
Research Article
Austin J Microbiol. 2021; 7(1): 1036.
Antibacterial Effect of Traditional Nepalese Drinking Water Copper Pot Against Clinically Isolated Multi Drug Resistant Escherichia coli
Soma Kanta B1#*, Nikita K1#, Indira P2 and Prem P3
1Manmohan Memorial Institute of Health Sciences, Kathmandu, Nepal
2Nepal Academy of Science and Technology (NAST), Kathmandu, Nepal
3Water/Waste Water Quality Assurance Division, Kathmandu, Nepal
#Contributed Equally to this Paper
*Corresponding author: Baral Soma Kanta, Manmohan Memorial Institute of Health Sciences, Kathmandu, Nepal
Received: February 18, 2022; Accepted: March 11, 2022; Published: March 18, 2022
Abstract
Microbiological contamination of drinking water is still a major issue in developing and under developing countries. Until it was safe to drink, water was typically stored in a variety of metal pots. Copper was discovered to be far more effective than the other metals. Ayurveda also recommends copper pots for drinking water. Therefore, the goal of this research is to see how copper affects numerous multi drug-resistant Escherichia coli clinical isolates. A total of 40 multi drug-resistant Escherichia coli were identified from various clinical specimens. For different periods of time, deionized water was put on a household copper pot having a capacity of 2 liters and a surface area of 860cm2 (2, 6, 12, 18, and 24 hours). Atomic absorption spectroscopy was used to measure the amount of copper leached. Luria Bertani broth was prepared using water stored in copper pot at different time interval. Diluted bacterial suspension was spreaded on Mac-Conkey agar plate surface for viable count. Copper leached from 24hour storage water was found to successfully suppress bacterial growth within the safety limit, followed by shorter time intervals. To combat multidrug-resistant Escherichia coli, this study recommends drinking water from a copper pot.
Keywords: Multi drug resistant; Atomic absorption spectroscopy; Viable count; Copper leached water
Introduction
In excess of 80% of all types of diseases are caused by impure water, according to World Health Organization [1]. Genes conferring resistance to various antibiotics have been identified in a wide range of water sources, including drinking water in developed and developing countries [2,3]. Escherichia coli are the most common commensal bacterium in the gastrointestinal tracts of animals and humans. Escherichia coli are also a member of feacal coliforms that contaminate drinking water due to human and animal feaces [4].
Heavy metals possess the ability in low concentration to exert lethal effects on bacterial, also known as antimicrobial action. Among various metals, silver, brass and aluminum have a lethal effect on bacteria, whereas copper has a comparatively greater effect [5]. The copper surface should kill bacteria by causing rapid membrane damage and DNA degradation which ultimately results in cell death [6]. The toxicity of copper ion is described by Fenton chemistry (reaction 1) combined with the Haber-Weiss cycle (reaction 2 and 3). These reactions result in the production of Reactive Oxygen species (ROS) under the aerobic condition which can inhibit the respiratory chain and can lead to irreversible damage to cellular components [7-9].
Cu_ + H2O2 → Cu2+ + OH_ (1)
H202 + OH- → H20 + O2 + H+ (2)
H2O2 + O2 → O2 + OH + OH (3)
Materials and Methods
Copper pot was purchased from kitchenware shop in Kathmandu, Nepal having capacity of 2L with surface area of 860cm2 and was spotless each time before use as in traditional Nepalese home practices. Deionized water was then stored in copper pot for 2, 6, 12, 18, 24 hours respectively.
Estimation of pH and copper content
Deionized water’s pH and copper concentration were tested before and after it was placed in a copper pot. Flame Atomic Absorption Spectroscopy was used to assess the concentration of copper leached in water at different time intervals (2-hour, 6-hour, 12-hour, 18-hour and 24-hour) in the department of food technology and quality control in Kathmandu, Nepal. Similarly, the pH of water placed in a copper pot was tested using a pH meter at different time intervals (2-hour, 6-hour, 12-hour, 18-hour and 24-hour).
Preparation of LB broth
Luria Bertani broth was prepared using water stored in copper pot at different time interval (2-hour, 6-hour, 12-hour, 18-hour and 24-hour) separately. 10ml of LB broth prepared from water stored in copper pot at different time interval was aliquoted in each tube separately.
