
Case Series
Austin J Microbiol. 2015; 1(2): 1006.
Three Cases of Clinical Pediatric Erythema Migrans in Southwest Virginia
James R. Palmieri1*, Kenneth R. McArtan II1, Russell Hendershot2, Scott King1 and Jenna Warehime1
¹Department of Microbiology, Edward Via College of Osteopathic Medicine, USA
²Department of Preventive Medicine and Population Health, Edward Via College of Osteopathic Medicine, USA
*Corresponding author: James R Palmieri, Department of Microbiology, Infectious and Emerging Diseases-Edward Via College of Osteopathic Medicine– Virginia Campus, 2265 Kraft Drive, Blacksburg, Virginia 24060, USA
Received: June 06, 2015; Accepted: June 30, 2015; Published: July 03, 2015
Abstract
Ticks are common outdoor pests. In Southwestern Virginia, there are five species of ticks, two of which are associated with Erythema Migrans (EM): the black legged tick (Ixodes scapularis) and the lone-star tick (Amblyomma americanum). The black legged tick is associated Borrelia burgdorferi the spirochete responsible for Lyme Diseases (LD). The lone star tick is affiliated with Southern Tick Associated Rash Illness (STARI) which has been associated with Borrelia lonestari. LD infects a person after a ticks feeds for 36 to 48 hours and presents in three stages. Stage 1 presents days to week after the initial bite with an EM rash and generalized sub-clinical symptoms. Stage 2, disseminated infection, presents weeks to months later. Stage 2 is characterized by neuropathy, such as facial palsy, and other organ system pathologies. Stage 3 takes months to a year to develop and is characterized by acrodermatitis chronica atrophicans and mono/oligo-arthritis. STARI presents with an EM rash and subclinical generalized symptoms 7 days after the lone star tick feeds on a person. We report 3 cases of pediatric EM. Case 1 presents a 19 month old female from Roanoke, VA with an erythematous rash on her right buttocks, sub-febrile temperature, and behavior change. Case 2 is a 29 month old boy from Pilot, Virginia who presents with an 8.5 cm erythematous rash on his left scapular/neck region. Case 3 is an 8 year old boy from Blacksburg, Virginia who presents with an erythematous rash that developed into an EM-like rash. Each case presented with and EM-like rash where a tick was the suspected cause. LD was either ruled-out or equivocal. STARI could be suspected in each case due to the atypical presentation of the illness. Though LD was not present or equivocal in each case, LD and STARI are important differentials when a patient presents with an EM-like rash.
Keywords: Appalachian trail; Borrelia burgdorferi; Erythema migrans; Lyme borreliosis; Lyme disease; Pediatric Lyme disease; Spirochete; STARI
Abbreviations
CDC: The United Stated Centers for Disease Control and Prevention; CSF: Cerebral Spinal Fluid; EM: Erythema Migrans; LB: Lyme Borreliosis; LD: Lyme Disease; PCP: Primary Care Provider; STARI: Southern Tick Associated Rash Illness; NNDSS: National Notifiable Disease Surveillance System
Introduction
Ticks are a common hazard of the outdoors to those who live and work around the forest edge [1]. In the Virginia area there are five common tick species known to feed on humans [1-3]. The blacklegged tick (Ixodes scapularis), well known as the “deer tick” is the common host of Lyme Disease (LD), anaplasmosis, and babesiosis [1,3,4]. The groundhog tick (Ixodes cookei) is the host of Powassan disease but only occasionally feeds on humans [1,3]. The lone star tick (Amblyomma americanum); which can cause ehrilichiosis, tularemia, and southern tick associated rash illness (STARI), has been associated with the uncultivable spirochete Borrelia lonestari [1,3,5,6]. The American dog tick (Dermacentor variablis) is the host to Rocky Mountain spotted fever (Rickettsia rickettsii) and tularemia [1,3]. The brown dog tick (Rhipicephalus saguineus) acts as the host to Rocky Mountain spotted fever, its most favored animal host is the dog [3]. Only two of these ticks are known to be associated with Erythema Migrans (EM): the black legged tick and the lone star tick [1,3]. The EM rash is associated with an infection caused by the spirochete bacteria Borrelia burgdoferi and Borrelia lonestari. Both spirochetes are associated with LD and Lyme disease – like illnesses [1,3,5,6].
Lyme disease
Lyme Disease, otherwise known as Lyme Borrelisosis (LB), was first documented in the United States in 1977 as part of an investigation of a cluster of pediatric arthritis cases among children living near and around Lyme, Connecticut [2,7,8]. Since its discovery and subsequent isolation years later, LD has become one of the most common vector borne illnesses in the United States with 20,000 cases annually [9,10]. In addition, LD is one of the most common tick borne illnesses in Europe [2,11] . LD is caused by five species of spirochete bacteria: Borrelia burgdorferi, Borrelia afzelii, Borrelia garninii, Borrelia spielmanii and Borrelia bavariensis [2]. In the United States, Borrelia burgdorferi (Sensu stricto) is the only known spirochete that causes LD [2].
