
Review Article
Austin Med Sci. 2023; 8(1): 1075.
Summary of Research and Development Progress of Oncolytic Viruses
Pengpeng Tian1,2*; Honghong Lu2,3
¹Institute of Bioengineering, Biotech Pharma Ltd, Beijing, China
²College of Science and Technology, Beijing Open University, Beijing, China
³College of Business, Beijing Open University, Beijing, China
*Corresponding author: Pengpeng Tian Institute of Bioengineering, Biotech Pharma Ltd, Rongjing East Street, Beijing Development Are (BDA), Beijing 100176, Beijing, China. Email: tianpeng363@163.com
Received: April 12, 2022 Accepted: May 11, 2022 Published: May 18, 2023
Abstract
Oncolytic viruses are viruses that have been genetically modified to specifically infect and kill cancer cells. They have significant potential in the field of cancer therapy because they possess many advantages over traditional treatments, such as selective killing of tumor cells, increasing the immunogenicity of tumor cells, and avoiding the side effects of chemotherapy and radiotherapy. Currently, several oncolytic viruses have shown good safety and efficacy in clinical trials. More combination therapy strategies are being explored, and it is expected that oncolytic viruses will be more widely used in cancer treatment in the future.
Keywords: Oncolytic viruses; Research and development progress; Immunotherapy
Introduction
Oncolytic Viruses (OVs) are currently a hot topic among researchers and have potential anti-tumor value. In 1999, Rabkin and colleagues discovered an Oncolytic Herpes Simplex Virus (oHSV) with tumor lysing activity that acted as an in situ tumor vaccine, activating anti-tumor immunity. This study found that OV delivery and tumor cell killing led to the generation of tumor-specific CD8+T cells. Subsequent studies have shown that tumor antigen-specific adaptive immunity plays an important role in OV-mediated tumor therapy. Based on these data, scientists believe that OVs have potential value as therapeutic tumor vaccines or as a new form of immunotherapy.
Characteristics of Oncolytic Viruses
Oncolytic Viruses (OVs) are a large group of viruses with anti-tumor effects. They selectively infect tumor cells and their stroma, replicate within them, and lyse the infected cells upon completion of replication. OVs do not infect or damage normal cells or tissues for the following reasons: 1) virus-specific receptors mediate virus-specific entry into tumor cells and their stroma; 2) compared to quiescent normal cells, tumor cells have high metabolic and replicative activity, which is advantageous for virus replication; 3) the tumor-driving mutations provide a natural selection advantage for virus replication. After tumor cells and their stroma are lysed by OVs, the newly replicated viruses continue to infect other tumor cells and their stroma [1].
Classification of Oncolytic Viruses
OVs viruses can be divided into two types: natural OVs and genetically engineered OVs. Genetically engineered OVs have enhanced cell toxicity and immune activation activity. Reovirus and vaccinia virus target tumor cells naturally through the Ras signaling pathway. Reovirus selectively replicates in Ras-activated cells, while vaccinia virus tends to replicate in cells with overexpressed EGFR, as it requires EGFR-Ras signaling for replication. Genetically engineered vaccinia virus, Pexa-Vec (JX-594), targets tumor cells through multiple mechanisms, including the EGFR/KRAS signaling pathway, the level of Thymidine Kinase (TK), and the resistance of tumor cells to type 1 interferon, which can activate viral replication. Vesicular Stomatitis Virus (VSV) can infect and destroy the tumor vascular system (without damaging the normal tissue vascular system), which is critical in the treatment of solid tumors.
Mechanisms of Oncolytic Virus Killing Tumor Cells
Oncolytic Viruses (OVs) kill tumor cells and inhibit tumor progression through four mechanisms: 1) lysis. OVs replicate inside tumor cells and ultimately cause tumor cell lysis; 2) Anti-tumor immunity. The virus, tumor antigens, and lysed cell debris are presented by Dendritic Cells (DCs) to activate local and systemic immunity; 3) Destruction of tumor tissue blood vessels, which can play an important role in solid tumor treatment; and 4) expression of transgenic products. Genetic engineering modifies OVs to load target genes, increase the expression of transgenic products, and enhance the killing function of OVs against tumor cells.
