
Research Article
Austin Med Sci. 2023; 8(1): 1076.
Eradication of Porphyromonas Gingivalis and Prevotella Intermedia Biofilms using the Chlorhexidine Gluconate and Matrica Mouthwash (Chamomile Extract)
Mahsa Jalili1,2; Fatemeh Sadat Abolhasani3; Parisa Bazin4; Somayeh Sharifi5; Morvarid Shafiei6*
1Department of Medical Microbiology, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
2Reference Laboratory of Public Health, Hamadan University of Medical Sciences, Hamadan, Iran
3Department of Microbiology, Faculty of Biological Sciences, Alzahra University, Tehran, Iran
4Dental Branch, Islamic Azad University of Medical Sciences, Tehran, Iran
5Department of Parasitology, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
6Department of Bacteriology, Pasteur Institute of Iran, Tehran, Iran
*Corresponding author: Morvarid Shafiei Department of Bacteriology, Pasteur Institute of Iran, Tehran, Iran. Tel: +98-9128047530, +98-2164112231 Email: Dr.m.shafiei@pasteur.ac.ir
Received: April 15, 2023 Accepted: September 29, 2023 Published: October 06, 2023
Abstract
Introduction: One of the critical pathogenic agents in oral and dental bacteria is biofilm formation. Porphyromonas gingivalis (P. gingivalis) and Prevotella intermedia (P. intermedia) biofilms critically contribute to the unsuccessful treatment of oral and dental illnesses, and their permanent presence in dental canals harms the recovery process after treatment. Today, dentists are seeking superior and more beneficial choices with less toxicity as appropriate disinfectants for the mouth and teeth.
Objective: The current research aimed to investigate the effects of Chlorhexidine (CHX) gluconate and matrica mouthwash on one-, three-, and five-day-old P. gingivalis and P. intermedia biofilms.
Method: The biofilm formation capabilities of P.gingivalis and P.intermedia were assessed using the microtiter plate method. Subsequently, the Minimum Inhibitory Concentration (MIC) of CHX gluconate and matrica mouthwash at various concentrations against biofilm formation in P. gingivalis and P. intermedia was specified using the microdilution method.
Results: The biofilm formation capabilities of P. gingivalis and P. intermedia were approved on a quantitative scale. Both CHX gluconate and matrica mouthwash were effective in eradicating biofilm formation in P. gingivalis and P. intermedia strains. Notably, according to the results, CHX mouthwash exhibited a greater efficacy in eradicating P. gingivalis biofilm. Furthermore, the matrica mouthwash capability to eradicate biofilms in P. gingivalis and P. intermedia was equivalent, with no significant difference observed.
Conclusion: The CHX mouthwash is recommended as a novel approach for controlling biofilm infections stemming from oral and dental bacteria, including P. gingivalis and P. intermedia
Keywords: Chlorhexidine gluconate mouthwash; Matrica mouthwash; Biofilm formation; Porphyromonas gingivalis; Prevotella intermedia
Introduction
Biofilms are structured communities of non-motile bacteria that can grow on various surfaces, including the oral cavity and teeth. These structured communities are highly resistant to the host immune system and conventional antimicrobial agents, causing critical risks to patients [1]. The capability to form biofilm in bacteria increases resistance to various drugs and antimicrobial substances and diminishes the metabolic activity of cells [2]. Biofilm formation has been extensively investigated in numerous studies due to its effect on over 80% of human bacterial infections [3]. Moreover, the oral cavity that is able to create an appropriate habitat for about 700 microbial species can create complex and dynamic biofilms known as dental plaque [4,5]. Among these, the Porphyromonas gingivalis (P. gingivalis) and Prevotella intermedia (P. intermedia) anaerobic and gram-negative bacteria that are capable of forming sub-gingival biofilms are involved in multiple important periodontal diseases, such as periodontitis and peri-implantitis [6]. The pathogenicity of P. gingivalis and P. intermedia is connected with various pathogenic agents contributing to the colonization of teeth, tissue destruction and interference with the host defense system [7,8]. These bacteria also contribute to tooth decay, lip fissures, salivary gland inflammation, etc. [9].