In the United States LD is transmitted almost exclusively by Ixodes ticks, on the East coast this is the black legged tick (Ixodes scapularis) [7,8,12]. Borrelia burgdorferi uses the tick as a vector to infect host species such as chipmunks, shrews, squirrels, birds, lizards, and especially deer and rodent species which serve an integral role in the tick’s life cycle [13-17]. The white tail deer (Odocoileus virginianus)is the most prevalent deer species in the eastern United States plays and important role in the adult and nymph ticks’ life cycle, but has little role in spreading B. burdorferi to uninfected ticks [18,19]. The deer acts as a host for B. burdorferi which allows the spirichetes to mature and to be transported to new areas [18,20]. The most critical and important hosts in the transmission of B. burgdorferi are mice and rodents, especially the white-footed mouse (Peromyscus leucopus) [13-16]. Mice act as the primary host during the larval and nymph stages of tick’s lifecycle [21]. Mice, especially the white-footed mouse, have been shown to be the most susceptible host for B. burdorferi and have the highest concentration of the spirochetes in their blood compared to other mammal models [21,22]. The black legged tick has a 2 year life span divided into 3 developmental stages (larval, nymph, and adult), and must feed only once each stage of its life cycle [13]. The nymph stage is reported to be the most important in the transmission of LD [7,13,21-23].
Once a human has been bitten by an Ixodes tick infected with B. burdorferi, the spirochetes are inoculated with a bacterial load that allows for survival in the human host [13,24]. In Stage 1 of the disease, also known as the early localized stage, it may take days or weeks to fully develop an EM rash at the site of initial infection. The rash is generally non-painful, but may be accompanied by some pruritus. In 10% to 30% of cases the EM rash is accompanied by nonspecific symptoms of malaise, subfebrile temperatures, and joint pain [11,25]. According to studies performed by Gerber, et al. (1996), 90% of children in their study presented with EM on their head and neck while older children most often present with EM on their arms and legs. Patients with lesions on the head or neck were significantly younger than those with lesions at other sites (mean age, 5.9 vs. 8.1 years) while patients with lesions on the arms or legs were significantly older than those with lesions at other sites (mean age, 9.2 vs. 7.4 years) [26].
If untreated, infection can progress over weeks or months to stage 2, the early disseminated stage. In stage 2, infection can spread to the meninges, brain, eyes, heart, joints, and muscles causing a wide range of symptoms [11]. The variability of symptoms may be linked to genetic variability of the LD spirochete; such differences are reported for LD in Europe [27-29]. In the early stages on dissemination, multiple skin lesions may appear [11]. Children may present with hypersensitivity in areas of the chest and/or waist accompanied by abdominal distention, joint pain, and mental status changes [6,30- 34]. In children, stage 2 is characterized by acute peripheral facial palsy with elevated protein and pleocytosis of the Cerebral Spinal Fluid (CSF) [11,35]. After 6 months of untreated infection, the disease enters stage 3, the late disseminated stage. Stage 3 consists of encephalitis, arteritis, polyneuropathy, acrodermatitis chronica atrophicans, and mono/oligo-arthritis. Like in stage 2, the symptoms are variable from person to person. In most cases, chronic arthritis and acrodermatitis chronica atrophicans are the hallmarks of stage 3 infection with many patients experiencing irreversible neurological deficits with varying severity [6,11,35-37]. In children, chronic LD can present as the classic arthritis and dermatological lesions reported in adults, but neurological changes can take the form of memory and behavioral alterations in addition to other neurological impairments. Memory and behavioral alterations may persist as a residual impairment even after successful treatment [9,38].
STARI
STARI (Southern Tick Associated Rash Illness), also known as Master’s Disease, is caused by the recently isolated Borrelia lonestari spirochete, and though this association is still debatable.This illness is vectored by the lone star tick Amblyomma americanum, first proposed as a possible vector of disease in 1984. The illness associated with the lone star tick is called “Lyme-like disease” but was not recognized to be distinct from LD until the late 1990’s [5,6,39]. The infection is spread via the lone-star tick after feeding for what is most likely a similar length of time as a LD transmitting Ixodes tick [39]. In a similar fashion to LD, STARI is believed to be spread via mice, the primary host of the larval and nymph tick stages [39,40]. The initial site of STARI infection can develop an EM rash similar to that of LD, although the borders in STARI are often less regular than those seen in LD infections [6,39]. The rash normally presents about 7 days after the initial tick bite [6]. Tissue biopsies show a greater degree of lymphocytic and neutrophilic reaction at the rash site. Unlike LD, the STARI EM rash may first appear as multiple rather than a singular classic “bull’s-eye” rash. Although there can be differences, the STARI EM rash is often too similar to that of LD to be distinguished by appearance alone [6,39,40]. According to the United States Centers for Disease Control and Prevention (CDC) there is not currently any approved diagnostic method to identify STARI [6]. Thus, the diagnosis of STARI is often made based on geographic association and clinically presenting symptoms [6]. Host exposure to tick habitats would support a STARI diagnosis but is not required according to the CDC. Patients develop similar non-specific, generalized symptoms of malaise and body aches but do not progress to the Stage 2 LD-like illness. Many patients who contract STARI will experience resolution in a few weeks with no lingering symptoms or permanent damage. Long-term studies on STARI have not been conducted at this time. STARI is often treated as LD due to their similar presentations; however, no approved treatments have been identified by the CDC as of June, 2015 [6,39,40].
