
Research Article
Austin J Microbiol. 2023; 8(1): 1041.
Screening of Trichoderma Isolates and Potential of Their Organic Extract to Control Phytophthora megakarya, the Causative Agent of Cocoa Black Pod Disease
Youassi YO; Ambatta AT; Dikongue NF; Davy MV; Ntah Ayong M; Tchameni NS*; Sameza ML
Faculty of Sciences, Department of Biochemistry, University of Douala, Cameroon
*Corresponding author: Séverin Nguemezi Tchameni Faculty of Sciences, Department of Biochemistry, University of Douala, Cameroon, PO BOX 24-157, Douala-Cameroon. Email: tchameni1@yahoo.fr
Received: April 04, 2023 Accepted: May 17, 2023 Published: May 24, 2023
Abstract
The aim of this work was to isolate Trichoderma agents of cocoa trees and to select the best antagonists able to reduce cocoa black pod disease caused by Phytophthora megakarya. Sampling was carried out in the Western region of Cameroon, locality of Bafang. Trichoderma and P. megakarya isolates were made on PDA medium in a Petri dish. In vitro antagonist activity of Trichoderma was evaluated by dual culture and antibiosis tests, and the best isolates were selected. The production of hydrolytic enzymes by the best isolates was done in specific solid media. The antimicrobial effect of the crude organic extract extracted from the selected Trichoderma isolates was performed in vitro by poisoned method. Results showed that 12 Trichoderma spp were isolated. Based on the rating scale of Bell, 4 isolates were selected, namely IS4, IS7, IS9 and IS11. These isolates were found to produce non-volatile and volatiles compounds as well as hydrolytic enzymes (cellulase, amylase, lipase and protease); Their organic crude extract significantly inhibited the growth of mycelial of P. megakarya with IC7 as the best isolates. IS7 Trichoderma isolate significantly produced cellulase, amylase, lipase and protease and at 100μg/ml, its organic crude extract total inhibited the mycelial growth of P. megakarya. It could be suggested that, Trichoderma IS7 isolate should be further investigated as promising biocontrol agent to manage P. megakarya infection.
Keywords: Phytophthora megakarya; Cocoa black pod disease; Trichoderma isolates; Hydrolytic enzymes; Organic extract
Introduction
Cocoa black pod, caused by the Oomycete Phytophthora megakarya is the most devasting disease of cocoa crops in the sub-tropical countries. Over the last 20 years, losses in cocoa yield in Cameroon were more than 50%, resulting in severe economies losses [1]. The severity of cocoa pod disease increases during the rainy season, as zoospores of P. megakarya are splashed and dispersed onto fresh cocoa, resulting in secondary infection [2]. To control the disease, farmers commonly use copper-based chemical pesticides. However, the repeated and hazard use of these chemicals has proven costly and their regular application has becomes ineffective [3]. In addition, systemic pesticides are not routinely used in cacao farming, due to high costs and risks of contaminating cacao beans, farmers and environment [4]. In areas of organic certified production of cocoa with restrictions to use of chemicals, biological control appears to be one of the few viable approaches [4]. Biological control by using antagonist microorganisms is an alternative to safely reduce the disease and the level of the use of synthetic pesticides [5]. The cocoa rhizosphere and phyllophere contains a varied set of microorganisms living as saprophytes, parasites or in symbiosis. These are bacteria and fungi that colonize different parts of the cocoa tree like pods and leaves [6]. Among them, Trichoderma is one of the most study filamentous endophyte fungi. Trichoderma spp have been widely studied and have been characterized for their high competitiveness for space and nutrients [11]. Trichoderma species are used effectively as antagonistic agents to control several pathogens in many important crops [10,12]. Trichoderma spp is considered to be an important reservoir of new bioactive secondary metabolites [7]. Also, these natural substances produced by Trichoderma spp which have various properties such as antibiotic, antifungal and antiviral activities [8,9]. Indeed, biological products are elicitors which are capable of stimulating the defense mechanisms of the plants, but also of producing the effector molecules during the attack of the pods by pathogens [10-12]. Most strains of Trichoderma are more competent at effectively controlling certain plant pathogens, while others may be largely ineffective [16]. Therefore, screening of biocontrol strains is essential for their further development and commercialization.
