
Review Article
Austin Med Sci. 2023; 8(1): 1072.
Probable Role of Antibody Targeting Nectin-4 in the Treatment of Cancer
Honghong Lu1,2; Pengpeng Tian2,3*
¹College of Business, Beijing Open University, Beijing, China
²College of Science and Technology, Beijing Open University, Beijing, China
³Institute of Bioengineering, Biotech Pharma Ltd, Beijing, China
*Corresponding author: Pengpeng Tian, Institute of Bioengineering, Biotech Pharma Ltd, Rongjing East Street, Beijing Development Are (BDA), Beijing 100176, Beijing, China. Email: tianpeng363@163.com
Received: February 23, 2022 Accepted: March 31, 2022 Published: April 07, 2023
Abstract
In recent years, more and more researches have shown that Nectin-4 is a new type of tumor associated antigen. It is highly expressed in breast, ovarian, lung, urothelial, esophageal, liver, colorectal, gastric, gallbladder, bladder and pancreatic cancers, and promotes the development and progression of tumors. Nectin-4 is highly correlated with the prognosis of various tumors. The investigations and mechanism of nectin-4 in various tumor types is reviewed below.
Keywords: Antibody; Nectin-4; Treatment; Cancer
Introduction
Nectin family proteins, also known as Poliovirus Receptor Related protein (PRR), belong to the immunoglobulin super family. As cell adhesion molecules, Nectin family proteins participate in the connection between epithelial cells and endothelial cells. The nectin family consists of four members: Nectin-1, Nectin-2, Nectin-3 and Nectin-4. Nectin-1,2 and 3 are widely expressed in adult tissues, while nectin-4 is only specifically expressed in human embryos and placenta.
Nectin-4, expressed by gene pvr14, is a type I transmembrane glycoprotein, which encoded by 510 amino acids with 66k Damolecular weight (Figure 1). It is a calcium independent alloantigen cell adhesion molecule. There is 92% similarity in human and mouse derived nectin-4. The extracellular domain (gly27-val351) of Nectin-4 is composed of three immunoglobulin like domains V-C-C. As a cell adhesion molecule, Nectin-4 is involved in the connection between epithelial cells and endothelial cells. Nectin-4 is also a receptor for measles virus infection of epithelial cells [1-3].
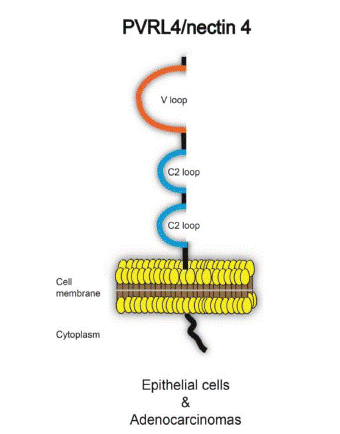
Figure 1: Structure pattern diagram of Nectin-4 [1].
The Investigation and Clinic Trials of Nectin-4 in Various Cancer
Breast Cancer
In a sample of 140 breast cancer samples, 63.5% of breast cancer tissues had Nectin-4 positive expression, and the increased Nectin-4 expression was significantly related to tumor grade (P<0.0001), tumor size (P<0.0001) and survival (P<0.0001) [4].
In another 5673 cases of invasive breast cancer, the expression of mRNA and immunohistochemical staining showed that Nectin-4 expression was not detected in adult healthy tissues (including breast). But the expression rate of three negative breast cancer samples was 61%, and the expression rate in basal like tumors (basal) was 62%. In non three negative breast cancer samples, or non basal like tumors (non-basal), the expression rate was 47%. Further statistical analysis showed that high expression of nectin-4 was much correlated with poor prognosis [4].
In addition to the expression on the surface of tumor cell membrane, high levels of soluble Nectin-4 molecules (43 kDa) were detected in the serum of patients with metastatic breast cancer. And soluble Nectin-4 was composed of extracellular segments of the whole Nectin-4.
Therefore, Nectin-4 can also is used as a specific serum marker for the diagnosis and follow-up of breast cancer and other kinds of cancer [5-10].
Lung Cancer
An immunohistochemical staining of 422 cases of Non-Small Cell Lung Cancer (NSCLC) showed that 245 cases (58.1%) displayed strong staining, 119 cases displayed mild staining (28.2%), 58 cases were not stained (13.7%). And no staining was found in adjacent normal lung tissues.