CDC guidelines
According to Centers for Disease National Notifiable Disease Surveillance System (NNDSS) guidelines, LD is described as a tickborne illness with dermatologic, rheumatologic, neurologic and cardiac symptomology or abnormalities [41]. The most common symptom being EM, which occurs in 60 - 80% of patients and is defined as anerythematous macule or papule rash that expands (over days to weeks) to form a large lesion, often with a clearing center, reaching greater or equal to 5 cm in largest diameter [9,41]. The presence of secondary lesions or annular erythematous lesions (hypersensitivity to the tick bite occurring within several hours of initial bite) does not rule out LD. Symptoms such as non-specific generalized malaise, fatigue, aches, headache, and fever support the diagnosis of LD and follow the presentation of EM. Late manifestations include musculoskeletal, nervous, or cardiovascular symptoms. Musculoskeletal symptoms of LD include recurrent, acute attacks of pronounced joint swelling in one or more joints, lasting weeks to months, sometimes followed by chronic arthritis in one or more joints. Neurologic involvement is defined by lymphocytic meningitis, cranial neuritis (facial palsy), radiculoneuropathy, or encephalomyelitis (confirmed by antibodies to B. burdorferi in the Cerebrospinal Fluid (CSF), higher titers must be in CSF compared to serum [41,42]. Cardiovascular symptoms include acute onset 2nd or 3rd degree atrioventricular blockage, which can resolve in days to weeks, and occasionally myocarditis. Once a diagnosis of EM is made, laboratory testing is suggested using approved techniques. which include: 1. positive culture, two-tier testing for IgM if symptoms are less than 30 days or IgG if symptoms were present for a longer period of time; 2. confirmation by western blot with 5 or more bands for IgG antigens, single-tier IgG; or 3.positive CSF antibody via Enzyme Immunoassay (EIA) or Immunofluorescence Assay (IFA) and CSF levels must be higher than that of serum [9,35,41,42]. If EM is present but with no known “exposure” such as having been in an endemic area where tick-human interaction is possible within 30 days of EM onset and supported with negative laboratory evidence, then the patient has a “suspected” case of LD. A “probable” case is defined by the before mentioned criteria with positive laboratory findings of an infection but lacking diagnostic criteria. In this case a diagnosis is made based on clinical suspicion. A “confirmed” case is defined by known exposure, laboratory evidence of positive titers with or without exposure, or at least one late manifested symptom with laboratory confirmation of disease [35,41-45].
Cases
The following cases are reported from primary care physicians in Montgomery and Roanoke Counties, Virginia.
Case Report 1: A 19 month old female from Roanoke, Virginia was brought to her Primary Care Provider (PCP) on October 25, 2013 for a concerning rash and change in behavior. Two weeks prior, the patient exhibited three days of fluctuating low-grade fever ranging between 100.0 to 100.7 Fahrenheit (F). Following the febrile episodes the child experienced restless nights where she would awake screaming and remain awake for several hours at a time. According to the parents, the child is usually a sound sleeper and this is unusual behavior for her. Seventeen days prior to presentation, the mother states that she removed two embedded ticks from the patient’s axilla. At the time the ticks were removed, the child was asymptomatic. Ten days after the ticks were removed the mother noticed the erythematous circular rash with central clearing on the lower left gluteal area. The patient’s past medical history included newborn jaundice treated with phototherapy for less than one day. The patient has no known allergies.
On physical examination the child was irritable but afebrile, with normal vital signs. The remainder of the physical examination was within normal limits with the exception of two skin lesions. The first lesion was a large, erythematous, macular, circular rash with central clearing located above the left buttocks (Figure 1). The other rash was identified under the patient’s chin and was also erythematous but lacked the “bull’s-eye” appearance. Neither lesion was measured during the physical examination. A two tiered test was performed on the child’s serum. The antibody test (IgM) for B. burgdorferi was elevated. Confirmatory testing via Western Blot demonstrated three of the ten bands displayed reactivity. IgG identification via Western Blot was considered negative with only three bands reactive. Five bands are necessary for confirmation of LD. Following the recommended CDC guidelines for LD diagnosis, the patient was prescribed Cefuroxime Axetil (Ceftin) 200 mg twice per day for twenty-one days. Following treatment, the child remains symptom free as of June2015.
