatment) diluted in the medium; PE cells treated with 100μM hydrogen peroxide for 0.5 hours and then with 20μM EPA/24h
(postreatment) diluted in the medium; PH - cells treated with 100μM hydrogen peroxide for 0.5 hours and then with 10μM DHA/24h (postreatment) diluted in the
medium; The values are means ± SD of three independent experiments (n=18). Statistical significance was determined by the One-way ANOVA with the Bonferroni
correction; NC vs. PD; PD vs. PH; PD vs. E; c - p <0.001; c* - p <0.001; (B) 100x magnification.
Effect of DHA and EPA postreatment on amounts of reactive oxygen species in stress-induced premature senescence in MRC-5 human lung fibroblasts
Next, we tested how postreatment with 10μM DHA as well as postreatment with 20μM EPA affects level of reactive oxygen species in cells in stress-induced premature senescence. We found that postreatment with 10μM DHA led to significantly reduced amounts of cells containing increased levels of reactive oxygen species (1.58 ± 0.45%) (Figure 3A) and to significantly elevated amounts of cells not containing increased levels of reactive oxygen species (96.64 ± 0.55%) (Figure 3B) compared to PD control. Similarly, postreatment with 20μM EPA led to significantly reduced amounts of cells containing increased levels of reactive oxygen species (1.56 ± 0.34%) (Figure 3A) and to significantly elevated amounts of cells not containing increased levels of reactive oxygen species (96.91 ± 0.64%) (Figure 3B) compared to PD control.
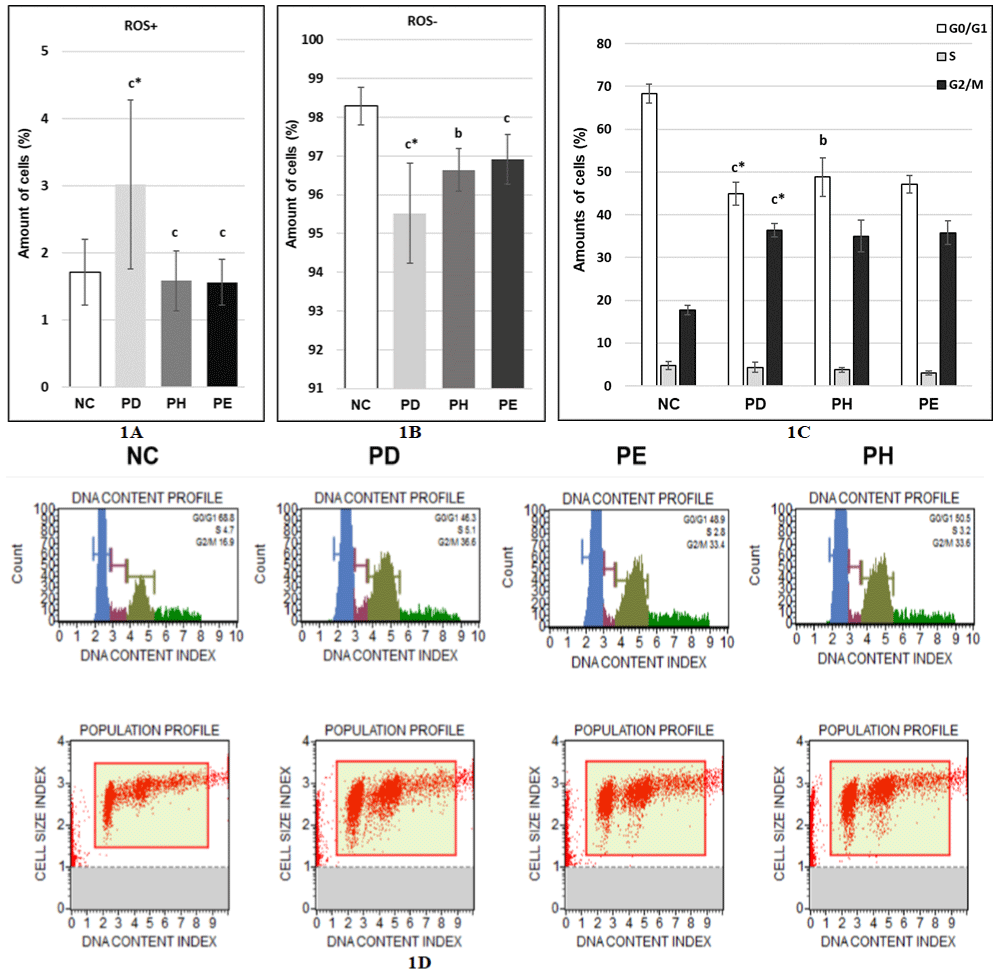
Figure 3: A, B) Effect of DHA and EPA postreatment on amounts of reactive oxygen species under stress inducing premature senescence in MRC-5 human lung
fibroblasts; C, D) Effect of DHA and EPA postreatment on cell cycle phases under stress inducing premature senescence in MRC-5 human lung fibroblasts; NC:
Normal cells treated with 1xPBS 1000x diluted in the medium for 0.5 hours; PD - cells treated with 100μM hydrogen peroxide for 0.5 hours and then with DMSO
2000x/24h (postreatment) diluted in the medium; PE cells treated with 100μM hydrogen peroxide for 0.5 hours and then with 20μM EPA/24h (postreatment) diluted
in the medium; PH - cells treated with 100μM hydrogen peroxide for 0.5 hours and then with 10μM DHA/24h (postreatment) diluted in the medium; The values are