Moreover, the high expression of Nectin-4 is closely related to the poor prognosis of lung cancer. And the survival time of patients with high expression of Nectin-4 is shorter than that of patients with no/weak expression of Necitn-4 [11].
Ovarian Cancer
In a trial using immunohistochemical staining to simultaneously analyze the specimens of 500 patients with ovarian cancer, 48.6% of ovarian cancer tissues showed positive expression of Nectin-4. In addition, soluble Nectin-4 was detected in 27(53%) of 51 patients with serous ovarian cancer, 4(29%) of 14 patients with clear cell carcinoma and 8(16%) of 51 patients with benign gynecological diseases [12]. Moreover, several studies have found that the high expression of Nectin-4 is closely related to the poor prognosis of ovarian cancer [9,10,13].
Liver Cancer
In a study of 107 liver cancer samples, it was found that the mRNA and protein levels of Nectin-4 were higher in liver cancer tissues than in adjacent non tumor tissues. Immunohistochemical staining showed that 67.82% (59/87) of HCC tissues were over expressed. The expression of Nectin-4 was significantly correlated with tumor size (P=0.029), metastasis status (P=0.023), vascular invasion (P=0.018) and tumor lymph node metastasis (P=0.003). In addition, Kaplan Meier survival analysis showed that positive Nectin-4 expression was closely associated with poor recurrence free survival (RFs, P=0.006) and overall survival (OS, P=0.005) [14].
Gastric Cancer
Nectin-4 can promote the growth and migration of human gastric cancer cells and promote the progression of gastric cancer. In a study of 64 patients with gastric cancer, the authors found that the high expression of Nectin-4 was significantly correlated with lymph node metastasis (P=0.025) and TNM stage (P=0.006). Among the 64 patients, 39 died, including 33 cases with high expression of Nectin-4 protein and 6 cases with low expression of Nectin-4 protein. The expression of Nectin-4 was higher in 13 surviving patients, lower in 12 surviving patients. Moreover, in patients with gastric cancer, the 5-year survival rate in the high Nectin-4 expression group was significantly lower than that in the low Nectin-4 expression group [15].
Esophageal Cancer
In a study of 94 esophageal cancer samples, the authors detected the mRNA levels of four genes: Nectin-1, Nectin-2, Nectin-3 and Nectin-4. It was found that only Nectin-4 was specifically expressed in esophageal cancer samples. Immunohistochemical staining showed that the positive staining of Nectin-4 could be seen on the cell membrane of esophageal cancer, while only weak or negative staining of Nectin-4 could be seen in normal esophagus. Survival analysis showed that the overall survival rate of patients with high expression was lower than that of patients with low expression (HR=1.704, 95% ci1.027-2.825, P=0.039) [16].
In addition, the expression of Nectin-4 in colorectal cancer [17], gallbladder carcinoma [18], bladder cancer [19] and pancreatic cancer [20] was closely related to the poor prognosis of patients.
Nectin-4 is a molecule that promotes the occurrence and progression of many kinds of tumors. It is widely expressed in breast cancer, ovarian cancer, lung cancer, urothelial carcinoma, esophageal cancer, liver cancer, colorectal cancer, gastric cancer, gallbladder cancer, bladder cancer and pancreatic cancer. Basically, it is not expressed in normal tissues. Therefore, it is a specific tumor associated antigen molecule.
The Function Mechanism of Nectin-4
Interaction with Afadin Protein
In epithelial cells, Nectin-4 and afadin are Co located at the adhesion junction dominated by cadherin. Nectin-4 interacts with the PDZ domain of afadin through the C-terminal intracellular motif [21]. Andafadin can interact with F-actin, tightly connected components (such as closed band ZO-1, connecting adhesion molecule jam), adhesion connecting components (such as ponsin), cytoskeleton regulatory protein profilin And Ras protein, participate in signal transduction and regulate cell proliferation. The high expression of Nectin-4 eventually causes the deformation of cancer cells, the extension of cell processes and the formation of lamellar pseudopodia, which improves the ability of tumor migration and invasion [11].