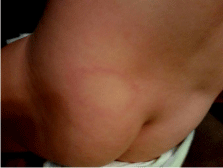
Figure 1: 19 month old female from Roanoke, Virginia with an erythematous
circular rash and central clearing located in the lower left lumbar-gluteal area.
Case Report 2: A 29 month old male from Pilot, Virginia was brought to his PCP on July 21, 2014 by his grandmother. She was concerned about an assumed insect bite and an enlarging rash. She describes the rash to be large in size on the patient’s left posterior scapular/neck region. She reports that the rash appeared about seventy-two hours prior to presentation to the PCP. The grandmother did mention that ten to twelve days prior, she removed several ticks from the child’s clothing. No ticks were found to be attached to the child’s skin. The grandmother stated that they live near a wooded lot in a rural farming community. The grandmother reports that he has been running a temperature but has had no further symptoms or abnormalities. He was not in any acute distress during the examination.
Physical examination revealed the child was running a low grade fever of 100.0 F. The described rash was an 8.5 cm diameter erythematous “bull’s-eye” lesion with central reddening, blanched inner ring, and irregular border (Figure 2). The lesion was located on the left scapular/ shoulder region with some extension to the neck. On palpation the lesion was warm to the touch. The child showed no further abnormalities and presented with a normal physical examination.
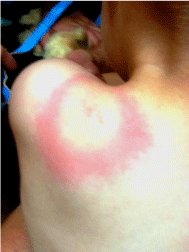
Figure 2: 29 month old male from Pilot, Virginia with a classic erythematous
migrans circular bulls-eye rash with central clearing (dorsal view) which
also contains a small centralized erythematous lesion, all located in the left
scapular/neck area.
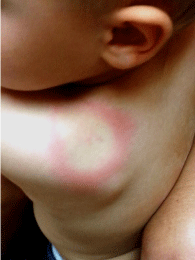
Figure 1: 29 month old male from Pilot, Virginia showing a classic
erythematous migrans circular bulls-eye rash with central clearing (anterior
view). Note for reference: the size of the lesion compared to the patients ear.
Following a discussion with the grandmother, she chose not to have any laboratory testing performed and desired treatment instead. The physician diagnosed the lesion as cellulitis and treated with Amoxicillin 500 mg tablets three times per day for fourteen days. The child has not, as of June, 2015, returned for follow-up.
Case Report 3: An 8 year old male from Blacksburg, Virginia was brought to his PCP on April 23, 2014 by his mother for a rash located on his left chest which began 24 hours prior to arrival. The rash was described as an erythematous, circular lesion that was warm to the touch. The patient’s mother noted he was running a temperature of 100.2 F. The patient had an otherwise normal review of systems. He has a known wheat allergy. When the mother was questioned about possible tick exposure, she mentioned that they live with several pet dogs in the household, but no ticks had been removed from the patient.
Physical examination revealed a child in no acute distress with an elevated temperature of 100.2 F. The rash was a 3.0 cm, circular, erythematous lesion with regular borders, located at the lateral side of the left nipple (Figure 3). The lesion was warm to the touch and at the time of initial presentation, the lesion had no central clearing.
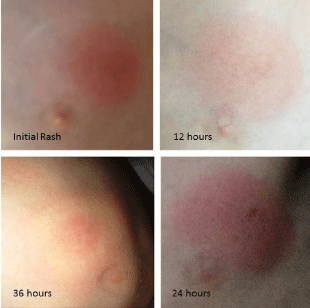
Figure 1: 8 year old male from Blacksburg, Virginia, with a 2.5 cm diameter
circular erythematous lesion located lateral to the left nipple (Initial rash) 12
hours prior to presentation to the family practitioner. At 24 hours the rash was
3.0 cm in diameter. At 36 hours, (24 hours following initial treatment) the rash
developed into the classic EM bulls-eye.
The physician suspected possible LD and ran an initial antibody test which proved to be negative using the Lyme Antibody interpretation EIA. The physician suspected a possible false negative result or a diagnosis of cellulitis and reactively treated the patient with Amoxicillin 250 mg 2 tablets twice per day for 14 days.
Once the treatment was started the rash began to change in nature. Within 24 hours of treatment, the rash showed signs of central clearing and by 36 hours presented with a classic “bull’s-eye” rash. The rash maintained its EM-like state but no expansion was noted.
The family left for vacation two days after starting the treatment. Three days later, the parents of the child stopped the treatment with Amoxicillin. According to the parents, they believed the newer form of the rash was due the Amoxicillin.
On June 4, 2014, the patient’s family returned from vacation. The rash was still present and in the current form. The family was concerned that the rash may be something more. The physician then treated the child with Doxycycline 50 mg twice a day for 14 days. The young boy remained symptom free as of June, 2015.