OVs also have the function of regulating the Tumor MicroEnvironment (TME), which can convert “cold tumors” into “hot tumors.” This is due to the release of Damage-Associated Molecular Patterns (DAMPs) and Pathogen-Associated Molecular Patterns (PAMPs) during the infection process, the production of cytokines, the infiltration of immune cells, the induction of Immunogenic Cell Death (ICD) of tumor cells, the activation of infiltrating immune cells, and the generation of anti-tumor immunity. To further enhance the immune activation function of OVs, the following methods can be considered: 1) Integrating immune activation genes (such as Th1 cytokine coding genes) into the virus carrier. Th1 cytokines include GM-CSF and IL-2. IL-10 can act as both a Th2 cytokine and a Th1 cytokine, enhancing anti-tumor immunity. IL-24 is a member of the IL-10 family and has potential anti-tumor activity when expressed by OVs. Zhu et al. showed that vaccinia virus (one of the OVs) expressing IL-2, IL-12, IL-15, IL-21, IL-23, and IL-36γ was safe and effective in multiple tumor models. OVs expressing IL-7 and IL-12 also have anti-tumor immune activation function; 2) Integrating ICOS ligand to enhance immune cell co-stimulation in TME. OVs can upregulate the expression of T cell ICOS receptors, theoretically amplifying anti-tumor immunity; 3) Integrating Immune Checkpoint Inhibitors (ICIs) to activate anti-tumor immunity; 4) Loading CD24 and CD47 antibodies. CD24 and CD47 are “do not eat me” signals of tumor cells, which allow them to escape immune inspection and clearance. CD24 and CD47 antibodies combined with OVs can enhance innate immunity, improve lysing function, and further promote immune regulation; 5) Loading tumor antigens to induce antigen-specific CD8+ CTL response; 6) Loading signaling pathway inhibitors to avoid the interaction between tumor cells and TME. The CXCR4/CXCR12 signal is blocked by CXCR4 antagonists to destroy tumor vascular tissue, induce ICD, reverse immune suppression in TME, enhance anti-tumor immunity, and inhibit tumor metastasis. 7) Loading T cell “engagers” to link initial T cells and tumor cells, activate T cells without the need for MHC, and eliminate tumor cells [2].
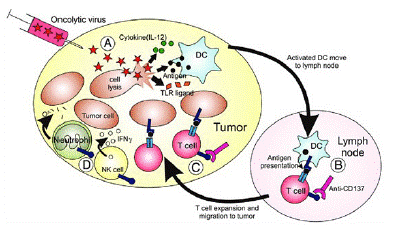
Figure 1: Oncolytic virotherapy.
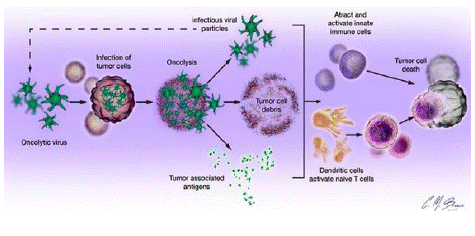
Figure 2: Oncolytic virus killing mechanism.
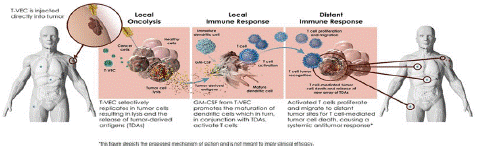
Figure 3: Mechanism of action of T-VEC.
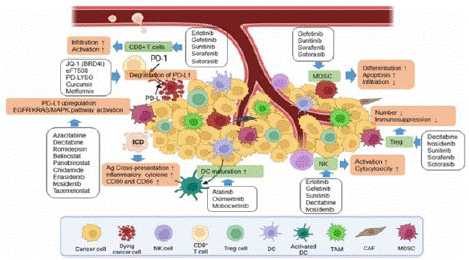
Figure 4: Small molecule inhibitors.
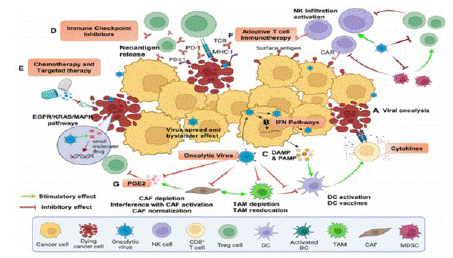
Figure 5: Combination therapy.
Clinical Approved Products and Current Status of Clinical Research
As of now, there are three OV (Oncolytic Virus) products approved for human cancer therapy: H101 (adenovirus) approved in China in 2005, T-VEC (HSV) approved in the United States in 2015, and Delytact (G47 Δ, HSV) approved in Japan in 2021. A recent review article summarized the current status of OV clinical trials: 97 completed trials involving 3,233 cancer patients and 119 published research reports. Among these studies, only 9% of patients had objective clinical responses and 21% had disease control, indicating that the therapeutic effect of OVs needs to be improved. However, three OVs carrying GM-CSF (HSV-1 [T-VEC], human adenovirus [CG0070], and vaccinia virus [Pexa-Vec]) have shown promising results. T-VEC can induce local and distant anti-tumor immunity. In a phase IIIc and IV melanoma patient study, T-VEC was approved by the FDA for the treatment of advanced melanoma based on its overall objective clinical response results from virus inoculation into the lesion. A recent real-world study of patients with unresectable metastatic melanoma at stages IIIB-IVM1a who could be injected but not resected showed that T-VEC treatment resulted in a high total remission rate (64%), with most patients (43%) achieving long-term complete remission. However, in a randomized, double-blind phase III clinical trial (NCT02263508), T-VEC combined with pembrolizumab did not show an advantage over pembrolizumab alone in the treatment of late-stage melanoma patients. In a phase II clinical trial for non-muscle invasive bladder cancer patients who did not respond to BCG, CG0070 was injected into the bladder, and after 6 months, 47% of patients achieved Complete Remission (CR). Subgroup analysis of in situ cancer patients showed a CR rate of 50%, and toxicity was acceptable. Currently, the BOND-003 phase III trial (NCT04452591) and a phase II trial combined with pembrolizumab are ongoing. Preliminary reports from the phase II trial showed a CR rate of 88% (14/16) at the 3-month evaluation. Pexa-Vec confirmed the oncolytic response, immune-mediated mechanism, tumor response, and dose-survival relationship in HCC patients in a phase II clinical trial. In the phase III PHOCUS clinical trial (NCT02562755), Pexa-Vec combined with Nexavar did not achieve the expected endpoint. However, 10 other phase I/II clinical studies are evaluating the role of Pexa-Vec in other solid tumors. A phase I/II clinical trial (NCT0320206073) of Pexa-Vec combined with ICI treatment for refractory colorectal cancer patients may prove the efficacy of this therapy. Regarding safety, to date, there have been no reports of significant toxicity or safety issues with OV in clinical trials. Influenza-like symptoms such as chills and fever may appear during local or systemic intravenous infusion, but the symptoms are mild [3].
