
Case Report
Austin Med Sci. 2022; 7(1): 1063.
Hashimoto Encephalopathy Presenting with New Onset Refractory Status Epilepticus and Clinical Hyperthyroidism
Aljaberi N¹, Elkambergy H¹, Achi E² and Dibu JR¹*
¹Neurologic Critical Care Unit, Critical Care Institute, Cleveland Clinic Abu Dhabi, United Arab Emirates
²Epilepsy Department, Neurological institute, Cleveland Clinic Abu Dhabi, United Arab Emirates
*Corresponding author: Jamil R. Dibu, Neurologic Critical Care Unit, Critical Care Institute, Cleveland Clinic Abu Dhabi, United Arab Emirates
Received: March 23, 2022; Accepted: April 14, 2022; Published: April 21, 2022
Abstract
NORSE occurs secondary to multiple etiologies including a rare diagnosis of Hashimoto Encephalopathy (HE). The mechanism of HE is not fully understood. Most reported patients with HE are hypothyroid or euthyroid at the time of presentation. Patients with HE commonly present with subacute clinical features, namely behavioral changes, and rarely with the acute presentation of status epilepticus. We report a case of a 14 years old female presenting with New Onset Refractory Status Epilepticus (NORSE) and clinical hyperthyroidism, subsequently developing behavioral symptoms with multiple relapses requiring various treatment modalities including pulse steroid therapy, intravenous immunoglobulin therapy, plasmapheresis and eventually partial thyroidectomy.
Keywords: Hashimoto Encephalopathy; Status Epilepticus; Hyperthyroidism
Background
Status epilepticus is a life-threatening medical emergency that occurs secondary to multiple etiologies including an uncommon but increasingly recognized diagnosis of Hashimoto Encephalopathy (HE). HE, also known as Steroid- Responsive Encephalopathy associated with Autoimmune Thyroiditis (SREAT), was originally described by Brain et al in 1966 [1], has an estimated prevalence of 2:100.000 [2] and is associated with Hashimoto thyroiditis with elevated antithyroid antibodies titers. The mechanism of HE is unknown however it is believed to be an immune mediated disorder with an inflammatory process affecting the central nervous system rather than the direct effect of an altered thyroid state on the brain [3] as most reported patients with HE were euthyroid at the time of presentation [2]. Patients with HE commonly present with subacute clinical features such as cognitive and behavioral changes in a hypothyroid state and rarely present acutely with status epilepticus [2]. We report a case of a 14-year-old female presenting acutely with New Onset Status Epilepticus (NORSE) and clinical hyperthyroidism.
Case Presentation
A 14-year-old female presented to an outside hospital with refractory convulsive status epilepticus resistant to initial therapy with intravenous lorazepam and phenytoin. She was subsequently intubated, loaded with levetiracetam and started on propofol infusion. Past medical history includes asymptomatic untreated hyperthyroidism and a goiter that was diagnosed approximately a year prior to initial presentation. She has a strong family history of autoimmune disease with multiple siblings diagnosed with hypothyroidism and type I diabetes mellitus.
On day 3 she was transferred to our hospital’s Neurologic Critical Care Unit for further evaluation and management. Upon arrival, continuous EEG revealed non-convulsive status epilepticus (Figure 1). The anti-epileptic treatment was hence optimized by increasing levetiracetam dose, adding lacosamide with a loading dose, and starting a midazolam infusion titrated up to seizures cessation, which was achieved on day 5.