Discussion
LD and STARI can be difficult diagnoses to make for many physicians. EM can look like other skin lesions such as cellulitis, erythema multiforme, granuloma annulare, hypersensitivity reactions, dermatitis, nummular eczema, methicillin-resistant Staphylococcus aureus infection, spider bite, tinea “ring worm,” and urticaria [9,33]. According to recent studies of Virginia physicians four or more pediatricians are often consulted before a diagnosis of EM is made [46]. Even the later symptoms of LD, such as neurological deficits, are often missed [35]. For this reason, it is important for PCPs to be educated about the identifying features of EM, LD, and STARI.
Case 1 showed a child with an obvious EM-like rash. The child presented with sub-clinical fever and appeared to be uncomfortable. The physician felt this was a possible LD case and ordered a LD screening, which proved to be negative. He proceeded to treat the child as if this was a case of LD or cellulitis by prescribing Ceftin for the rash. There are several problems with this case. First, the physician did not measure or trend the erythematous site that produced an EMlike rash [41,42]. Second, the ticks found were not at the site of the rash, nor were the ticks retained for later examination [41,42]. Third, Western Blot ruled out LD and an interpretation of sub-levels of LD infection is not advised [44,45]. The physician did treat the case as a cellulitis and treated it appropriately [24]. The patient’s symptoms resolved but there is no way of knowing the actual cause of the rash since no skin biopsy or culture was performed.
Case 2 presented as a more typical and classic case of EM where measurements were performed. The diagnosis of EM could be made with a lesion diameter of 8.5 cm and a central clearing [41]. There was no clinical recording of enlargement. Since the grandmother opted to not test the child, no positive identification could be made and the lesion could only be diagnosed as a cellulitis. The treatment of Amoxicillin 500 mg three times per day is an excessive dose for a 29 month old child [24]. Such a high dose of medication can have its own set of problems. Since the patient has not returned as of June, 2015, no further clinical determination can be made from this case.
Case 3 presented with a fever and rash that later developed into an EM-like lesion. This case initially presented as cellulitis and was treated appropriately. There were no tick bites mentioned but a suspicion of tick contact is plausible due to the interaction between the child and their dogs. After 24 hours of treatment, the rash developed into an EM-like lesion with central clearing. This rash remained until the family returned to the physician, at which point Doxycycline was prescribed. The EM-like lesion showed signs of enlargement but no mention of it growing to a reportable size. No further diagnostic or laboratory tests were performed to confirm LD, making this an equivocal case. According to the CDC the presence of a bull’s-eye rash warrants treatment for LD [41].
In all three cases, LD was suspected even though Virginia is not considered an endemic region [47]. Areas endemic to LD include: Connecticut, Delaware, Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Wisconsin [9]. Virginia ranks 14th in number of reportable cases of LD and borders endemic regions, leaving one to suspect a possible migration of LD carrying Ixodes ticks [47]. Each case presented with a more “clinical” definition of EM but none met the criteria for reportable diseases [41]. All three cases were fairly equivocal for LD due to lack of testing or Western Blot test rule-out [41]. Each case demonstrated a break in the diagnostic chain for agreed upon criteria of reportable LD [48]. One child did show laboratory findings of B. burdorferi antigen reactivity but this cannot be used as a definitive means of diagnosis for a subclinical case of LD [41,42]. All three reported cases could fall under the diagnosis of STARI, for which confirmatory laboratory testing has not yet been made standard. All three cases contained a clinical EM rash which STARI could be responsible for. STARI is endemic to the region and features nonspecific, generalized symptoms making this a possible diagnosis [39,40]. In the three cases we report, the rash was suspected to be caused by a tick but none were found in the vicinity of where the rash appeared. It is plausible that the nymph-stage tick was present but undetected due to its small size. A nymph-stage tick could easily have fed on these children for the amount of time necessary to transfer clinical or subclinical levels of spirochetes, resulting in the rashes seen in all three cases (Figures 1, 2 and 3) [7,13,23]. In addressing the three reported cases, adherence to reporting protocols and recognition of mimics is important. According to Rose, et al. (1994) in a case study of 227 pediatric cases of reported LD, 138 were found to be negative for LD upon reevaluation at a LD treatment center, supporting the fact that adherence to symptomology and reportable criteria is important to avoid misdiagnosis of LD [49].
LD and STARI differential
LD can become a serious disease if it is allowed to progress to dissemination. Though, LD is not endemic to Virginia, it is important to recognize that Virginia borders several LD endemic states and is ranked 14th in the nation for reportable LD cases [20,47,50,51]. LD in Virginia has been increasing in number and frequency, with a 3-fold increase since 2002. According to the CDC, from 1990 to 2012, there have been 8,787 reported cases of LD in Virginia alone [20,51,52]. This increase in incidence could be due to habitat loss in the northern, more endemic regions of the state, forcing deer and tick populations south [19,20,52]. One model suggests that deforestation in the more southern states is causing an increase in Virginia’s deer population, a favored host for the black legged tick. Another possible explanation is the increase in mice populations that serve as mammalian hosts for tick larvae and nymphs [19,20,22]. Though LD is not thought to be present in the more southern states, LD is still being reported and treated in those areas [8,13,14,47,52-54]. Examples of such cases are noted in the article “Lyme Borreliosis in human patients in Florida and Georgia” by Clark KL, et al. (2013) [54].