The present study was carried out to screen and to evaluate potential isolates of Trichoderma spp for biocontrol of P. megakarya, causing cocoa black pod.
Materials and Methods
Plant Pathogen
Phytophthora megakara was isolated from cocoa pods showing the typical symptoms of black pod disease. They were washed in distilled water and disinfected with 1% NaOCl (sodium hypochlorite). The pods were cut (3cm) and incubated on PDA medium (supplemented with penicillin and ampicillin) and incubated at 28°C. Dishes were observed daily and suspected pathogenic mycelia were transferred to fresh medium. The pathogen was identified according to the protocol used by Tchameni [13]. The isolated pathogen was maintained in PDA medium at 4°C until use.
Sample Collection and Isolation of Trichoderma spp
Fresh leaves, roots, trunk and pods samples were collected from the cocoa trees farms at Bafang (Western region of Cameroon) in September 2019. The samples were packed into sterilized polyethylene bags and transferred to the Biochemistry Laboratory of the University of Douala. The isolation of Trichoderma was done in a Potato Dextrose Agar (PDA) medium supplemented with 150 mg/l of penicillin and ampicillin according to the protocol described by Pimentel et al. [14]
Healthy leaves and pods were washed 3 times with sterilized distilled water, dried thoroughly between sterilized fi lter paper, and cut into 1cm 2-segments. Five segments of each sample were placed on the surface of PDA medium in each plate. Three replicates were used for each sample, and then, the plates were incubated at 25°C. Emerging mycelia of Trichoderma colonies were replicated after 48h of incubation into a new PDA medium and purifi ed by successive transfers. Each purified isolate was maintained in PDA medium and stored at 4°C.
Screening for Antagonistic Activity of Trichoderma Isolates
Antagonistic activity was performed by dual culture, according to the protocol used by Tchameni et al [15]. Five millimeters plug of each Trichoderma isolate (2 days old) was inoculated on one side of Petri dishes containing PDA medium. A 3cm mycelial plug from a 3-day-old PDA culture of P. megakarya was inoculated from the opposite side. The control was carried out by plates inoculated with the pathogen agent. All plates were incubated at 28°C for 7 days. The inhibition of mycelial growth of the pathogen was recorded according to the formula: I (%) = ((Do-De)/Do))×100 (1), where Do is the growth diameter of the pathogen and De is the growth diameter of the pathogen in paired culture. Each treatment consisted of three repetitions and the experiment was repeated twice. The level of antagonism of each Trichoderma isolate in paired culture was made using the R1 to R5 rating scale as described by Bell et al [27]. R1 is the rate when Trichoderma has completely invaded the pathogen colony and covered the entire PDA plate; R2 the rate when it colonizes at least two-thirds of the surface of the medium. In the case of R3, the pathogen and the antagonist each colonized approximately half of the medium. For R4, the pathogens invaded Trichoderma and covered two-thirds of the culture medium and concerning R5, the pathogen colonized the entire plate.
Evaluation of the Effect of Volatile and Non-Volatile Compounds
The effect of volatile compounds produced by selected Trichoderma isolates was done according to the method used by Bedine et al [16]. Five millimeters of 3-old days of mycelial disc of Trichoderma isolate was incubated on the centre of PDA plate (9cm). The lid of the Petri dish was replaced with dish centrally inoculated with P. megakarya. Paraffin paper was used to seal the two plates. In the control plates, the antagonist was replaced by an agar disc. All the plates were incubated at 28°C for 7 days.
In the case of non-volatile compounds, each Trichoderma isolate was grown for 48h on a sterile cellophane disc laying on PDA in 9cm Petri dish. In the control, the antagonist was replaced by an agar disc. The cellophane with the mycelia was then removed and the test pathogen was inoculated for an incubation period of 7 days at 28°C [16].
For volatile and non-volatile assays, each treatment consisted of 3 replicates, and the experiments were repeated twice. At the end of incubation, the colony diameter of P. megakarya was measured and the inhibition of mycelial growth (% IMG) was evaluated using the previous formula (1).