means ± SD of three independent experiments (n (reactive oxygen species)=11-13; n (cell cycle phases)=6-10). Statistical significance was determined by the
One-way ANOVA with the Bonferroni correction. NC vs. PD; PD vs. PH; PD vs. PE; b-p <0.01; c-p <0.001; c*-p <0.001.
Effect of DHA and EPA postreatment on cell cycle phases in stress-induced premature senescence in MRC-5 human lung fibroblasts
Senescent cells cannot proliferate anymore and this state is called permanent cell cycle arrest. Therefore, we detected how cells are distributed in cell cycle phases after postreatment with 10μM DHA as well as postreatment with 20μM EPA in stress-induced premature senescence. After postreatment with 10μM DHA the number of cells in G0/G1 phase increased to 48.82 ± 4.5% compared to PD control (44.96 ± 2.69%) and the number of cells in S phase as well as in G2/M phase decreased to 3.77 ± 0.59% and 35.00 ± 3.71%, respectively compared to PD control (4.31 ± 1.18% - S phase; 36.37 ± 1.63% - G2/M phase) (Fig. 4A, 4B). However, the decrease was not statistically significant (p(S) = 0.65; p(G2/M) = 0.25). After postreatment with 20μM EPA 47.13 ± 2.03% of cells were in G0/G1 phase, whereas 3.03 ± 0.49% of cells were in S phase and 35.78 ± 2.77% of cells were in G2/M phase (Figure 3C and 3D). These results show that also after postreatment with 20μM EPA the number of cells in G0/G1 phase increased and the number of cells in S as well as G2/M phase decreased compared to PD control but these changes were not statistically significant (p(G0/G1) = 0.01; p(S) = 0.33; p(G2/M) = 0.65).
Effect of DHA and EPA postreatment on p21 protein level in stress-induced premature senescence in MRC-5 human lung fibroblasts
Further, we performed a western blot to detect expression of p21 on the protein level after postreatment with 10μM DHA and 20μM EPA because increased expression of p21 is an important hallmark of senescence causing cell cycle arrest. We detected significantly decreased expression of p21 after postreatment with 10μM DHA as well as after postreatment with 20μM EPA. The expression of p21 in cells treated with DHA was reduced to 73.86 ± 14.17% while the expression of p21 in cells treated with EPA was lowered to 88.4 ± 6.52% compared to PD2 control (Figure 4A and 4B).