STARI is still considered a newer tick borne illness and few long term studies have been completed on the lasting complications STARI has on its patients. Most patients recover without incident, even without treatment. STARI, being a LD mimic, may account for an increasing number of cases of EM in southern states, especially in areas where the lone-star dog tick is more prevalent [6,39,40]. STARI can explain why many patients with EM and sub-clinical symptoms test negatively for LD. It is also plausible that there is some cross reactivity to immunological testing, especially if STARI is proven to be caused by the spirochete B. lonestari [6,39,40,55]. Cross reactivity to LD testing has already been reported between other spirochete related diseases such as syphilis and Rocky Mountain spotted fever [56].
Although LD has only been proven to be transmitted by ticks, LD spirochetes have been isolated in breast milk, umbilical tissue, semen, vaginal secretions, and other bodily fluids [57]. This leads one to question whether other modes of transmission are plausible [57]. Other spirochetes, such as leptospirosis and syphilis, have modes of transmission using bodily fluids of humans and/or animals. STARI may have other modes of transmission through the utilization of bodily fluids as well [58,59].
Treatment
B. burdorferi and other Borrelias are highly susceptible to cefotaxime, ceftriaxone, and macrolides. There is some sensitivity to amoxicillin, other aminopenicillins, and tetracycline but to a lesser degree. Generally in Stage 1, Doxycycline 100 mg b.i.d. for 14 days is the standard of care for adults. Children under nine generally receive amoxicillin in a modified dose (the adult dose being 500 mg t.i.d. for 14 days). For later development of LD, as in neuroborrellosis and symptomatic Lyme carditis; ceftriaxone or ceftotaxime 2g IV qd is given for 14 to 28 days (in stage 3 neuroborreliosis a 28 day course is required). In Lyme arthritis Doxycycline is used at 100 mg b.i.d. for 28 days. Children with Lyme arthritis should receive a modified dose of Amoxicillin for 28 days [6,9,11].
Conclusion
EM is a difficult diagnosis to make especially with EM-like mimics such as STARI. Though EM is often used in conjunction with a diagnosis of LD, STARI is an alternative diagnosis in many cases. Both LD and STARI are tick borne illnesses with overlapping endemic regions; however neither illness is isolated to just those regions. It is important for PCPs to be aware of the signs and symptoms of both diseases. With LD, PCPs need to utilize standardized diagnostic techniques and follow generally accepted criteria for identifying the disease. PCPs should always measure, mark, and photograph an EM rash. Marking and measuring are important criteria for the reportable diagnosis of EM. PCPs must be vigilant for atypical cases and the possibility that such atypical cases of LD may actually be STARI. In the same regard, patients must be aware of ticks and other vectors that may be present around their homes. Patients should keep any attached ticks they remove for at least a month. These ticks should be presented to their physician if the patient becomes sick or develops a rash. Hypersensitivity rashes to tick bites are common, but EM is not a normal occurrence. Parents and guardians of children should be aware of the areas where their children are playing. Inspecting the child’s skin on a regular basis and maintaining good hygiene is a must for those whom live near or around areas where ticks are present. It is also important to continue any antibiotic regimen to completion under the guidance of a physician.
Although LD has been studied in great detail since its discovery in 1977, STARI has not been well researched. The long term effects of STARI on humans still require study. Further research is necessary to explain why LD cases are present in outlying regions and whether these cases are actually STARI. Additional research is also necessary to better solidify STARI as an illness caused by Borrelia lonestari and whether this spirochete has any cross-reactivity to LD testing.
Acknowledgement
The authors thank the Edward Via College of Osteopathic Medicine for support for this study.
References
- Virginia Department of Health. Centers for Disease Control and Prevention. Preventing Tick-Borne Disease in Virginia. 2014.
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012; 379: 461–473.
- Tick borne Disease of the United States: A Reference Manual for Health Care Providers. 2nd edn. Centers for Disease Control and Prevzention. 2014.
- Tick Borne Diseases of the United States. A Reference Manual for Healthcare Providers. 2nd edn. 2014.
- Varela AS, Luttrell MP, Howerth EW, Moore VA, Davidson WR, Stallknecht DE, et al. First culture isolation of Borrelia lonstari, putative agent of Southern Tick-Associated Rash Illness. J Clin Microbiol. 2004; 42: 1163.
- Symptoms, Diagnosis and Treatment. Centers for Disease Control and Prevention. 2014.