Evaluation of Extracellular Enzyme Production
Hydrolytic activities of cellulase, amylase, lipase and protease were detected on specifi c solid media. Trichoderma isolate was grown on a medium containing the enzyme substrate and the zone of degraded substrate (halos) formed around the colony was measured after 3 days of incubation. Cellulase activity was done according to the method described by Bedine et al [16]. The detection medium was prepared using (g/l): NaCl (0.5), H2PO4 (1), MgSO4, 7 H2O (0.5), MnSO4, H2O (0.01), NH4NO3, (0.3), FeSO4, 7H2O (0.01), carboxymethylcellulose (10), agar (15), all adjusted to pH 7.0 and autoclaved at 121°C for 15 min. Cellulases was revealed by treating the cultures with a Congo Red solution (0.1%) for 10-15 min, then with a 1M NaCl solution for 10 minutes. The formation of a clear halo around the fungal colonies indicated hydrolysis of carboxymethylcellulose.
Amylase activity was determined by using a plate screening medium (glucose yeast extract peptone) containing 2% of soluble starch [17]. The inoculated plates were incubated at 28°C and the formation of halo around the fungal colonies indicated the digestion of starch.
The detection of lipases was evaluated using a chromogenic medium supplemented with phenol red. The chomogenic medium contained: 0.01% phenol red, 1% lipid substrate (Tween 80), 10mM CaCl2, 2% agar, the whole was sterilized and adjusted to pH 7.4 with 0.1N NaOH. After solidification of the culture medium in the Petri dishes, a mycelial pellet of the antagonist was inoculated and the whole incubated at 28°C for 3 days. After incubation, the formation of a clear halo around the colonies indicates lipase activity.
The detection of proteases was evaluated by the method used by Bedine et al [17]. Indeed, the specific protease medium was prepared from (g/L): K2HPO4 (2), 10g of glucose, 5g of peptone, 15g of gelatin.
Liquid Culture and Extraction of Organic Crude Extract
Five agar plugs from 2 days preculture of Trichoderma isolate were introduced into 1L conical flask containing 200ml of Potatoes Dextrose Broth (PDB). The flask was incubated at 25°C in dark under stationary condition for 30 days. Cultures were then filtered on vacuum using Whatman paper N°4. The filtrate extracted twice with ethyl acetate and evaporated under reduced pressure at 40°C. The crudes residues obtained were stored at 4°C until used
In Vitro Antimicrobial Activities of Crude Extract
Phytophthora megakarya plugs from 3-days preculture was placed at the centre of V8 agar plate. The antimicrobial activities were done according to the method describe by Vinale et al. (2006). The crude organic extract was diluted in Dimethyl Sulfoxide (DMSO) at the concentration ranging from 25 to 100μg/ml/l/ and 10μl of each concentration were applied on the top of each plug. The control was made by applying the same volume of DMSO on the pathogen plug. Plates were incubated at 25°C for 5 days and the pathogen growth inhibition calculated according to the formula: %IPG = (Do-Dx)/Do)x100 were Do = pathogen growth diameter on control and Dx = pathogen growth diameter on treated plate. Each treatment consisted of three replicates and the experiment was repeated two times.
Statistical Analyzes
The data were analyzed with SPSS software version 18.0 for Windows (SPSS, Chicago, IL, USA). Quantitative variables were presented as the mean ± Standard Deviation (SD). One-way ordered analysis of variance (ANOVA) was used to compare mean values between multiple groups (n≥2). Subsequently, Fisher's Pair Least Significant Difference (PLSD) post hoc test was used to make pairwise comparisons if the ANOVA result was significant. Homogeneity of variance test was previously used at ANOVA to compare the variance between groups. Thus, ANOVA was used if this test showed equality of variances (null hypothesis retained). The significance level was set at p-value<0.05. The person correlation was done to evaluate the correlation between des parameters.
Results
In Vitro Confrontation Profile of Biocontrol Agents Against P. Megakara
From Fresh leaves, roots, trunk and pods collected in cocoa trees, 12 isolates of Trichoderma spp were isolated. It was 4 isolates from trunks (IS2, IS3, IS4 and IS5); 3 isolates from roots (IS1, IS9 and IS10) and 5 isolates from pods (IS6, IS7, IS8, IS11 and IS12). All these fungi significantly (p≤0.5) inhibited the mycelial growth of P. megakarya. The inhibition rate varied from 50% to 68%, depending of Trichoderma isolates. The highest-level rate of antagonist (R1) was recorded at the proportion of 33.3% (Figure 1). The isolates IS4, IS7, IS9 and IS11 which exhibited this R1 class antagonism against the pathogen were selected for the further study (Figure 2 & Figure 3).