The Activation of PI3K/Akt Signal Pathway
In a breast cancer metastasis model, Nectin-4 is a marker of Breast Cancer Stem Cells (BCSCs). Nectin-4 activates the WNT/beta-Catenin signaling pathway through PI3K/AKT kinase molecules to achieve self-renewal of breast cancer stem cells. In vitro, in vivo and clinical pathological data show that the upreg ulation of Nectin-4 expression is closely related to the activation of Wnt/beta catenin signaling pathway, the proliferation of cancer stem cells, Epithelial Mesenchymal Transformation (EMT), the metastasis and invasion of tumor cells [22]. In a study of gastric cancer model, it was found that the gastric cancer tumor cell line SGC-7901 over expressing Nectin-4 showed faster cell growth ability and sensitivity to cell growth. And the migration ability of SGC-7901 cells over expressing Nectin-4 was enhanced compared with the control group. In the experiment of tumor bearing mouse model, the weight and volume of tumor growth were significantly larger than those in the control group after injection of gastric cancer cell line over expressing Nectin-4. The results of further biochemical experiments also show that nectin-4 participates in the proliferation and migration of gastric cancer cells through PI3K/Akt pathway. And nectin-4 may play a potential role in promoting cell proliferation [15]. Investigations in gallbladder cancer have also shown that Nectin-4 promotes the proliferation, migration and tumor growth of gallbladder cancer cells through PI3K/Akt pathway [18].
The Promotion of Angiogenesis
The intratumoral angiogenesis model established by highly metastatic breast cancer cells and human umbilical vein endothelial cells (HUVECs) can be used to research the contribution of Nectin-4 to angiogenesis systematically. In vivo and in vitro evidence shows that Nectin-4 can promote angiogenesis. Metastatic Breast Cancer Stem Cells (mBCSCs) drive ADAM-17 expression after hypoxia, resulting in the exfoliation of Nectin-4 into the microenvironment. It interact with integrin-beta 4 on endothelial cells. This interaction promotes angiogenesis within tumors through Src, PI3K, AKT, and iNOS pathways [23].
Marketed Drugs
ADC: Enfortumab Vedotin
The Antibody Drug Conjugate (ADC) Enfortumab vedotin, developed jointly by Seattle genetics and Astellas Pharma, is formed by coupling the human IgG1 monoclonal antibody enfortumab (clone No.: ags-22ce) targeting Nectin-4 with the cytotoxic agent MMAE (monoclonal auristatin E), which is connected to the antibody of Nectin-4 through protease cleavage linker. It is used to treat patients with locally advanced or metastatic urothelial carcinoma. Information on several clinical trials is summarized below.
Ev-101 (clinicaltrials.gov No.: nct02091999): Ev-101 is a phase I clinical trial to evaluate the pharmacokinetics, immunogenicity, safety and antitumor activity of enfortumab vedotin in patients with urothelial carcinoma and other malignant solid tumors expressing Nectin-4. According to the clinical trial of 112 patients, the ORR of Enfortumab vedotin was 42% (Cr, n=5; PR, n=42). ORR was 42% (95% CI 31.2-52.5) and 36% (95% CI 20.4-54.9) in patients treated with checkpoint inhibitor and liver metastasis, respectively. The 1-year Overall Survival (OS) was 51.6% (95% CI 40.3-61.8) and 42% (95% CI 25.0-58.0), and the median OS was 12.2 months (95% CI 8.5-17.1) and 10.4 months (95% CI 6.4-14.1); Median Progression Free Survival (PFS) was 5.4 months (95% CI 5.1-6.3) and 3.5 months (95% CI 1.6-6.6).
The most common adverse reactions were fatigue (53%), hair loss (46%) and loss of appetite (42%). Grade 3 adverse reactions occurred in ≥5% of patients, including anemia (8%), hyponatremia (7%), urinary tract infection (7%) and hyperglycemia (6%). In addition, 4 cases of death caused by side effects of drugs (respiratory failure, urinary tract obstruction, diabetic ketoacidosis and multiple organ failure) were reported in [24,25].
Ev-201 (clinicaltrials.gov No.: nct03219333): Ev-201 is a global, clinical phase II, two cohort, single group trial to evaluate the safety and efficacy of intravenous injection of 1.25mg/kg Enfortumab vedotin in patients with advanced urothelial cancer who have previously been treated with platinum chemotherapy and checkpoint inhibitors. The results showed that among 303 enrolled patients, the ORR was 44% (95% CI 35.1-53.2), the CR rate was 12%, and the PR rate was 32% [24].