- Murray TS, Shapiro ED. Lyme disease. Clin Lab Med. 2010; 30: 311–328.
- Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease--United States, 1992-2006. Department of Health & Human Services, Centers for Disease Control and Prevention. 2008; 57: 1-9.
- Wright WF, Riedel DJ, Talwani R, Gilliam BL. Diagnosis and management of Lyme disease. Am fam Physician. 2012; 85: 1086-1093.
- Garro A, Marcellin L. First Consult: Lyme Disease. Clinical Key. Elsevier. 2013.
- Nau R, Christen HJ, Eiffert H. Lyme Disease – Current state of knowledge. Dtsch Arztebl Int. 2009; 106: 72-82.
- Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J Infect Dis. 1996; 173: 403-409.
- Palmieri JR, King S, Case M, Santo A. Lyme disease: case report of persistent Lyme disease from Pulaski County, Virginia. Int Med Case Rep J. 2013; 6: 99-105.
- Meek JI, Roberts CL, Smith EV, Cartter ML. Underreporting of Lyme disease by Connecticut physicians. J Public Health Manag Pract. 1996; 2:61-65.
- Meyerhoff JO. Lyme disease. 2013.
- Centers for Disease Control and Prevention (CDC). Summary of notifiable diseases – United States, 2010. Morb Mortal Wkly Rep. 2012; 59: 1–111.
- Anderson JF, Mangarelli LA. Avian and mammalian host for spirochete-infected ticks and insects in a Lyme disease focus in Connecticut. The Yale Journal of Biology and Medicine. 1984; 57: 627-641.
- Telford SR, Mather TN, Moore SI, Wilson ML, Spielman A. Incompetence of deer as reservoirs of the Lyme disease spirochete. The American Journal of Tropical Medicine and Hygiene. 1988, 39: 105-109.
- Bosler EM, Ormiston BG, Coleman JL, Hanrah JP, Benach JL. Prevalence of the Lyme disease spirochete in populations of white-tailed deer and white-footed mice. The Yale Journal of Biology of Medicine. 1984; 57: 651-659.
- Shelus V. Tick-borne Disease Risk along the Appalachian Trail. 2012.
- LoGiudice K, Osteld RS, Schmidt KA, Keesing F. The ecology of infectious disease: Effects of host diversity and community composition of Lyme disease risk. PNAS. 2003; 100: 567-571.
- Anderson AF, Magnarelli LA. Avian and mammalian hosts for spirochete-infected tick and insects in a Lyme disease focus in Connecticut. The Yale Journal of Biology and Medicine. 1984; 57: 627-641.
- Bockenstedt L. Lyme disease. Firestein G, Budd R, Harris E Jr, McInnes I, Ruddy S, Sergent J, editors. In: Kelley’s Textbook of Rheumatology. St. Louis, Missouri, USA: W.B. Saunders. 2009; 1715–1725.
- Hamilton R J. Tarascon Pocket Pharmacopoeia. 14th edn. Philadelphia, PA: Jones & Bartlet Learning. 2013.
- Comparison Chart of Lyme Disease and Co-infections Symptoms. 2010.
- Gerber M, Shapiro ED, Burke G, Parcells VJ, Bell GL. Lyme disease in children in southeastern Connecticut. New England J Med. 1996; 335: 1270-1274.
- Hanincova K, Mukherjee P, Ogden NH, MagosG, Wormser GP, Reed KD, et al. Multilocus sequence typing of Borrelia burgdorferi suggest existence of lineages with differential pathogenic properties in humans. PLoS One. 2013; 8: e73066.
- Wilske B. Diagnosis of Lyme Borreliosis in Europe. Vector-Borne and Zoonotic Diseases. 2003; 3: 215-227.
- Schutzer SE, Coyle PK, Dunn JJ, Luft BJ, Brunner M. Early and specific antibody response to OspA in Lyme disease. J Clin Invest. 1994; 94: 454-457.
- Fried MD, Schairer J, Madigan G. Bartonella henselae is associated with heartburn, abdominal pain, skin rash, mesenteric adenitis, gastritis and duodenitis. J Pediatr Gastroenterol Nutr. 2002; 35: 3.
- Fried MD, Adelson ME, Mordechai E. Simultaneous gastrointestinal infections in children and adolescents. J Practical Gastroenterology. 2004; 78-81.
- Fried, Martin D, Abel M, Pietruccha D, Bal A. The spectrum of gastrointestinal manifestations in children and adolescents with Lyme disease. J Spirochetal Tick-borne Dis. 1999; 29: 375-528.
- Daffner KR, Saver JL, Biber MP. Lyme polyradiculoneuropathy presenting as increasing abdominal girth. Neurology. 1990; 40: 373-375.
- Wormser GP. Early Lyme Disease. N Engl J Med. 2009; 354: 2794-2801.