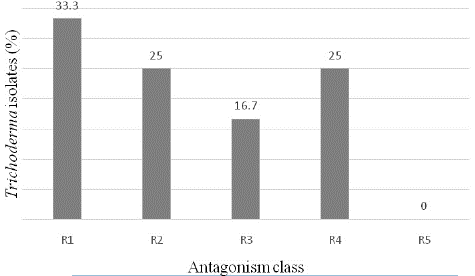
Figure 1: Proportion of different antagonist classes obtained during in-vitro dual culture with P. megakarya.
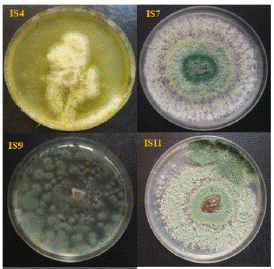
Figure 2: Morphological aspect of the 4 selected Trichoderma isolates on PDA medium.
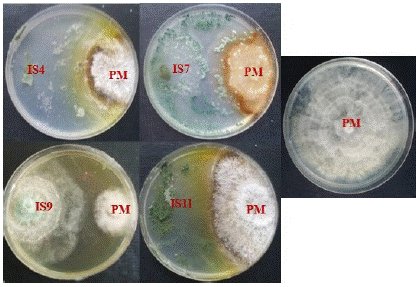
Figure 3: Antagonistic effect of 4 selected Trichoderma isolates on dual culture test.
PM: Phytophthora megakarya
Antibiosis Profi les of the Selected Antagonist Agents
The 4 selected isolates of Trichoderma were tested for the production of non-volatile and volatile compounds. The results showed that, all these isolates produced non-volatile and volatile components with significantly inhibition of the pathogen (Table 1). The inhibition rate varies from 82.3 to 100% and from 55.3 to 60.5% respectively, for non-volatile and volatile compounds, depending on the antagonists’ isolates.
Mycelial Growth Inhibition (%)
Trichoderma isolates
IS4
IS7
IS9
IS11
Non-volatile compounds
100.0±0a
92.4±0.75ab
82.3±0.85b
86.7±091b
Volatile compounds
55.3±0.64ab
57.4±0.80ab
60.5±3.25a
58.2±1.07a
In the same line, means ± Standard Deviations with same letter are not significantly different according to Duncan test at 5%.
Table 1: Inhibitory effect of Trichoderma isolates on P. megakarya through production of non-volatile and volatile metabolites.
Production of Cell Wall Degrading Enzymes
Hydrolytic enzymes activities were characterized by halo zone formation around Trichoderma isolates colony. The zone diameter ranged from 0.0cm to 4.03cm for cellulase, 0.0mm to 7.03cm for amylase, 2.5cm to 8.23cm for lipase and for protease 0.0cm to 7.03cm for protease Table 2. The highest activities of the 4 hydrolytic enzymes production were recorded with IS7 Trichoderma isolates.
Holo-enzyme zone (cm)
Isolates
Cellulases
Amylases
Lipases
Proteases
IS4
0±0.00a
6.03±0.76c
5.23±0.25b
0±0.00a
IS7
4.03±0.15a
7.03±1.75b
8.23±0.25c
7.03±0.15c
IS9
0±0.00 a
0±0.00a
8.03±0.2c
0±0.00a
IS11
0±0.00a
2.23±0.2b
2.5±0.2b
2.23±0.37b
In the same line, means ± Standard Deviations with same letter are not significantly different according to Duncan test at 5%.
Table 2: Holo-enzyme activities exhibited by selected isolates of Trichoderma.