Ev-103 (clinicaltrials.gov No.: nct03288545): Ev-103 is a clinical phase I, dose increasing and dose expanding trial. Enfortumab vedotinis used in combination with chemotherapy or immunotherapy in patients with urothelial carcinoma. The preliminary results of Ev-103 showed that in most patients, the combination of Enfortumab vedotin and pembrolizumab reduced the tumor, with an ORR of 71% (32/45; 95% confidence interval 55.7-83.6). The CR rate was 13% (6/45); 58% (26/45) had PR, and 22% (10/45) had stable progression. ≥grade 3 treatment-related adverse reactions were rash (11%; 5/45), hyperglycemia (7%), and peripheral neuropathy (4%; 2/45). These rates are similar to those of monotherapy using Enfortumabvedotin [24,26].
Padcev submitted applications for two clinical trials in China October 2020. Padcev is an antibody drug conjugate targeting Nectin-4. The indication approved in the United States is to treat locally advanced or metastatic urothelial carcinoma (UC, the most common type of bladder cancer).
9mw2821 of Mabwell Biology is a new ADC drug targeting Nectin-4 based on ADC drug development platform and automatic high-throughput hybridoma antibody molecular discovery platform. 9mw2821 has uniform structure and is easy to be industrialized. It showed good drug formation in vitro pharmacodynamic activity, in vivo metabolic properties and preliminary safety. It has good anti tumor effect in a variety of animal tumor models. Its clinical value will be revealed in the next clinical trial.
Antibody
Sbt6290 is an antibody candidate drug targeted by Nectin-4 developed by Silverback Therapeutics using its proprietary immunotac technology platform that can be delivered systematically. At the 2021AACR conference, the company announced the preclinical research results of the candidate drug, indicating that Sbt6290 can activate human myeloid cells and showed single drug antitumor activity in mouse trials.
Bicycle Toxin Conjugate
Bt8009, which is a BTC drug targeting Nectin-4, is under development by Bicycle Therapeutic [27]. It is currently in phase 1/2 clinical trial, which is carried out in patients with advanced solid tumors related to the expression of Nectin-4.
As a cutting-edge biotechnology company, Bicycle Therapeutics is developing a new type of drugs - Bicyclic Peptides, also known as bicycles. It is reported that the molecular weight of these drugs is 50-100 times smaller than that of typical antibody coupled drugs. They are expected to completely penetrate the tumor and have more advantages in tumor antigen targeting. Bt8009 has shown better anti-tumor activity in preclinical research.
Conclusion
In short, Nectin-4 has been a fully validated tumor antigen, especially the birth of the first ADC drug targeting Nectin-4, which proves that it is an effective target for the development of tumor drugs. It also lays a foundation for the development and identification of ADC drugs and other new drug categories.
Although the number of R&D pipelines targeting nectin-4 has not been reported so far. However, with the further advancement of relevant research, it is expected that more antibody drugs, ADC drugs and other new drugs targeting Nectin-4 will enter clinical trials and shine in more cancer treatment fields in the future.
Author Statements
Conflicts of Interest
There are no conflicts of interest.
References
- Delpeut S, Noyce RS, Richardson CD. The tumor-associated marker, PVRL4 (nectin-4), is the epithelial receptor for morbilliviruses. Viruses. 2014; 6: 2268-86.
- Noyce RS, Bondre DG, Ha MN, Lin LT, Sisson G, et al. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011; 7: e1002240.
- Noyce RS, Richardson CD. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 2012; 20: 429-39.
- Rabet MM, Cabaud O, Josselin E, Finetti P, Castellano R, et al. Nectin-4: a new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann Oncol. 2017; 28: 769-776.
- Fabre-Lafay S, Garrido-Urbani S, Reymond N, Goncalves A, Dubreuil P, et al. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J Biol Chem. 2005; 280: 19543-50.
- Fabre-Lafay S, Monville F, Garrido-Urbani S, Berruyer-Pouyet C, Ginestrier C, et al. Nectin-4 is a new histological and serological tumor associated marker for breast cancer. BMC Cancer. 2007; 7: 73.
- Rajc J, Gugic D, Frohlich I, Marjanovic K, Dumencic. Prognostic role of Nectin-4 expression in luminal B (HER2 negative) breast cancer. Pathol Res Pract. 2017; 213: 1102-1108.
- Zeindler J, Soysal SD, Piscuoglio S, Ng CKY, Mechera R, et al. Nectin-4 Expression Is an Independent Prognostic Biomarker and Associated With Better Survival in Triple-Negative Breast Cancer. Front Med (Lausanne). 2019; 6: 200.
