
Editorial
Austin J Microbiol. 2015;1(1): 1005.
Undetected Lyme Disease (LD) and Southern Tick-Associated Rash Illness (STARI) in Populations of Southwest Virginia, Leading to Delayed Treatment and an Increased Incidence of Chronic Infection
James R. Palmieri¹*, Jenna Warehime¹, Scott King¹, Amy Doolan², Daniel Reynolds¹, Luke Perry¹ and Elliot Martin¹
¹Department of Microbiology, Edward Via College of Osteopathic Medicine, USA
²Department of Family and Sports Medicine, Edward Via College of Osteopathic Medicine, USA
*Corresponding author: James R Palmieri,Department of Microbiology, Infectious and Emerging Diseases, Edward Via College of Osteopathic Medicine–Virginia Campus, 2265 Kraft Drive, Blacksburg, Virginia 24060 USA
Received: June 24, 2015; Accepted: July 01, 2015; Published: July 02, 2015
Editorial
Lyme Disease (LD) is a multisystem infectious tick-borne zoonotic disease involving multiple systems. Lyme disease is known as Lyme borreliosis, Erythema Migrans (EM) with polyarthritis, Lyme arthritis, or Tickborne meningopolyneuritis. Lyme disease is characterized by a distinctive skin lesion, a red macule or papule that expands in an annular manner (Figure 1) [1]. The systemic symptoms include polyarthritis with neurologic and cardiac involvement. Other symptoms of LD include malaise, fatigue, fever, headache, stiff neck, mylagia, migratory arthralgia, or lympadenopathy lasting several weeks and may precede lesions. Neurological and cardiac abnormalities may occur weeks to months after onset of EM [2,3,4]. Chronic arthritis may also develop. LD is the most common tickborne infection in the United States and Europe. The disease is caused by a spirochete of the genus Borrelia. Borrelia burgdorferi is primarily responsible for LD in the United States while B. afzelii and B. garinii are the primary causing agents in Europe and Asia, respectively. These bacterial agents are transmitted by the bite of infected Ixodes ricinus complex ticks. Rodents, such as the white-footed mouse, are the main reservoirs of Borrelia species in the northeastern United States. The ticks that transmit LD are frequently encountered in backyards and outdoor recreational areas. The incidence of LD in the United States has been increasing steadily since 2002 [1,5]. Early appropriate treatment will increase chances of eradicating the disease and may prevent a patient from developing chronic LD [5-8].
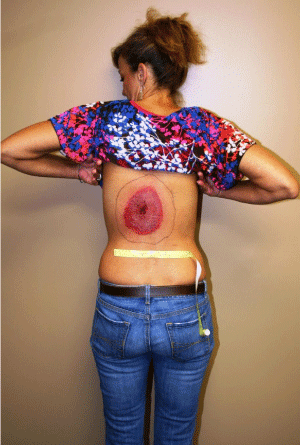
Figure 1: Back of 50 year old female patient from Southwest Virginia showing
erythema migrans bulls-eye, the characteristic rash of Lyme disease; Note:
the rash had decreased from the black outlined area to the reddened area
measuring 11cm x 14cmatday 9 post infection following four full days of
antibiotic treatment [1].
Southern Tick Associated Rash Illness (STARI) or Master’s Disease is an emerging infectious disease in the southeastern and south-central United States. STARI is spread by tick bites and is caused by the recently isolated Borrelia lonestari spirochete, though this association is still debatable [9-11]. STARI is vectored by the lonestar tick Amblyomma americanum [11]. In a similar fashion to LD, STARI is believed to be spread via mice which serve as the primary host for tick larva and nymph stages [11,12]. In humans the initial site of infection can develop an EM rash similar to that of LD, except the lesion borders are more irregular. The rash normally presents about 7 days after the initial tick bite. Unlike LD, the STARI EM may first appear as multiples rather than a singular “bull’s-eye” rash. The STARI rash is too similar to LD to be distinguished via appearance alone [10-12]. Unfortunately, there is no approved diagnostic method to identify STARI, thus the diagnosis is often based on geographic association and clinical symptoms [10]. Patients with STARI develop similar non-specific, generalized symptoms of malaise and body aches but do not progress into the Stage 2 Lyme disease-like illness. Patients who have STARI will have symptoms which often resolve in a few weeks with no permanent damage. Due to its likeness to LD, STARI is often treated as LD; however, no approved treatments have been identified [11-12].