Biomarkers
Clinical studies have shown that only some patients benefit from OVs therapy, and the identification of biomarkers can effectively guide the application of OVs therapy, thus improving the therapeutic effect. Generally speaking, biomarkers are divided into predictive and responsive types. For M1 adenovirus, four predictive biomarkers have been identified to evaluate its OVs activity increase: Zinc finger Antiviral Protein (ZAP), inositol kinase 1a, Ras homolog family member Q, KRAS mutation and activation. Serum HMGB1 may be a predictive biomarker for oncolytic adenovirus immunotherapy. Currently, the understanding of OV-mediated responsive biomarkers is limited. Immunoglobulin-Like Transcript 2 (ILT2) may be a responsive biomarker for vaccinia virus. Laboratory studies have found that the expression of ILT2 in tumors is negatively correlated with clinical response. Further studies have shown that ILT2 is a marker of regulatory CD4+ (Treg) and inhibitory CD8+ T cell responses, and the downregulation of ILT2 can predict the patient’s treatment response. Scientists believe that some biomarkers are tumor type-specific and/or OV-specific, but each OV is unique, so they should be studied separately [4].
Outlook on Oncolytic Virus Combination Therapy
Given the limited efficacy of OVs as monotherapy, combination with other anti-tumor therapies is generally preferred. Tumor cells enhance cell proliferation, tumor progression, and metastasis by altering signal transduction pathways. These changes at the cellular and molecular level have been used in the design of small molecule targeted drugs. Small molecule targeted drugs that regulate key cell signaling pathways involved in tumor cell survival and proliferation play an important role in tumor drug therapy, revealing the interactions between tumor and immune cells in the TME [5]. 1. Regulators targeting the key cell signaling pathways that control tumor cell survival and proliferation can induce ICD of tumor cells, enhance tumor immunogenicity, and subsequently anti-tumor immunity. 2. In addition to inducing tumor cell death, kinase inhibitors targeting tumors can also reverse immune suppression in the TME. 3. Changes in metabolism and epigenetics of tumor and immune cells in the TME can be suppressed by regulators that correspond to key enzymes involved in this process, inhibiting tumor growth and proliferation and restoring normal immune cell function.
Based on the above characteristics of tumor regulation and treatment, signal transduction regulators have become an ideal choice for combination therapy with OVs. EGFR-KRAS, PI3K-AKT-mTOR, ERK-MEK, JAK-STAT, p53, PD-1/PD-L1, as well as epigenetics or immune pathways (cGAS-STING), are all hot topics in current research on OV combination therapy [6].
Conclusion
OVs, as a novel anti-tumor therapy, have great development value. Its main mechanism of action is anti-tumor immunity, in addition to playing an important role in destroying tumor tissue blood vessels and regulating TME in solid tumor treatment, which is the application advantage of OVs therapy. The study of OVs biomarkers is of great significance in patient selection and drug efficacy evaluation. The combination of OVs and signal transduction small molecule inhibitors may become the focus of future OVs research. It is difficult to deal with complex and challenging tumor tissues with a single therapy alone.
References
- Bommareddy PK, Shettigar M, Kaufman HL. Integrating oncolytic viruses in combination cancer immunotherapy. Nature reviews Immunology. 2018; 18: 498-513.
- Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Molecular therapy: the journal of the American Society of Gene Therapy. 2010; 18: 251-263.
- Martin NT, Bell JC. Oncolytic Virus Combination Therapy: Killing One Bird with Two Stones. Molecular therapy : the journal of the American Society of Gene Therapy. 2018; 26: 1414-1422.
- Twumasi-Boateng K, Pettigrew JL, Kwok YYE, Bell JC, Nelson BH. Oncolytic viruses as engineering platforms for combination immunotherapy. Nature reviews Cancer. 2018; 18: 419-432.
- Guo ZS, Bartlett DL. Oncolytic viruses as platform for multimodal cancer therapeutics: a promising land. Cancer gene therapy. 2014; 21: 261-263.
- Zhu Z, McGray AJR, Jiang W, Lu B, Kalinski P, et al. Improving cancer immunotherapy by rationally combining oncolytic virus with modulators targeting key signaling pathways. Mol Cancer. 2022; 21: 196.