Bacterial strain
Escherichia coli isolated from various clinical specimen including urine, sputum, pus, blood from Department of clinical Microbiology, Manmohan Memorial Teaching Hospital, Kathmandu, Nepal. Antimicrobial susceptibility test was performed on Mueller Hinton Agar using Kirby Bauer disk diffusion technique as recommended by Clinical and Laboratory Standard Institute guideline-2015. Zone measurement was done in millimeters. The result was interpreted as sensitive and resistant based on zone size interpretative chart and differentiated as MDR E. coli. Experiment using E. coli was performed in Microbiology laboratory of Manmohan Memorial Institute of Health Sciences.
Inoculum preparation
3-5 well isolated colonies were taken from the culture plate and passed in tube containing 5ml of nutrient broth. Broth was incubated for 4-6 hours at 37°C to bring in log phase. The turbidity was adjusted to 0.5 McFarland standards by comparing in a file with black lines.
Inoculum transfer to LB broth
0.1ml of bacterial suspension equivalent to 0.5 McFarland standards was transferred to each LB broth prepared from 2-hour, 6-hour, 12-hour, 18-hour and 24-hour copper pot stored water. Bacterial suspension was incubated overnight at 37°C.
Viable count by spread plate method
1ml of bacterial suspension after overnight incubation was diluted in 9 ml of nutrient broth and was continued to 10-8.1 ml of each diluted series was transferred to two Mac-Conkey agar plates. Each plate was incubated at optimum temperature and plate with 25- 250 colonies was choose and counted while rest was discarded. The colony counter was used to determine the viable count and colony count was determined in CFU/ml by using formula:
CFU/ml = no of colonies/dilution factor*volume of sample
Ethical consideration
Ethical approval was taken from Institutional Review Committee (Ref. No. 77/35/MMIHS/2077) of Manmohan Memorial Institute of Health Sciences (MMIHS), Kathmandu. Informed written consent was taken from every participant after explaining the objective of the study.
Statistical analysis
All results were entered in the database and analysis was done by using Statistical Package for Social Science (SPSS) version 20.0 IBM Statistics and Microsoft Excel. Statistical analysis of the data was performed by Shapiro-Wilk normality test to determine normal or asymmetric data distribution. Wilcoxon signed-rank test to compare mean viable count of bacteria in normal water and different time interval copper leach water. Log reduction calculated using Microsoft Excel.
Results
Over the course of three months, a laboratory-based cross sectional investigation was conducted on a total of 40 clinical isolates of MDR Escherichia coli from patients visiting a tertiary care hospital in Kathmandu, Nepal (November 2020-Janaury 2021). Various concentrations of copper were leached from a copper pot over a period of time (2 hours, 6 hours, 12 hours, 18 hours, and 24 hours) and quantified using atomic absorption (Figure 1). A pH meter was also used to measure the pH of deionized water (Figure 2). LB broth was made from water that had been held in a copper pot for various periods of time. The mean viable count of MDR E. coli isolated from various clinical samples (urine, pus, sputum, blood) was compared using normal water as a reference and water held in a copper pot for various time intervals (2hrs, 6hrs, 12hrs, 18hrs and 24hrs) as a test. Viable count was greatly reduced as concentration of copper leach increases i.e. with increasing holding time which was statistically highly significant (Table 1). The appropriate holding time for drinking water in domestic copper pot against clinically isolated MDR E. coli were inhibited with maximum holding time of 24- hour. Survival of test organisms in different copper eluted water was expressed as Difference between mean starting inoculum and mean viable count (Δ log10 cfu/ml). Log reduction increases along with increase concentration of copper i.e. as holding time increases (Table 2). Copper pot showed 99.999% bacterial reduction in MDR E. coli isolated from pus, sputum, blood whereas 99.99% bacterial reduction from urine with 24-hour holding time.
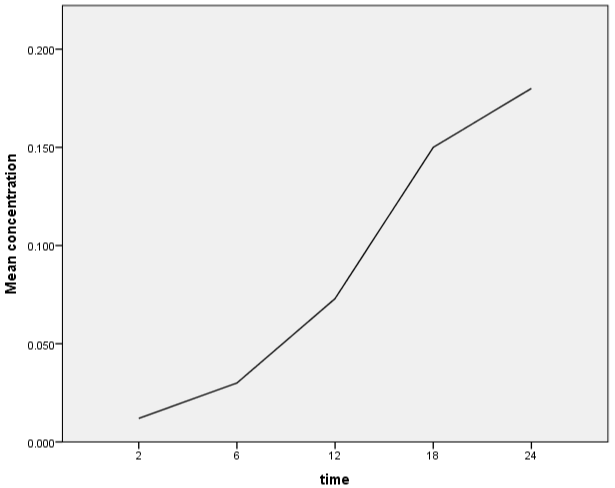
Figure 1: Elution of copper ions as a function of leaching time.