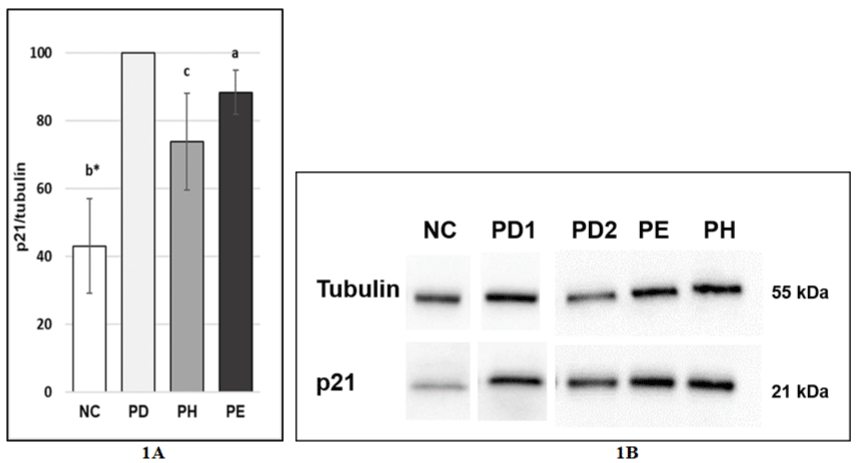
Figure 4: A, B) Effect of DHA and EPA postreatment on p21 protein level under stress inducing premature senescence in MRC-5 human lung fibroblasts NC:
Normal cells treated with 1xPBS 1000x diluted in the medium for 0.5 hours; PD1 - cells treated with 100μM hydrogen peroxide for 0.5 hours and then with DMSO
2000x/24h (postreatment) diluted in the medium; PD2 - cells treated with 100μM hydrogen peroxide for 0.5 hours and then with DMSO 2000x/24h (postreatment)
diluted in the medium; PE cells treated with 100μM hydrogen peroxide for 0.5 hours and then with 20μM EPA/24h (postreatment) diluted in the medium; PH - cells
treated with 100μM hydrogen peroxide for 0.5 hours and then with 10μM DHA/24h (postreatment) diluted the medium; NC vs. PD1 = membrane 1; PD2 vs. PE vs.
PH = membrane 2; The values are means ± SD of three independent experiments (n=3-5). Statistical significance was determined by the One-way ANOVA with
the Bonferroni correction. NC vs. PD; PD vs. PH; PD vs. PE; a-p <0.05; c-p <0.001; c*-p <0.001.
Discussion
Senescent cells accumulate in many tissues and organs with age and contribute to decline of physiological functions and development of cancer, chronic inflammation and age-related pathologies [21-26]. Therefore, it is important to find out how to prevent or diminish their formation. In our study we have focused on the investigation of the effects of docosahexaenoic (DHA) and eicosapentaenoic (EPA) acid on the development of stress-induced senescence since both DHA and EPA have important functions in metabolism [29-33]. Previously, we established a model of peroxide induced senescence using MRC-5 human lung fibroblasts [34, 35]. Addition of hydrogen peroxide to cells leads to an increased formation of reactive oxygen species and consequently may result in the development of senescence [35,36]. Here, we showed that treatment with DHA as well as EPA performed immediately after incubation of cells with hydrogen peroxide was able to suppress the development of peroxide induced senescence. Compared to controls (without EPA and DHA), we have observed slightly increased cell growth, decreased percentage of SA-β-gal positive cells, reduced levels of reactive oxygen species and reduced p21 protein expression after the postreatment with DHA or EPA.
Similarly, Yamagata et al. (2016) observed a decreased formation of senescent cells (decreased SA-β-galactosidase mRNA level and p21 protein level) after the cotreatment of human endothelial cells ISO-HAS with DHA and TNF-a. TNF-a was the inductor of senescence [34]. In another study, the treatment of aortic endothelial cells (HAECs) with EPA as well as DHA led to the reduced SA-β-galactosidase activity in peroxide induced senescence [37]. Furthermore, EPA was able to reduce expression of matrix metaloproteinase-1 (MMP-1) in cells growing in TNFa- or UV-induced stress conditions [38] and secretion of the cytokine interleukin-8 (IL-8) in cells growing in UV-induced stress conditions [39]. MMP-1 and IL-8 are often overexpressed in senescent cells and belong to components of senescence-associated secretory phenotype (SASP) [1,17,40]. These results indicate that ability of DHA as well as EPA to suppress the development of stressinduced senescence may not be cell type specific.
Our experiments revealed that after the postreatment with DHA as well as EPA, levels of reactive oxygen species (ROS) decreased. Hydrogen peroxide which was used as an inductor of senescence, leads to an increased levels of ROS which consequently may cause the development of senescence, as was mentioned above [35,36] (section 4). Silva et al. (2016) have recently found that the treatment of skeletal muscle cells C2C12 with EPA increased a protein expression of two antioxidant enzymes Mn-SOD and catalase [29]. Moreover, aortic endothelial cells (HAECs) that were treated with EPA or DHA and subsequently damaged by incubation with hydrogen peroxide, showed decreased ROS levels and increased mRNA level of antioxidant enzymes such as Mn-SOD, tioredoxin reductase-1 and heme oxygenase-1. Further, that study has unveiled an important role of nuclear factor erythroid 2-related factor 2 (Nrf-2) which is a transcription factor responsible for the induction of intracellular antioxidant enzymes [37,41]. Silencing of Nrf-2 abrogated the decrease in ROS levels and the increase in mRNA levels of antioxidant enzymes mediated by EPA or DHA [37]. These findings indicate that the development of peroxide induced senescence in our cell model could also be suppressed through the induction of antioxidant enzymes.