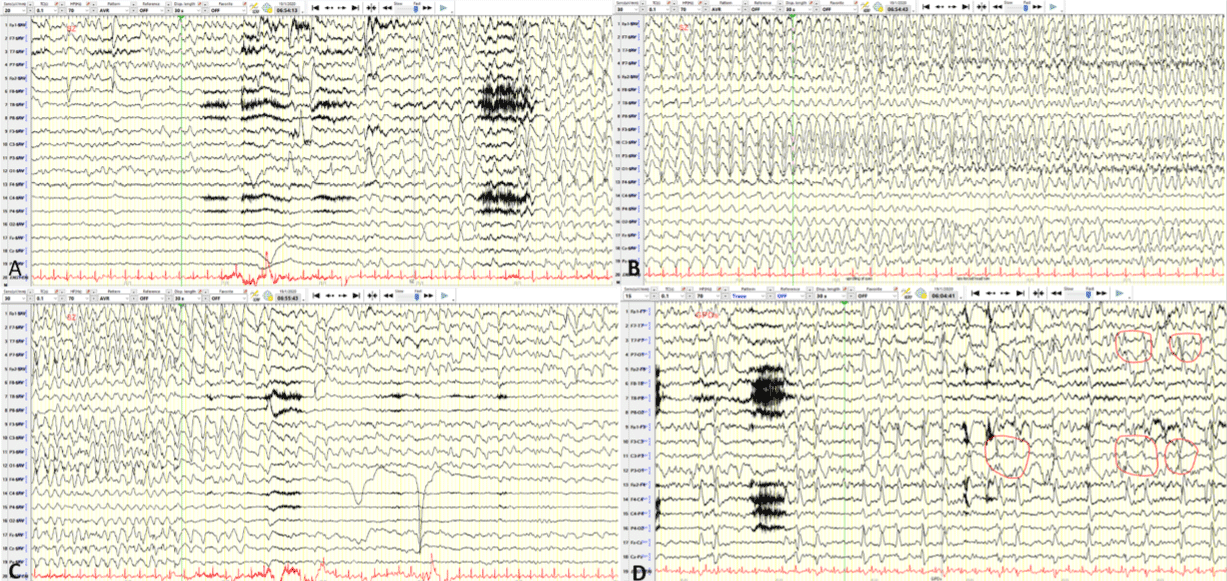
Figure 1: Setting: 30sec/epoch; Sensivity 20-30 microvolts/mm; montage-Average-left referential montage. A) Baseline background consisting of a fundamental
rhythm of 3-4 Hz delta with rare intermixed 6-7 Hz theta activity is seen in the first 10 seconds followed by a muscle artifact and a sudden appearance of left
posterior quadrant very-high-voltage 1.5Hz sharply contoured delta activity (O1, P3, P7) with intermixed spikes in the left posterior temporal region (P7/O1) rapidly
involving the left hemisphere. B) Ictal discharge evolves to very-high-voltage (600 microvolts peak-to-peak) well-organized rhythmic 2Hz delta activity with a spike
component (1st half of the epoch) and evolves further to a 2.5Hz less organized high-voltage (400 microvolts) generalized delta activity with a left hemisphere
predominance. C) Ictal discharge terminals abruptly (mid-epoch) with a return of the previously seen baseline encephalopathic background. D) LB montage:
Interictal generalized quasi-periodic discharge with left hemisphere predominance, and with maximal spike negativity in the left posterior quadrant (P7/P3/O1).
Initial work up upon ICU admission revealed suppressed TSH: 0.005milli IU/L (Ref range 0.51-4.30 milli IU/L), normal free T3: 4.7pmol/L (Ref range 4.77-7.52 pmol/L), high free T4: 26.8pmol/L (Ref range 11.6-19.6 pmol/L), elevated thyroid peroxidase antibody 120IU/ml (Ref range less than 60IU/ml), normal TSH receptor antibody <0.3 (Ref range <1.75IU/L) and elevated thyroglobulin antibody 475IU/ml (Ref range less than 60IU/ml).
The patient underwent further investigations including a lumbar puncture with cerebrospinal fluid showing no evidence of infection (1 nucleated cell) and negative meningitis and encephalitis panel. In addition, serum and CSF autoimmune epilepsy and paraneoplastic autoantibody panels were negative.
MRI brain with IV contrast showed abnormal hyperintense T2 signal of the left posterior thalamus without abnormal enhancement or diffusion restriction (Figure 2).
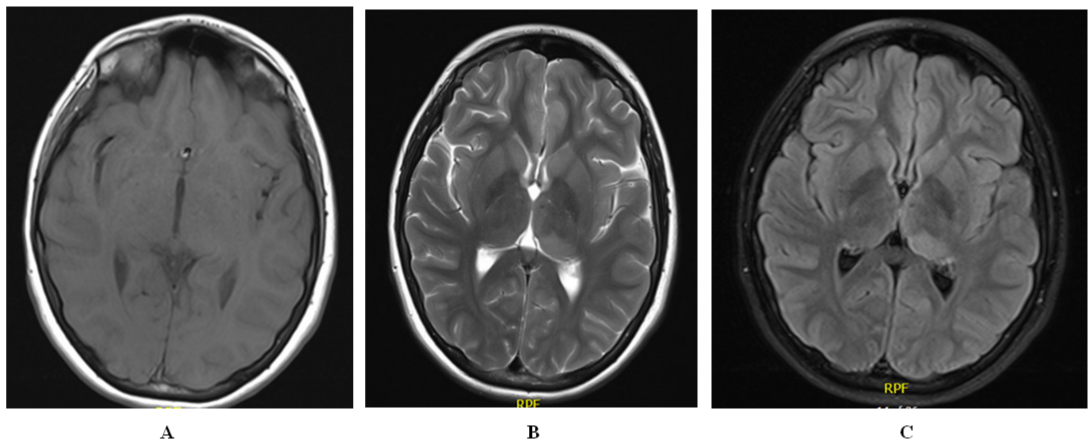
Figure 2: MRI Brain: A) No contrast enhancement; B) T2 slide showing hyperintensity over left posterior thalamus; C) FLAIR slide showing hyperintensity over left
posterior thalamus.