Chlorhexidine (CHX), a commonly used antibacterial mouthwash, is recommended by dentists because of its antimicrobial, antifungal, and antiviral effects. Moreover, CHX gluconate exhibits particularly strong and long-lasting antimicrobial activities, making it a gold standard and the most highly effective anti-plaque agent [10]. However, evidence suggests that CHX gluconate may cause various complications, such as tooth discoloration, burning sensation, and dry mouth [11]. Matrica herbal mouthwash, which contains chamomile extract, is an essential herbal mouthwash produced by Barij Essential Pharmaceutical Company in Iran [12]. Chamomile, the main ingredient of this mouthwash, possesses antibacterial activities against various bacteria. The anti-inflammatory properties of chamomile can be attributed to its main compounds, i.e., flavonoids, including apigenin, luteolin, quercetin, and patuletin, and lipophilic substances such as sesquiterpenes [11,12]. Some studies have also demonstrated the vital role of matrica herbal mouthwash in controlling the growth of dental microbial plaque, disinfecting the gums and oral cavity, reducing inflammatory lesions of the gums and oral cavity, and combating oral-gum inflammations and infections [13]. Therefore, the current study aimed to assess the anti-biofilm activities of CHX gluconate and matrica mouthwash against biofilm formation in P. gingivalis and P. intermedia isolated strains.
Materials and Method
Bacterial Isolation and Identification
The clinical strains of P. gingivalis and P. intermedia utilized in previous studies were also employed in the current research. These strains, which were obtained from 38 patients who referred to the Faculty of Dentistry, Hamedan University of Medical Sciences, were cultured on Brucella agar supplemented with 10% defibrinated horse blood, 5 μg/mL hemin, and 0.4 μL/mL vitamin K1, and incubated under anaerobic conditions (5% CO2, 5% H2, and 90% N2) for 7 to 10 days. The identification of the anaerobic bacteria was accomplished using API 20 and rapid ID 32A biochemical tests, as well as gas chromatographic analysis of fatty acids [14]. Following identification, isolated colonies were diluted in fresh Brain Heart Infusion (BHI) broth to achieve a McFarland standard turbidity of 0.5. Additionally, a small quantity of this bacterial suspension was employed for subsequent experiments.
Biofilm Formation by Crystal Violet Assay
Biofilm formation was conducted following the protocol outlined by Kuboniwa et al. [16]. The biofilm formation was determined using the microtiter plate test. In a nutshell, anaerobically grown bacteria from an overnight culture were cultured on BHI broth at a concentration of 0.5 McFarland standards, as determined by absorbance readings (0.08-0.1 at 625 nm), using a spectrophotometer (Shimadzu, model UV-120-01, Japan). The BHI broth medium was supplemented with 1% horse serum, hemin, and vitamin K.
The plate containing the bacterial suspension was then placed in an anaerobic chamber at 37°C for 72 hours to allow for the growth of anaerobic bacteria under anaerobic conditions and the formation of biofilm on the bottom of the wells. Following incubation, the contents of each well were aspirated, and the wells were washed with sterile physiological saline to eliminate non-adherent cells. The attached bacteria were fixed using absolute methanol for 10 minutes. Crystal violet (1% w/v) was used to stain the plates. The excess stain was removed by washing, and the plates were rinsed using tap water. The bound dye was re-solubilized with 200 μL glacial acetic acid (33% v/v). Each well’s Optical density (OD) was measured at 650 nm using an Enzyme-Linked Immunosorbent Assay (ELISA) reader. The un-inoculated wells containing media were used as blanks. A three-grade scale was employed to assess the biofilm formation capability. The first grade represented negative results, with ODs below 0.500. The second grade indicated positive results, with ODs ranging from 0.500 to 1.500. Finally, the third grade denoted strongly positive results, with ODs exceeding 1.500. All experimental steps were repeated three times.
Anti-Biofilm Activities of Chlorhexidine and Matrica Mouthwash
In this research, the impacts of matrica herbal mouthwash and CHX on the formation of biofilms were investigated. The biofilms were established in microtiter plates (Sigma Aldrich, St. Louis, Missouri, USA), as described previously in section 2.2. Following a 72-hour incubation period in an anaerobic atmosphere, the media were thrown away, and the plates were thoroughly washed with Phosphate-Buffered Saline (PBS) (pH=7.4) to eliminate all unattached cells. Subsequently, various dilutions of matrica herbal mouthwash (100%, 50%, and 20%) and CHX (2%, 1%, and 0.5%) were prepared and added separately to the pre-formed biofilms. Control wells were also included, with each medium added separately to a subset of the wells. Additionally, each microtiter plate possessed three wells for sterility controls and three wells for growth controls, each single well for different concentrations of matrica herbal mouthwash and CHX. The plates were then incubated for 24 hours at a temperature of 37°C in an anaerobic atmosphere and stained using crystal violet.