Ability of crude organic extracts from Trichoderma isolates to inhibit the mycelial growth of P. megakarya
All the crude extracts exhibited a significant inhibition (P≤0,05) of mycelial growth against the pathogen compared to the control (Table 3). The total inhibition (100%) of organic extract from IS7 and IS9 Trichoderma isolates was recorded at 100μg/ml. At the same concentration, the inhibition growth was 80.23% and 62.23% respectively, for IS4 and IS11 Trichoderma isolates.
IsoIates
IS4
IS7
IS9
IS11
Extract (μg/ml)
Inhibtion (%)
0
0.00 ± 0.00a
0.00 ± 0.00a
0.00 ± 0.00 a
0.00 ± 0.00a
25
15.03 ± 0.76b
40.00± 0.10b
25.23 ± 0.25b
10.03 ± 0.00b
50
65.10 ± 0.50c
75.05± 0.15c
80.03 ± 0.2c
45.06 ± 0.00c
100
80.23 ± 0.2d
100.00 ± 0.00d
100 ± 0.00d
62.23 ± 0.37d
In the same column, means ± Standard Deviations with same letter are not statistically different according to Duncan test at 5%.
Table 3: Effect of organic crude extract of Trichoderma isolate on the mycelial growth of P. Megakarya
Discussion
In the present work, several isolates of Trichoderma agents were purified from the various samples collected from cocoa farms. The colonization of Trichoderma on different parts of the plant responds to a need both physiological and immune, showing their capacity to respond effectively by a systemic mechanism to stress or to cryptogamic attacks to which it is exposed. Previous study by Hanada et al [10] shows the diversity of endophytes isolated from cocoa pods as Trichoderma sp. These results confirmed the persistence of Trichoderma in various saprophytic soils and, the cosmopolitan character of this fungus colonizing a wide range of habitats and ecological niches as elucidated by Ntah et al [18].
The strong antagonist activity observed in antimicrobial tests could be due to the ability of the Trichoderma to react through several mechanisms such as antibiosis by direct confrontation, production of non-volatile and volatile substances. The direct confrontational antibiotic test was found to be very effective against the growth of P. megakarya. The significant inhibitory effects were observed with isolates of Trichoderma sp. is thought to be due to the rapid growth rate of Trichoderma allowing the competition for space and nutrients [11].
In this study, the results showed the production of inhibitory compounds and enzymes as well as direct coils of Trichoderma isolates around the pathogen. Similar findings were reported by Khaledi and Taheri [19] who studied the antagonism of T. harzianum against M. phaseolina and suggested the production of volatile compounds as one of the main mechanisms. Similarly, Cherkupally et al [20], Tchameni et al [21] also reported the involvement of volatile and non-volatile compounds produced by Trichoderma spp against plant pathogens. Cell wall degrading enzymes are known to be responsible for the destruction of the cellular integrity of pathogens by Trichoderma [22]. However, according to Tahía et al [23], biocontrol results from the combination of diff erent mechanisms which include physical contact, competition for nutrients and space, production of lytic enzymes and antibiotics. These mechanisms are used sequentially or/and simultaneously [8]. Despite the fact that it is very diffi cult to predict the behavior in natural environments of a given strain with biological control activity in the laboratory, in vitro tests are useful for determining antagonistic profiles and selecting potential biological control agents. against specific plant pathogens before assessing greenhouse production and field trials [24].
The antimicrobial potential of crude extract from Trichoderma agents against P. megakarya was evaluated. The results showed that our extract reduced significantly the mycelial growth of the pathogen. Indeed, secondary metabolites present in our extracts are known for their fungicidal, bactericidal and insecticidal actions. Gadji et al [25] showed the sensitivity of Phytophthora sp. toward organic products. However, under optimal conditions P. megakarya is able to pass through the cortex and infect the beans which are the major stage of spoilage as the beans are intended for consumption. In response, the metabolites present in extract would act through an antibiotic effect, able to diffuse through the cell surface and affect the cell activity of the pathogen by preventing the repair of previous damage to the wall or even death. This death by contact is linked to the necrotrophic action of antibiotic molecules which prevent the formation of the cell membrane, the formation of vesicles, the reduction in the diameter of the hyphae and the degradation of the cell wall by lytic enzymes such as protease, lipase and cellulase [16, 26]
Conclusion
This study shows that Trichoderma colonize the rhizosphere of the cocoa tree. The isolates of Trichoderma sp. had significant antagonistic effects and antimicrobial activity toward P. megakarya. These could be explained by production of non and volatile compounds as well as hydrolytic enzymes. The best antagonist was FC7 isolate of Trichoderma. However, investigation of organic extract as well as field experiment are needed.