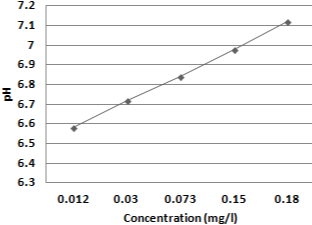
Figure 2: pH changed at different concentration of copper.
Clinical samples
Number of MDR E. coli isolates
Mean Viable count
P value
Normal water
Copper leached water
2 hour
6 hour
12 hour
18 hour
24 hour
Urine
15
2.87E+11
3.97E+10
3.94E+09
4.81E+08
4.06E+07
3.93E+06
<0.001
Pus
10
5.86E+11
5.50E+10
3.80E+09
3.64E+08
2.53E+07
5.28E+06
<0.001
Sputum
8
8.00E+11
8.77E+10
1.46E+09
2.60E+08
2.81E+07
3.59E+06
<0.001
Blood
7
8.07E+11
6.24E+10
2.02E+09
1.74E+08
2.64E+07
2.72E+06
<0.001
P-value was calculated using Wilcoxon signed rank test.
Table 1: Comparison of Mean viable count of MDR E. coli on normal water and copper leached water at different time intervals.
Clinical Samples
Time
Δ log10 cfu/ml of E. coli isolates
2 hours
6 hours
12 hours
18 Hours
24 hours
Urine
-0.85
-1.86
-2.78
-3.85
-4.86
Pus
-1.03
-2.19
-3.21
-4.37
-5.05
Sputum
-0.96
-2.74
-3.49
-4.45
-5.35
Blood
-1.11
-2.6
-3.67
-4.48
-5.47
A negative sign represents a reduction of inoculums than at time 0.
Table 2: Difference between mean starting inoculum and mean viable cell count (Δ log10 cfu/ml) of E. coli isolates at different time intervals.
Discussion
Copper’s antibacterial action on bacteria, viruses, and fungi has been established in several lab investigations as contact killing action of metallic copper [10]. Copper pot was found to be more effective than silver pot and brass pot in a study by Shrestha et al., 2010 [11]. Preethi Sudha et al. discovered that the mean copper content and pH of an overnight water kept copper pot were 426.83 +/_ 33.64 ppb and 7.16+/-, respectively, compared to zero ppb copper concentration and pH 6.80 initially [12]. Similarly, in the study conducted by Sheeba Ganesan, concentration of copper leached from copper pot was 177 +/_ 16 ppb and also pH was slightly increased from 7.83 +/_ 0.4 to 7.93 +/_ 0.3 after incubation for 16 hours [12]. However in our study, concentration of copper leached at different interval of time i.e. 2 hours, 6 hours, 12 hours, 18 hours, 24 hours were 0.012mg/l, 0.030mg/l, 0.073mg/l, 0.15mg/l and 0.18mg/l respectively which was within safety limit of WHO which was initially zero as deionized water was used. Similarly, pH was slightly increasing in our study as time duration increased i.e. 6.58, 6.72, 6.84, 6.98, and 7.12 after 2 hours, 6 hours, 12 hours, 18 hours, 24 hours’ time interval respectively which was initially pH 6.50.
In the study by Rajani Shrestha et al., the load of E. coli was completely reduced with 4 hours of holding time in copper pot. However, load of MDR E. coli isolated from water was lately inhibited as this was achieved only after 48 hours of holding time with in copper pot [11].
In the study by Matthew Domek et al., copper concentration of 0.05mg/L, showed greater than 90% and >99.5% injury of water isolated E. coli within 2 days and 5 days respectively [13]. As per study conducted by Cristina Molteni et al., 55μM copper leached in water caused complete killing of wild type in 6 hours [14]. In the study conducted by Maria Souli et al., second and third generation resistant E. coli isolates showed reduction of initial inoculum by 2 log10 cfu/cm² at 3 hours of incubation in Cu 99% copper coupon and bactericidal effect at 6 hours [15]. As per the study conducted by G. Steindal et al., MDR E. coli showed three-fold log reduction of viable after 30 minute of exposure in copper coupon and five-fold log reduction within 60min of exposure of CTX-M-15 producing E. coli in copper coupon [16]. Bacterial isolates were shown to be reduced by one-fold in 2-hour copper leached water, two-fold in 6-hour copper leached water, three-fold in 12-hour copper leached water, four-fold in 18-hour copper leached water, and five-fold in 24-hour copper leached water in our investigation.