Statistical Analysis
The data analysis was performed using SPSS software version 18 and the analysis of variance (ANOVA) test.
Results
Biofilm Formation in Porphyromonas Gingivalis and Prevotella Intermedia
Microtiter plate techniques were employed in order to assess the biofilm capacity of P. gingivalis and P. intermedia strains. The findings revealed a robust positive correlation between these strains and biofilm formation. Furthermore, the varying composition of matrix materials is the reason for the intensity of biofilm formation in these bacterial isolates [15].
Anti-Biofilm Activities of Chlorhexidine and Matrica Mouthwash
In the present study, the anti-biofilm activities of matrica herbal mouthwash and CHX against P. gingivalis and P. intermedia strains were carried out using the standard broth dilution method. Different concentrations of CHX gluconate (2%, 1%, and 0.5%) were tested against these isolates. According to Figure 1, CHX gluconate exhibited significant inhibitory activities against the biofilms of P. gingivalis. Moreover, at higher dilutions, CHX could effectively demolish a substantial amount of biofilms in this bacterium (Figure 1B). It was observed that higher concentrations of CHX easily eradicated a significant portion of the biofilm structure in this bacterium. Besides, matrica mouthwash was found to eliminate the biofilm structure in these bacteria, although the CHX anti-biofilm characteristic exhibited more pronounced impacts (Figure 1A).
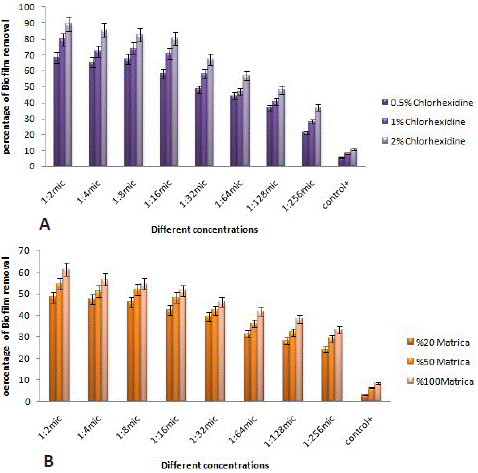
Figure 1: The percentage of biofilm elimination in different concentrations of chlorhexidine gluconate (A), and matrica mouthwash on Porphyromonas gingivalis biofilm (B).
The results also indicated that CHX possessed anti-biofilm capabilities against P. intermedia biofilm, albeit with weaker impacts compared to P. gingivalis (Figure 2A). Additionally, at higher concentrations, CHX exhibited anti-biofilm properties against P. intermedia (Figure 2B). Matrica mouthwash also demonstrated the capability to eliminate the biofilm structure in these bacteria (Figure 2B). Overall, based on the findings presented in Figures 1 and 2, it can be concluded that Matrica mouthwash revealed the same effect on biofilm of P. gingivalis and P. intermedia isolates.
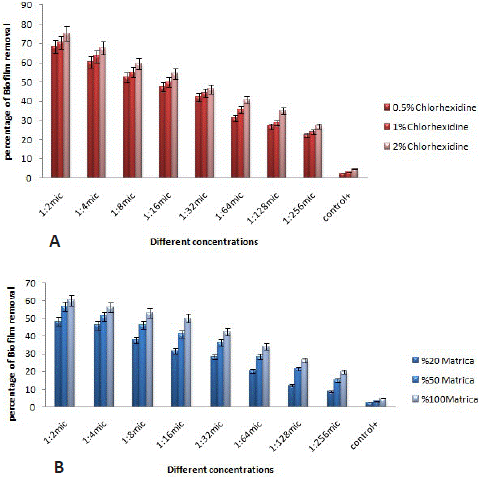
Figure 2: The percentage of biofilm elimination in different concentrations of chlorhexidine gluconate (A), and matrica mouthwash (B) on Prevotella intermedia biofilm.