- Buchanan PC, Boylan KLM, Walcheck B, Heinze R, Geller MA, et al. Ectodomain shedding of the cell adhesion molecule Nectin-4 in ovarian cancer is mediated by ADAM10 and ADAM17. J Biol Chem. 2017; 292: 6339-6351.
- Derycke MS, Pambuccian SE, Gilks CB, Kalloger SE, Ghidouche A, et al. Nectin 4 overexpression in ovarian cancer tissues and serum: potential role as a serum biomarker. Am J Clin Pathol. 2010; 134: 835-45.
- Takano A, Ishikawa N, Nishino R, Masuda K, Yasui W, et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009; 69: 6694-703.
- Boylan KL, Buchanan PC, Manion RD, Shukla DM, Braumberger K, et al. The expression of Nectin-4 on the surface of ovarian cancer cells alters their ability to adhere, migrate, aggregate, and proliferate. Oncotarget. 2017; 8: 9717-9738.
- Nabih ES, Abdel Motaleb FI, Salama FA. The diagnostic efficacy of nectin 4 expression in ovarian cancer patients. Biomarkers. 2014; 19: 498-504.
- Ma J, Sheng ZY, Lv Y, Liu WB, Yao QY, et al. Expression and clinical significance of Nectin-4 in hepatocellular carcinoma. Onco Targets Ther. 2016; 9: 183-90.
- Zhang Y, Chen P, Yin W, Ji Y, Shen Q, et al. Nectin-4 promotes gastric cancer progression via the PI3K/AKT signaling pathway. Hum Pathol. 2018; 72: 107-116.
- Deng H, Shi H, Chen L, Zhou Y, Jiang J, et al. Over-expression of Nectin-4 promotes progression of esophageal cancer and correlates with poor prognosis of the patients. Cancer Cell Int. 2019; 19: 106.
- Zhang J, Liu K, Peng P, Li S, Ye Z, et al. Upregulation of nectin-4 is associated with ITGB1 and vasculogenic mimicry and may serve as a predictor of poor prognosis in colorectal cancer. Oncol Lett. 2019; 18: 1163-1170.
- Zhang Y, Li S, Wang L, Wu Y, Hao J, et al. A novel PI3K/AKT signaling axis mediates Nectin-4-induced gallbladder cancer cell proliferation, metastasis and tumor growth. Cancer Lett. 2016; 375: 179-189.
- Targeting Nectin-4 in Bladder Cancer. Cancer Discov. 2017; 7: OF3.
- Nishiwada S, Sho M, Yasuda S, Shimada K, Yamato I, et al. Nectin-4 expression contributes to tumor proliferation, angiogenesis and patient prognosis in human pancreatic cancer. J Exp Clin Cancer Res. 2015; 34: 30.
- Reymond N, Fabre S, Lecocq E, Adelaide J, Dubreuil P, et al. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J Biol Chem. 2001; 276: 43205-15.
- Siddharth S, Goutam K, Das S, Nayak A, Nayak D, et al. Nectin-4 is a breast cancer stem cell marker that induces WNT/beta-catenin signaling via Pi3k/Akt axis. Int J Biochem Cell Biol. 2017; 89: 85-94.
- Siddharth S, Nayak A, Das S, Nayak D, Panda J, et al. The soluble nectin-4 ecto-domain promotes breast cancer induced angiogenesis via endothelial Integrin-beta4. Int J Biochem Cell Biol. 2018; 102: 151-160.
- Hanna KS. Clinical Overview of Enfortumab Vedotin in the Management of Locally Advanced or Metastatic Urothelial Carcinoma. Drugs. 2020; 80: 1-7.
- Rosenberg J, Sridhar SS, Zhang J, Smith D, Ruether D, et al. EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients With Nectin-4-Positive Solid Tumors, Including Metastatic Urothelial Carcinoma. J Clin Oncol. 2020; 38: 1041-1049.
- Rosenberg JE, O’Donnell PH, Balar AV, McGregor BA, Heath EI, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2019; 37: 2592-2600.
- Bicycle Therapeutics Announces First Patient Dosed in Phase I/II Trial of Bicycle® Toxin Conjugate BT8009 in Patients with Advanced Solid Tumors. Retrieved Sep 10 2020. https://investors.bicycletherapeutics.com/news-releases/news-release-details/bicycle-therapeutics-announces-first-patient-dosed-phase-iii-0