Finally, as the holding period and copper content were increased, bacterial viability was significantly reduced. The 24-hour holding period was found to be the most acceptable and safe for preserving water. Copper pots for drinking water storage are advised for controlling MDR E. coli.
Acknowledgments
We would like to express our gratitude to all of the patients who took part in this research. We would like to express our gratitude to everyone of the laboratory workers, administration, and administrators at Manmohan Memorial Teaching Hospital Kathmandu for allowing us to conduct this research.
References
- Ellis H, Schoenberger E. On the Identification of Associations between Five World Health Organization Water, Sanitation and Hygiene Phenotypes and Six Predictors in Low and Middle-Income Countries. PloS one. 2017; 12: e0170451.
- Marathe NP, Pal C, Gaikwad SS, Jonsson V, Kristiansson E, Larsson DGJ, et al. Untreated urban waste contaminates Indian River sediments with resistance genes to last resort antibiotics. Water Research. 2017; 124: 388- 397.
- Mezrioui N, Baleux B. Resistance patterns of E. coli strains isolated from domestic sewage before and after treatment in both aerobic lagoon and activated sludge. Water Research. 1994; 28: 2399-2406.
- Odonkor ST, Addo KK. Prevalence of Multidrug-Resistant Escherichia coli Isolated from Drinking Water Sources. International Journal of Microbiology. 2018; 2018: 7204013.
- Shrestha R, Joshi DR, Gopali J, Piya S, et al. Oligodynamic action of silver, copper and brass on enteric bacteria isolated from water of Kathmandu Valley. Nepal Journal of Science and Technology. 2009; 10: 189-193.
- Vincent M, Duval RE, Hartemann P, Engels-Deutsch M, et al. Contact killing and antimicrobial properties of copper. Journal of Applied Microbiology. 2018; 124: 1032-1046.
- Liochev SI, Fridovich I. The Haber-Weiss cycle-70 years later: an alternative view. Redox Report. 2002; 7: 55-57.
- Warnes SL, Caves V, Keevil CW, et al. Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environmental Microbiology. 2012; 14: 1730-43.
- Hong R, Kang TY, Michels CA, Gadura N, et al. Membrane lipid peroxidation in copper alloy-mediated contact killing of Escherichia coli. Appl Environ Microbiol. 2012; 78: 1776-1784.
- Grass G, Rensing C, Solioz M, et al. Metallic copper as an antimicrobial surface. Appl Environ Microbiol. 2011; 77: 1541-1547.
- Shrestha R, Joshi DR, Gopali J, Piya S, et al. Oligodynamic Action of Silver, Copper and Brass on Enteric Bacteria Isolated from Water of Kathmandu Valley. Nepal Journal of Science and Technology. 2010; 10: 189-193.
- Sudha VB, Singh KO, Prasad SR, Venkatasubramanian P, at al. Killing of enteric bacteria in drinking water by a copper device for use in the home: laboratory evidence. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009; 103: 819-822.
- Domek MJ, LeChevallier MW, Cameron SC, McFETERS GA, et al. Evidence for the role of copper in the injury process of coliform bacteria in drinking water. Appl Environ Microbiol. 1984; 48: 289-293.
- Molteni C, Abicht HK, Solioz M, et al. Killing of bacteria by copper surfaces involves dissolved copper. Appl Environ Microbiol. 2010; 76: 4099-4101.
- Souli M, Galani I, Plachouras D, Panagea T, Armaganidis A, Petrikkos G, et al. Antimicrobial activity of copper surfaces against carbapenemase-producing contemporary Gram-negative clinical isolates. Journal of Antimicrobial Chemotherapy. 2013; 68: 852-857.
- Steindl G, Heuberger S, Springer B, et al. Antimicrobial effect of copper on multidrug-resistant bacteria. Wiener Tierarztliche Monatsschrift. 2012; 99: 38-43.