However, the effect of CHX mouth wash on the removal of P. gingivalis biofilm was significant.
Discussion
Periodontal diseases are now highly prevalent worldwide, affecting up to 90% of the global population [17]. Periodontitis, a chronic inflammatory disease, can cause the progressive destruction of teeth, alveolar bone, and ultimately tooth loss [18,19]. The destruction of soft and bone tissues in periodontal disease primarily stems from chronic infection due to the colonization and invasion of certain bacteria. According to numerous studies, forming bacterial biofilms, particularly P. gingivalis and P. intermedia, are known as agents of periodontal disease [20]. These biofilms play a crucial role in the development and progression of periodontal disease, accounting for most tissue and tooth damage [19,20]. Moreover, the prevalence rate of opportunistic infections has enhanced due to the high incidence of Acquired Immunodeficiency Syndrome (AIDS) and various types of cancer [21]. Consequently, investigations have been conducted to identify effective solutions to combat P. gingivalis and P. intermedia. One approach to eliminate this issue is using various antiseptics, disinfectants, and mouthwashes, such as CHX gluconate and matrica mouthwash [22]. In this study, we evaluated the anti-biofilm impacts of two mouthwash types, namely CHX gluconate and matrica mouthwash on biofilm of P. gingivalis and P. intermedia. Our findings indicate that CHX exhibits greater efficacy than matrica mouthwash against preformed biofilms. Additionally, our study indicates that CHX is particularly effective in eliminating P. gingivalis biofims. Therefore, by using CHX mouthwash preformed P. gingivals biofilms can be efficiently removed. This finding is consistent with the findings of Esfahanizadeh et al. study, reporting that 2% CHX mouthwash is more effective than Matrica mouthwash in eradicating biofilm formed by P. gingivalis and also in controlling microbial plaque [23]. Furthermore, numerous natural products extracted from traditional and herbal medicines possess antibacterial properties, making them an appropriate option to treat periodontal disease. These herbal mouthwashes can serve as effective antibacterial and anti-biofilm agents. However, there is evidence that researches on herbal mouthwashes is limited, and the antimicrobial spectrum of these mouthwashes remains unclear [24]. Hence, it is recommended that further studies for evaluating antibacterial and antibiofilm properties of herbal mouthwashes should be considered.
Conclusion
The findings of this study indicated that CHX mouthwash exhibited an even stronger antibiofilm effect than matrica mouthwash. However, it should be noted that CHX mouthwash has some disadvantages, including an unpleasant taste and the potential to temporarily alter the sense of taste, as well as the ability to cause a brown discoloration on teeth [25]. Consequently, more studies are requied to solve these disadvantages.
Author Statements
Ethical Approval
This study was approved by the ethics committee of the Pasteur Institute of Iran (IR.PII.REC.1400.004).
References
- Fourrier F, Dubois D, Pronnier P, Herbecq P, Leroy O, Desmettre T, et al. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit: a double-blind placebo-controlled multicenter study. Crit Care Med. 2005; 33: 1728-35.
- Yamamoto T, Kita M, Oseko F, Nakamura T, Imanishi J, Kanamura N. Cytokine production in human periodontal ligament cells stimulated with Porphyromonas gingivalis. J Periodont Res. 2006; 41: 554-9.
- Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011; 90: 294-303.
- Jalili M, Mahmoodabadi KA, Sayehmiri K. Relationship between Helicobacter pylori and periodontal diseases: A meta-analysis study and systematic review. Open Dent J. 2020; 14: 362-8.
- Patra JK, Kim ES, Oh K, Kim HJ, Dhakal R, Kim Y, et al. Bactericidal effect of extracts and metabolites of Robinia pseudoacacia L. on Streptococcus mutans and Porphyromonas gingivalis causing dental plaque and periodontal inflammatory diseases. Molecules. 2015; 20: 6128-39.
- Tsuzuno T, Takahashi N, Yamada-Hara M, Yokoji-Takeuchi M, Sulijaya B, Aoki-Nonaka Y, et al. Ingestion of Porphyromonas gingivalis exacerbates colitis via intestinal epithelial barrier disruption in mice. J Periodont Res. 2021; 56: 275-88.
- Kacírová J, Madar M, Štrkolcová G, Madari A, Nemcová R. Dental biofilm as etiological agent of canine periodontal disease. Bacterial biofilms. IntechOpen. 2019.