EPA and DHA are also able to inhibit the activation of NFκβ [42] - a transcription factor which mediates the expression of some SASP components and autocrine regulation of senescence [17,43].
According to our results 10μM DHA was able to suppress the development of peroxide induced senescence whereas EPA of the same concentration had no significant effect on it. Only EPA at the concentration of 20μM had a similar effect on the senescence as 10μM DHA. EPA was found to be β-oxidized more significantly than DHA which could explain why 10μM DHA had an effect on the senescence and 10μM EPA had not [44,45]. Another difference between EPA and DHA is their accumulation in lipid rafts. Lipid rafts are 10-200 nm membrane domains containing tightly packed cholesterol and sphingolipids which regulate intracellular signaling and gene expression [47]. DHA has a much greater tendency to incorporate into lipid rafts than EPA. Therefore, DHA has a much greater potential to affect cell signaling by modifying the composition of these lipid rafts [47,48].
Further, EPA and DHA are enzymatically metabolized into electrophilic fatty acid oxo-derivates (EFOXs) 5-oxoEPA and 7-oxoDHA, respectively. EFOXs were shown to activate Nrf-2- dependent antioxidant gene expression [47,49]. Enzymes converting omega-3 fatty acids into EFOXs can have different affinity for different omega-3 fatty acids [50]. Thus, amounts of EFOXs formed would depend on omega-3 fatty acids from which they were synthesized. In addition, various EFOXs could activate Nrf-2-dependent antioxidant gene expression to a different extent.
Several other studies have reported that DHA of the same concentration as EPA leads to a more pronounced effect on the suppresssion or induction of various enzymes and cytokines [51- 53]. However, there are also studies showing that EPA of the same concentration as DHA has a more pronounced effect on the suppression or induction of some enzymes and cytokines [30,54].
Finally, we also found that pretreatment with DHA as well as pretreatment with EPA did not significantly affect cell growth. Consistent with these results we assume that pretreatment with DHA as well as pretreatment with EPA does not affect the development of peroxide induced senescence since increased cell growth is one of non-senescent hallmarks. One possible explanation could be the phospholipid turnover [55]. We hypothesize that higher concentrations of EPA and DHA or longer incubation time with these two fatty acids could lead to the suppression of the development of peroxide induced senescence in our cell model. Sakai et al. (2017) applied 100μM DHA or 100 μM EPA to aortic endothelial cells 36 hours before induction of senescence with hydrogen peroxide and detected the suppression of the senescence development [37].
Conclusion
In conclusion, our present study has shown that postreatment with DHA as well as EPA is able to affect the development of stressinduced senescence with DHA being effective at lower concentrations than EPA.
Declaration
Acknowledgement: This work was supported by the EU grant from the CBC programme, Interreg V-A-NutriAging [V-0014].
Author contributions: Maria Janubova: Conceptualization, Methodology, Data curation, Software, Writing – Original draft preparation; Katarina Konarikova, Helena Gbelcova, Zuzana Szentesiova: Methodology; Ingrid Zitnanova: Funding acquisition, Project administration, Visualization, Supervision, Writing- Reviewing and Editing. All authors approved the final version.
Funding: This work was supported by the EU grant from the CBC programme, Interreg V-A-NutriAging [V-0014].
Compliance with ethical standards: In this work, we did not use humans or animals as objects of research.
References
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007; 8: 729-740.
- Shelton DN, Chang E, Whittier PS, Choi D, Funk WD. Microarray analysis of replicative senescence. Curr Biol. 1999; 9: 939-945.
- Poulsen RC, Watts AC, Murphy RJ, Snelling SJ, Carr AJ, Hulley PA. Glucocorticoids induce senescence in primary human tenocytes by inhibition of sirtuin 1 and activation of the p53/p21 pathway: in vivo and in vitro evidence. Ann Rheum Dis. 2014; 73: 1405-1413.
- Blomquist E, Westermark B, Pontén J. Ageing of human glial cells in culture: increase in the fraction of non-dividers as demonstrated by a minicloning technique. Mech Ageing Dev. 1980; 12: 173-182.