Thyroid ultrasound revealed a large 4 x 4.1 x 2.9 cm partially cystic nodule involving most of left lobe and isthmus. Her CT chest and MRI abdomen and pelvis were unremarkable.
During hospitalization, the patient was cared for by a multidisciplinary team including neurocritical care, epilepsy, and endocrinology.
Midazolam and propofol infusion were then gradually tapered after 24 hours of seizures cessation and stopped on day 7 and the patient was successfully extubated. Additional treatment included: intravenous methylprednisolone 1g for 3 days, thereafter 1mg/kg oral prednisone and 5-day course of intravenous immunoglobulin (0.4mg/kg). Patient was also started on carbimazole and propranolol for clinically symptomatic hyperthyroidism. She was transferred out of ICU to the regular ward on day 11. At the time, she had no focal deficits except ataxia on neurological exam; however she continued to have waxing and waning behavioural and psychiatric symptoms including psychosis, hyperactivity, disinhibition and aggression, which were gradually improving over the course of the hospitalization. She was discharged on day 23 to a rehabilitation facility as she had ataxic gait, thought to be a motor sequela of HE with a slow and gradual prednisolone taper regimen.
The patient was diagnosed with probable Hashimoto’s encephalopathy causing refractory status epilepticus and cognitive dysfunction. Throughout the next 8 months she had frequent relapses manifested by seizures and behavioral changes requiring hospitalizations, despite optimizing her oral anti-epileptic and thyroid medications regimens in the outpatient follow ups with her epileptologist and endocrinologist. Beginning of month 9, while continuing on her oral steroid maintenance dose, her condition worsened, and she was re-admitted to the hospital with multiple seizures and worsening behavioral and cognitive function with rapid progression to obtundation requiring intubation and mechanical ventilation. EEG did not show any status epilepticus rather severe slowing. Her repeat work up included repeat CSF studies with increased CSF protein level (255mg/dL) but normal nucleated cells, and negative CSF and serum autoimmune encephalitis workup, a serum TPO of 142IU/mL, a thyroglobulin level of 192IU/mL, a TSH of 0.468milli IU/L, a free T4 of 12.8pmol/L, and a thyroid receptor antibody level of 1.22IU/L (<1.75IU/L normal). Ultrasound of the pelvis and ovaries was unremarkable. She received pulse steroid therapy and plasmapheresis resulting in progressive improvement in mental status and was eventually extubated. Subsequently she was placed on immunosuppressive therapy and planned for partial left thyroidectomy.
Discussion
Status Epilepticus is a neurological emergency, carrying high morbidity and mortality if left untreated which could be mitigated by rapid and appropriate treatment [4].
HE has a female predominance with female to male ratio of 4:1 [6,7], and a relatively greater frequency in the adult population [5,6]. The age of presentation reported in the literature ranges from 2 years to 86 years and the mean age of presentation in children is 12-14 years [7].
The pretreatment criteria of Hashimoto Encephalopathy (HE) published by Mattozzi et al. [8], included the following:
• Subacute onset of cognitive impairment, psychiatric symptoms, or seizures.
• Euthyroid or mild hypothyroidism.
• Serum thyroid peroxidase antibodies (TPO-Ab) more than 200IU/ml.
• Absent neuronal antibodies in serum and CSF.
• No other etiologies like toxic, metabolic, infectious, vascular or neoplastic process identified that could explain the clinical findings.
Looking further into demographic data and clinical features of these 24 patients, 6 patients had presented with NORSE as the main presenting clinical feature, with a median age of 24 (range 11-53), 3 were female, 2 of them had increased signal in medial temporal lobes, 2 patients had pleocytosis in CSF. All had received steroid for treatment and only 2 demonstrated complete recovery after treatment with steroids. Most of their patients were euthyroid or mildly hypothyroid.
In their literature review, Ercoli et al. [2] analyzed 135 patients, of which 104 had symptoms other than status epilepticus and 31 patients had status epilepticus. Amongst the patients with status epilepticus, 12 (41%) were euthyroid, 7 (24%) had clinical hypothyroidism, 2 (7%) had clinical hyperthyroidism. The prevalence of elevated TPO-Ab was in 9 (29%) patients, elevated thyroglobulin antibody (TG-Ab) in 3 (10%) and elevated TPO-Ab plus TG-Ab combined in 19 (61%) patients. Alternatively, Chong JY et al. [9] included 85 patients with HE, 2 had subclinical hyperthyroidism and 4 had overt hyperthyroidism.