- Santino I, Comite P, Gandolfo GM. Borrelia burgdorferi, a great chameleon: know it to recognize it . Neurol Sci. 2020; 31: 193-196.
- Huppertz HI, Karch H, Suschke HJ, DöRing E, Ganser G, Thon A, et al. Lyme arthritis in European children and adolescents. Arthritis & Rheumatism, 1995; 38: 361-368.
- Seltzer EG, Gerber MA, Cartter ML, Freudigman K, Shapiro ED. Long-term outcomes of persons with Lyme disease. JAMA. 2000; 283: 609-616.
- Cameron DJ. Proof that chronic Lyme disease exists. Interdisciplinary Perspectives on Inf Dis. 2010; 1-4.
- Masters EJ, Grigery CN, Masters RW. STARI or Masters Disease: Lone star tick-vectored lyme-like illness. Infec Dis Clin N Am. 2008; 22: 361-376.
- Zajkowska J, Moniuszko AM, Czupryna P, Pancewicz SA, Grygorczuk S, Kodrusik M. STARI-a new tick borne spirochetosis. Przegl Epidemiol. 2009; 63: 19-22.
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System (NNDSS). Lyme disease (Borrelia burgdorferi). 2014.
- Centers for Disease Control and Prevention. Lyme disease transmission. 2013.
- Wilske B, Fingerle V, Schulte-Spechtel U. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunology & Medical Microbiology. 2007; 49: 13-21.
- Centers for Disease Control and Prevention. Understanding the EIA Testing. 2011.
- Centers for Disease Control and Prevention. Understanding the Immunoblot Test. 2011.
- Virginia Lyme Disease Fact Sheet. 2014.
- Centers for Disease Control and Prevention. Lyme Disease, Reported cases of Lyme disease by state or locality, 2003-2012. 2014.
- Huppertz HI, Bartmann P, Heininger U, Fingerle V, Kinet M, Klein R, et al. Rational diagnostic strategies for Lyme borreliosis in children and adolescents: recommendations by the Committee for Infectious Diseases and Vaccinations of the German Academy for Pediatrics and Adolescent Health . European J Ped. 2012; 171: 1619-1624.
- Rose CD, Fawcett PT, Gibney KM, Doughty RA. The over diagnosis of Lyme disease in children residing in an endemic area. Clin Peadiatr (Phila). 1994; 33: 663-668.
- Shapiro ED. Lyme disease. Finnland, Pollard AJ, editors. In: Hot Topics in Infection and Immunity in Children IV. Heidelberg: Springer. 2008: 185–195.
- Centers for Disease Control and Prevention. Final 2010 Reports of Nationally Notifiable Infectious Diseases. Morbidity and mortality weekly report. 2011; 60: 1088-1101.
- Lyme Disease Case Maps. Lyme Disease Association. 2014.
- Centers for Disease Control and Prevention. Surveillance for Lyme Disease --- United States, 1992—2006. Surveillance Summaries. Morbidity and Mortality Weekly Report. 2008; 58: 1-9.
- Clark KL, Leydet B, Hartman S. Lyme borreliosis in human patients in Florida and Georgia, USA. Int J Med Sci. 2013; 10: 915-931.
- Pediatrics Consultant Live. Southern Tick –Associated Rash Illness. Pediatric Skin Diseases. 2009.
- Magnarelli LA, Anderson JF, Johnson RC. Cross-reactivity in serological test of Lyme disease and other spirochetal infections . J Infect Dis. 1987; 156: 183-188.
- Schmidt BL, Aberer E, Stockenhuber C, Klade H, Breier F, Luger A. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in the urine and breast milk of patients with Lyme borreliosis. Diag Micro Inf Dis. 1995; 21: 121-128.
- O’Flanagan D, Couturier E, Doherty L, Fenton K. Evidence of increased transmission of syphilis among homosexual men and heterosexual men and women in Europe. Eurosurveillance. 2000; 4: 1472.
- Bharti AR, Nailly JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, et al. Leptospirosis: a zoonotic disease of global importance. The Lancet Infectious Disease. 2003; 3: 757-771.
- Huppertz HI, Bartmann P, Heininger U, Fingerle V, Kinet M, Klein R, et al. Rational diagnostic strategies for Lyme borreliosis in children and adolescents: recommendations by the Committee for Infectious Disease and Vaccinations of the German Academy for Pediatrics and Adolescent Health. Eur J Pediatr. 2012; 171: 1619-1624.
- Ghayad Z, Hou C. Erythema migrans in early disseminated Lyme disease. JAOA. 2012; 112: 748.
- Huppertz HI, Bartmann P, Heininger U, Fingerle V, Kinet M, Klein R, et al. Rational diagnostic strategies for Lyme borreliosis in children and adolescents: recommendations by the Committee for Infectious Diseases and Vaccinations of the German Academy for Pediatrics and Adolescent Health. European journal of pediatrics, 2012; 171: 1619-1624.