- Lopes MP, Cruz áA, Xavier MT, Stöcker A, Carvalho-Filho P, Miranda PM, et al. Prevotella intermedia and periodontitis are associated with severe asthma. J Periodontol. 2020; 91: 46-54.
- Caufield PW, Griffen AL. Dental caries: an infectious and transmissible disease. Pediatr Clin North Am. 2000; 47: 1001-19.
- Tabary N, Chai F, Blanchemain N, Neut C, Pauchet L, Bertini S, et al. A chlorhexidine-loaded biodegradable cellulosic device for periodontal pockets treatment. Acta Biomater. 2014; 10: 318-29.
- Bagis B, Baltacioglu E, özcan M, Ustaomer S. Evaluation of chlorhexidine gluconate mouthrinse-induced staining using a digital colorimeter: an in vivo study. Quintessence Int. 2011; 42: 213-23.
- Sadeghi M, Bahramabadi R, Assar S. Antibacterial effects of Persica and Matrica herbal mouthwashes on common oral microorganisms: an in vitro study. J Mashhad Dent Sch. 2011; 35: 107-14.
- Salehi P, Kohanteb G, Momeni Danaei S. Comparison of the antibacterial effects of Persica and matrica, two herbal mouthwashes with chlohexidine mouthwash. J Dent. 2005; 6: 63-72.
- Sanai Y, Persson GR, Starr JR, Luis HS, Bernardo M, Leitao J, et al. Presence and antibiotic resistance of Porphyromonas gingivalis, Prevotella intermedia, and Prevotella nigrescens in children. J Clin Periodontol. 2002; 29: 929-34.
- Yoshida A, Kuramitsu HK. Streptococcus mutans biofilm formation: utilization of a gtfB promoter–green fluorescent protein (PgtfB::gfp) construct to monitor development. Microbiology (Reading). 2002; 148: 3385-94.
- Kuboniwa M, Tribble GD, James CE, Kilic AO, Tao L, Herzberg MC, et al. Streptococcus gordonii utilizes several distinct gene functions to recruit Porphyromonas gingivalis into a mixed community. Mol Microbiol. 2006; 60: 121-39.
- Clinical, institute LS. Performance standards for antimicrobial susceptibility testing. Wayne, PA: Clinical and Laboratory Standards Institute. 2017.
- Mota-Meira M, Lapointe G, Lacroix C, Lavoie MC. MICs of mutacin B-Ny266, nisin A, vancomycin, and oxacillin against bacterial pathogens. Antimicrob Agents Chemother. 2000; 44: 24-9.
- Petersen PE. The burden of oral disease: challenges to improving oral health in the 21st century. SciELO public health; 2005.
- Barbosa GM, Colombo AV, Rodrigues PH, Simionato MRL. Intraspecies variability affects heterotypic biofilms of Porphyromonas gingivalis and Prevotella intermedia: evidences of strain-dependence biofilm modulation by physical contact and by released soluble factors. PLOS ONE. 2015; 10: e0138687.
- Jalili M, Sharifi S, Abdal K, Daneshyar F. Eradication of biofilm formation by Crocus sativus alcoholic extract in Streptococcus mutans clinical isolates. Journal of Medicinal plants and By-product. 2021; 10: 39-42.
- Jalili M, IrajPakzad S, Ghafourian S, Badakhsh B. In vitro eradication of Pseudomonas aeruginosa persister cell Producers by Peganum harmala. Clin Lab. 2022; 68.
- Jalili M, Amraei M, Sadeghifard N, Ghafourian S. Evaluation of biofilm formation and anti-biofilm properties of Peganum Harmala and Crocus sativus in Shigella flexneri clinical isolates. Open Microbiol J. 2019; 13: 297-300.
- Gupta D, Nayan S, Tippanawar HK, Patil GI, Jain A, Momin RK, et al. Are herbal mouthwash efficacious over chlorhexidine on the dental plaque? Pharmacogn Res. 2015; 7: 277-81.
- Duran-Pinedo AE, Baker VD, Frias-Lopez J. The periodontal pathogen Porphyromonas gingivalis induces expression of transposases and cell death of Streptococcus mitis in a biofilm model. Infect Immun. 2014; 82: 3374-82.