LD and STARI cases in Virginia have increased in frequency since 2005. Unique to the northeastern states with the highest prevalence of LD is the 2,175 mile Appalachian Trail (AT). Its route transects the eastern United States, passing through Virginia to Springer Mountain, Georgia, and is an ideal geographic area for LD acquisition because of the presence of the tick vector and the rodent and deer hosts. Many parents with infants and young children use the AT for day trips or short weekend camping. Epidemiological data of LD reported for counties along the AT shows that the highest rates of LD occur in New York, Connecticut, and New Jersey, and incidence rates in these states have increased since 1992 [13-16]. Presently the incidence of LD in Virginia has increased three-fold since 2002, expanding from northern and eastern Virginia westward [17-19]. According to the CDC, between 1990 and 2012, there have been 8,787 reported cases of LD in the western and southern bordering states of Virginia which include West Virginia, Kentucky, Tennessee and North Carolina; each reporting 1,243; 377; 682, and 1,782 cases of LD respectively; substantially fewer reported cases of LD when compared to Virginia. During the same timeframe, 460,682 cases of LD were reported by the CDC for all 52 states [19].
Presently at the Edward Via College of Osteopathic Medicine, Virginia Campus (VCOM), the Department of Microbiology, Infectious And Emerging Diseases Research Group, is studying LD and STARI in the local human populations of Southwest Virginia. VCOM, along with the Virginia-Maryland Regional College of Veterinary Medicine and Virginia Tech-Carilion School of Medicine, are jointly conducting studies on several aspects of LD and STARI which include: potential damage to nerve cells due to antibodies in a patient infected with Lyme disease; pediatric Lyme disease and STARI in southwest Virginia; and undetected Lyme disease in at risk adult populations in southwest Virginia, leading to delayed treatment and increased incidence of chronic infections. Future studies include: the determination of Borrelia burgdorferi in vaginal secretions of patients infected with acute or chronic LD; and determination of increased prevalence of LD in dark skinned populations in southwest Virginia.
A study by Middelveen (2104) suggests that LD may be acquired through sexual transmission [20]. The study reported semen samples and vaginal secretions from three groups of patients: control subjects without evidence of LD, random subjects who tested positive for LD, and married heterosexual couples engaging in unprotected sex who tested positive for LD. As expected, all of the control subjects tested negative for Borrelia burgdorferi in semen samples or vaginal secretions. In contrast, all women with LD tested positive for Borrelia burgdorferi in vaginal secretions, while about half of the men with LD tested positive for the Borrelia burgdorferi spirochete in semen samples. Furthermore, one of the heterosexual couples with LD the identical strains in married couples strongly suggests that sexual transmission of LD occurs. showed identical strains of the Lyme spirochete in their genital secretions. The presence of the Lyme spirochetes in genital secretions and the identical strains in married couples strongly suggests that sexual transmission of LD occurs.
Several recent studies suggest that LD may be more prevalent in African Americans. Of patients diagnosed with LD, Caucasians were nearly six times more likely than African Americans to have a detected EM rash, since the darker skin color is more likely hide the bull’s-eye rash, the most tell-tale and diagnostic sign of LD and STARI [21]. Because their skin tone hides this key indicator, dark skinned individuals including African Americans may remain untreated longer and therefore suffer more complications from chronic LD.