Reflecting on our case, we believe it is important to report its unusual presentation and findings that are unique, namely her pediatric age with acute presenting symptom of NORSE, her TPOAb level, and less commonly reported clinical hyperthyroidism state on presentation.
The evidence for the target TPO-Ab level above which the diagnosis of Hashimoto’s encephalopathy remains unclear. While Mattozzi et al specify a cutoff of >200IU/mL for the pre-treatment criteria of HE in their experience [8], our patient’s TPO-Ab level was lower, but nonetheless met the other required exclusion criteria for other possible diagnoses such as autoimmune, paraneoplastic diseases or other toxic-metabolic etiologies. In addition, thyroid peroxidase antibodies, which are a prerequisite to the diagnosis of HE by the criteria published by Mattozi et al. [8], have poor disease-specificity as 13% of healthy individual may be antibody positive [10]. Lastly, whereas most of the patients reported in the literature are typically euthyroid or hypothyroid, our patient was in clinical hyperthyroid state upon her presentation.
HE is diagnosis by exclusion as demonstrated in our case with other etiologies-toxic, infectious, metabolic, neoplastic ruled out and a negative autoimmune encephalitis serum and CSF panels. Furthermore, her response to steroids, intravenous immunoglobulin and plasmapheresis strongly favors the diagnosis of HE.
Learning Points
We emphasize the importance of considering HE as a cause for NORSE even when it defies the typical subacute presentation of cognitive and behavioral dysfunction. Abnormal TPO levels below 200 IU/mL as well as a clinical hyperthyroidism state should not exclude HE as a diagnosis as shown in our case after ensuring that all other possible etiologies are excluded. The correct diagnosis of HE is important as patients are unlikely to respond to anti-epileptic medications alone and will require steroids and possibly other concomitant immunomodulatory treatment. We believe our case increases a clinician’s awareness of HE presentation with unusual features, leading to a prompt diagnosis and early intervention for this disorder.
What is New?
• Status epilepticus can occur secondary to multiple aetiologies including an uncommon but increasingly recognized diagnosis of Hashimoto Encephalopathy (HE).
• Most reported patients with HE is euthyroid to mildly hypothyroid at the time of presentation. Thyroid peroxidase antibodies levels positive threshold in these patients is not well defined.
• HE patients commonly present with subacute clinical features such as cognitive and behavioural changes and rarely acutely with status epilepticus.
• We report a case of a 14-year-old female presenting acutely with New Onset Refractory Status Epilepticus (NORSE) and clinical hyperthyroidism, subsequently developing behavioural symptoms with multiple relapses requiring various treatment modalities.
References
- Brain L, Jellinek EH, Ball K. Hashimoto’s disease and encephalopathy. Lancet. 1966; 2.
- Ercoli T, Defazio G, Muroni A. Status epilepticus in Hashimoto’s encephalopathy. Seizure. 2019; 70.
- Kothbauer-Margreiter I, Sturzenegger M, Komor J, Baumgartner R, Hess CW. Encephalopathy associated with Hashimoto thyroiditis: Diagnosis and treatment. J Neurol. 1996; 243.
- Vanhaerents S, Gerard EE. Epilepsy Emergencies: Status Epilepticus, Acute Repetitive Seizures, and Autoimmune Encephalitis. CONTINUUM Lifelong Learning in Neurology. 2019; 25.
- Sadan O, Seyman E, Ash EL, Kipervasser S, Neufeld MY. Adult-onset temporal lobe epilepsy, cognitive decline, multi-antiepileptic drug hypersensitivity, and Hashimoto’s encephalopathy: Two case studies. Epilepsy Behav Case Reports. 2013; 1.
- Mahmud FH, Lteif AN, Renaud DL, Reed AM, Brands CK. Steroid-responsive encephalopathy associated with Hashimoto’s thyroiditis in an adolescent with chronic hallucinations and depression: Case report and review. Pediatrics. 2003; 112.
- Montagna G, Imperiali M, Agazzi P, D’Aurizio F, Tozzoli R, Feldt-Rasmussen U, et al. Hashimoto’s encephalopathy: A rare proteiform disorder. Autoimmunity Reviews. 2016; 15.
- Mattozzi S, Sabater L, Escudero D, Ariño H, Armangue T, Simabukuro M, et al. Hashimoto encephalopathy in the 21st century. Neurology. 2020; 94.
- Chong JY, Rowland LP, Utiger RD. Hashimoto encephalopathy: Syndrome or myth? Archives of Neurology. 2003; 60.
- Litmeier S, Prüss H, Witsch E, Witsch J. Initial serum thyroid peroxidase antibodies and long-term outcomes in SREAT. Acta Neurol Scand. 2016; 134.