- Bandyopadhyay D, Timchenko N, Suwa T, Hornsby PJ, Campisi J, Medrano EE. The human melanocyte: a model system to study the complexity of cellular aging and transformation in non-fibroblastic cells. Exp Gerontol. 2001; 36: 1265-1275.
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, et al. Stemcell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006; 443: 421-426.
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961; 25: 585-621.
- DiLeonardo A, Linke SP, Clarkin K, Wahl GM. DNA damage triggers a prolonged p53- dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994; 8: 2540-2551.
- Chen QM, Bartholomew JC, Campisi J, Acosta M, Reagan JD, Ames BN. Molecular analysis of H2O2-induced senescent-like growth arrest in normal human fibroblasts: p53 and Rb control G1 arrest but not cell replication. Biochem J. 1998; 332: 43-50.
- Ziegler DV, Wiley CD, Velarde MC. Mitochondrial effectors of cellular senescence: beyond the free radical theory of aging. Aging Cell. 2015; 14: 1-7.
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p 53 and p16/INK4a. Cell. 1997; 88: 593-602.
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995; 92: 9363-9367.
- Hein N, Sanij E, Quin J, Hannan KM, Ganley A, Hannan RD. The Nucleolus and Ribosomal Genes in Aging and Senescence. Nagata T, editor. In: Senescence. InTech. 2012: 172-208.
- Passos JF, Nelson G, Wang C, Richter T, Simillion C, Proctor CJ, et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010; 6: 1-14.
- Herbig U, Jobling WA, Chen BP, Chen DJ, Sedivy J. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53 and p21 (CIP1), but not p16(INK4a). Mol Cell. 2004; 14: 501-513.
- Narita M, Nunez S, Heard E, Narita M, Lin AW, Hearn SA, et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell. 2003; 113: 703-716.
- Davalos AR, Coppe PJ, Campisi J, Desprez Yp. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010; 29: 273-283.
- Kullman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008; 133: 1019-1031.
- Kumar S, Millis AJ, Baglioni C. Expression of interleukin 1-inducble genes and production of interleukin 1 by aging human fibroblasts. Proc Natl Acad Sci USA. 1992; 89: 4683-4687.
- Naylor RM, Baker DJ, van Deursen JM. Senescent cells: a novel therapeutic target for aging and age-related diseases. Clin Pharmacol Ther. 2013; 93: 105-116.
- van Deursen JM. The role of senescent cells in ageing. Nature. 2014; 509: 439-446.
- Tominaga K. The emerging role of senescent cells in tissue homeostasis and pathophysiology. Pathobiol Aging Age-Relat Dis. 2015; 5: 27743.
- Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006; 66: 794-802.
- Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLos Biol. 2008; 6: 2853-2568.
- Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich B, Morton JP et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013; 15: 978-990.
- Proshkina EN, Solovev IA, Shaposhnikov MV, Moskalev AA. Key Molecular Mechanisms of Aging, Biomarkers, and Potential Interventions. Mol. Biol. (Mosk). 2020; 54: 883-921.
- Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003; 126: 1-27.
- Champigny CM, Cormier RPJ, Simard CJ, St-Coeur PD, Fortin S, Pichaud N. Omega-3 Monoacylglyceride Effects on Longevity, Mitochondrial Metabolism and Oxidative Stress: Insights from Drosophila melanogaster. Mar Drugs. 2018; 16: 453.
- Neuman JC, Fenske RJ, Kimple ME. Dietary polyunsaturated fatty acids and their metabolites: Implications for diabetes pathophysiology, prevention, and treatment. Nutrit Health Agi. 2017; 4: 127-140.
- da Silva Jr. EP, Nachbar RT, Levada-Pires AC, Hirabara SM, Lambertucci RH. Omega-3 fatty acids differentially modulate enzymatic anti-oxidant systems in skeletal muscle cells. Cell Stress Chaper. 2016; 21: 87-95.
- Lalia AZ, Dasari S, Robinson MM, Abid H, Morse DM, Klaus KA, et al. Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging (Albany NY). 2017; 9: 1096-1129.
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010; 142: 687-698.
- Grimm H, Mayer K, Mayser P, Eigenbrodt E. Regulatory potential of n-3 fatty acids in immunological and inflammatory processes. Br J Nutr. 2002; 87: S59-67.