In summary, there is a need for diagnostic tests sensitive and specific enough to identify LD in all stages of infection and to be able to differentiate LD from STARI. Some clinicians will find controversy in the diagnosis and treatment of patients presenting with signs and symptoms of LD but lacking any dermatological presentation of the EM rash or in patients presenting with negative serological tests. This confusion may be amplified when considering patients with STARI who may present with Lyme disease-like EM but lack positive serological test results for LD. Those with clinical presentations distorted by coinfections will also likely experience a delay in treatment. Clinicians who hesitate to treat patients who do not display all of the diagnostic criteria required by the Infectious Diseases Society of America and by the United States Centers for Disease Control and Prevention, may see their patients continue to progress from a subclinical phase to a more advanced chronic phase of LD.
References
- Palmieri JR, King S, Case M, Santo A. Lyme disease: case report of persistent Lyme disease from Pulaski County, Virginia. International medical case reports journal. 2013; 6: 99-105.
- Tick Borne Diseases of the United States. A Reference Manual for Healthcare Providers. 3rd edn. 2015.
- Eugene DS. Borrelia burgdorferi (Lyme Disease). Centers for Disease Control and Prevention, National Notifiable Diseases Surveillance System (NNDSS). 2014; 35: 500-509.
- Centers for Disease Control and Prevention. Lyme disease transmission. 2015.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012; 379: 461–473.
- Wright WF, Riedel DJ, Talwani R, Gilliam BL. Diagnosis and management of Lyme disease. Am fam Physician. 2012; 85: 1086-1093.
- Garro A, Marcellin L. Lyme Disease. Clinical Key. Elsevier. 2014.
- Nau R, Christen HJ, Eiffert H. Lyme Disease – Current state of knowledge. Dtsch Arztebl Int. 2009; 106: 72-82.
- Masters EJ, Grigery CN, Masters RW. STARI, or Masters disease: Lone star tick-vectored lyme-like illness. Infec Dis Clin N Am. 2008; 22: 361-376.
- Centers for Disease Control and Prevention. Understanding the EIA Testing. 2015.
- Lyme Disease Case Maps. Lyme Disease Association, Inc. 2015.
- Centers for Disease Control and Prevention. Surveillance for Lyme Disease --- United States, 1992-2006. Surveillance Summaries. 2008; 57: 1-9.
- Shapiro ED. Lyme disease. Finn A, Pollard AJ, editors. In: Hot Topics in Infection and Immunity in Children IV. Springer. 2008.
- New York State Department of Health, Information for a healthier New York. Be Tick Free A- Guide for Preventing LymeDisease. 2011.
- Conn's Current Therapy. Edward T. Bope, Rick D. Kellerman. 1st edn. Elsevier. 2014.
- Tick Borne Diseases of the United States. A Reference Manual for Healthcare Providers. 2nd edn. 2014.
- Shelus V. Tick-borne Disease Risk along the Appalachian Trail (Doctoral dissertation, Duke University). 2012.
- Centers for Disease Control and Prevention, Final 2010 Reports of Nationally Notifiable Infectious Diseases. Morbidity and mortality weekly report. 2011; 60: 1088-1101.
- Lyme Disease Association. 2015.
- Middelveen MJ, Bandoski C, Burke J, Sapi E, Mayne PJ, Stricker RB. Isolation and detection of Borrelia burgdorferi from human vaginal and seminal secretions. In Journal of Investigative Medicine. 2014; 62: 280-281.
- Fix AD, Peña CA, Strickland GT. Racial differences in reported Lyme disease incidence. American journal of epidemiology. 2000; 152: 756-759.
Citation: Palmieri JR, Warehime J, King S, Doolan A, Reynolds D, et al. Undetected Lyme Disease (LD) and Southern Tick-Associated Rash Illness (STARI) in Populations of Southwest Virginia, Leading to Delayed Treatment and an Increased Incidence of Chronic Infection. Austin J Microbiol. 2015;1(1): 1005. ISSN 2471-0296