- Yamagata K, Suzuki S, Tagami M. Docosahexaenoic acid prevented tumor necrosis factor alpha-induced endothelial dysfunction and senescence. Prostaglandins Leukot Essent Fatty Acids. 2016; 104: 11-18.
- Janubova M, Hatok J, Konarikova K, Zitnanova I. γ- and d-Tocotrienols interfere with senescence leading to decreased viability of cells. Mol Cell Biochem. 2021; 476: 897-908.
- Kim JY, Lee JS, Han YS, Lee JH, Bae I, Yoon YM, et al. Pretreatment with lycopene attenuates oxidative stress-induced apoptosis in human mesenchymal stem cells. Biomol Ther (Seoul). 2015; 23: 517-524.
- Sakai C, Ishida M, Ohba H, Yamashita H, Uchida H, Yoshizumi M, et al. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS One. 2017; 12: e0187934.
- Kim HH, Shin CM, Park CH, Kim KH, Cho KH, Eun HC et al. Eicosapentaenoic acid inhibits UV-induced MMP-1 expression in human dermal fibroblasts. J. Lipid Res. 2005; 46: 1712-1720.
- Storey A, McArdle F, Friedmann PS, Jackson MJ, Rhodes LE. Eicosapentaenoic acid and docosahexaenoic acid reduce UVB- and TNFalpha- induced IL-8 secretion in keratinocytes and UVB-induced IL-8 in fibroblasts. J Invest Dermatol. 2005; 124: 248-255.
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008; 133: 1019-1031.
- Levonen AL, Inkala M, Heikura T, Jauhiainen S, Jyrkkänen HK, Kansanen E, et al. Nrf2gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler Thromb Vasc Biol. 2007; 27: 741-747.
- Farías JG, Carrasco-Pozo C, Carrasco Loza R, Sepúlveda N, Álvarez P, Quezada M, et al. Polyunsaturated fatty acid induces cardioprotection against ischemia-reperfusion through the inhibition of NF-kappaB and induction of Nrf2. Exp Biol Med (Maywood). 2017; 242: 1104-1114.
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory Networks during Cellular Senescence: Causes and Consequences. Trends Mol Med. 2010; 16: 238-246.
- Kaur G, Molero JC, Weisinger HS, Sinclair AJ. Orally administered [14C]DPA and [14C]DHA are metabolized differently to [14C] EPA in rats. Br J Nutr. 2013; 109: 441-448.
- Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid beta-oxidation of eicosapentaenoic acid in mouse brain: an in situ study. Prostaglandins Leukot Essent Fatty Acids. 2009; 80: 157-163.
- Pal A, Metherel AH, Fiabane L, Buddenbaum N, Bazinet RP, Shaikh SR. Do Eicosapentaenoic Acid and Docosahexaenoic Acid Have the Potential to Compete against Each Other? Nutrients. 2020; 12: 3718.
- Dyall SC. Long-chain fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015; 7: 52.
- Williams JA, Batten SE, Harris M, Rockett BD, Shaikh SR, Stillwell W, et al. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys J. 2012; 103: 228-237.
- Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010; 6: 433-441.
- Russell FD, Bürgin-Maunder CS. Distinguishing health benefits of eicosapentaenoic and docosahexaenoic acids. Mar Drugs. 2012; 10: 2535- 2559.
- Gorjão R, Azevedo-Martins AK, Rodrigues HG, Abdulkader F, Arcisio- Miranda M, Procopio J, et al. Comparative effects of DHA and EPA on cell function. Pharmacol Ther. 2009; 122: 56-64.
- Verlengia R, Gorjão R, Kanunfre CC, Bordin S, Martins De Lima T, Martins EF, et al. Comparative effects of eicosapentaenoic acid and docosahexaenoic acid on proliferation, cytokine production, and pleiotropic gene expression in Jurkat cells. J Nutr Biochem. 2004; 15: 657-665.
- Brown ER, Subbaiah PV. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on human skin fibroblasts. Lipids. 1994; 29: 825-829.
- Verlengia R, Gorjão R, Kanunfre CC, Bordin S, Martins de Lima T, Martins EF. Effects of EPA and DHA on proliferation, cytokine production, and gene expression in Raji cells. Lipids. 2004; 39: 857-864.
- Patton-Vogt J, de Kroon AIPM. Phospholipid turnover and acyl chain remodeling in the yeast ER. Biochim Biophys Acta Mol Cell Biol Lipids. 2020; 1865: 158462.