References
- Ndoumbe`-Nkeng M, Cilas C, Nyemb E, Nyasse´ S, Bieysse D, et al. Impact of removing diseased pods on cocoa black pod caused by Phytophthora megakarya and on cocoa production in Cameroon. Crop Protection. 2004; 23: 415-424.
- Tchameni NS, Nwaga D, Nana Wakam L, Ngonkeu MEL, Fokom R, et al. Growth enhancement, amino acid synthesis and reduction in susceptibility towards Phytophthora megakarya by arbuscular mycorrhizal fungi inoculation in cocoa plants. J Phytopathol. 2012; 160: 220-228.
- Efombagn MIB, Marelli JP, Ducamp MC, Cilas C, Nyasse S, et al. Eff ect of fruiting traits on the fi eld resistance of cocoa (Theobroma cacao L.) clones to Phytophthora megakarya. J Phytopathol. 2004; 152: 557-562.
- Flood J, Guest D, Holmes KA, Keane P, Padi B, Sulistyowati E. Cocoa under attack. In: Flood, J., Murrphy, R. (Eds.), Cocoa Futures. CABI-FEDERACAFE, Chininà, Colombia, 2004; 164.
- Harman GE. Overview of mechanism and uses of Trichoderma spp. Phytopathology. 2008; 96: 190 -194.
- Kamal AM Abo-Elyousr, Sobhy II Abdel-Hafez, Ismail Abdel-Rahim. Isolation of Trichoderma and Evaluation of their Antagonistic Potential against Alternaria porri. J Phytopathol. 2013; 162: 567-574.
- Vinale F, Sivasithamparam K, Ghisalberti EL, Woo SL, Nigro M, et al. Trichoderma secondary metabolites active on plants and fungal pathogens. Open Mycol J. 2014; 8: 127-139.
- Vinale F, Sivasithamparam K, Ghisalberti EL, Marra R, Woo SL, Lorito M. Trichoderma–plant pathogen interactions. Soil Biol Biochem. 2008; 40: 1-10.
- Benitez T, Rincon AM, Limon MC, Codon AC. Biocontrol mechanisms of Trichoderma strains. Int Microbiol. 2004; 7: 249–260.
- Hanada RE, Pomella AWV, Soberanis W, Loguercio LL, Pereira, JO. Biocontrol potential of Trichoderma martiale against the black-pod disease (Phytophthora palmivora) of cacao. Biol Cont. 2009; 50: 143- 149.
- Tchameni NS, Sameza ML, O’donovan A, Fokom R, Ngonkeu MEL, et al. Antagonism of Trichoderma asperellum against Phytophthora megakarya and its potential to promote cacao growth and induce biochemical defense. Mycology. 2017; 8: 84-92.
- Siebatcheu EC, Wetadieu D, Youassi YO, Bedine Boat AM, Kibrom GB, et al. Secondary metabolites from an endophytic fungus Trichoderma erinaceum with antimicrobial activity towards Pythium ultimum. Natural Product Research. 2022; 37: 657-662.
- Tchameni NS. Growth enhancement and cocoa defense stimulation against Phytophthora megakarya: Interest of mycorrhizal fungi and a biological control agent. Ph.D. Thesis 2014; University of Yaoundé I, Cameroon. 2012; 160.
- Pimentel IC, Glienke-Blanco C, Gabardo J, Stuart RM, Azevedo JL. Identification and colonization of endophytic fungi from soybean (Glycine max (L.) Merril) under different environmental conditions. Brazilian Archives of Biology and Technology. 2006; 49: 705-711.
- Vinale F, Marra R, Scala F, Ghisalberti EL, Lorito M, Sivasithamparam K. Major secondary metabolites produced by two commercial Trichoderma strains active against different phytopathogens. Lett. Appl. Microbiol. 2006; 43: 143-148.
- Bedine BMA, Sameza ML, Iacomi B, Tchameni NS, Fekam BF. Screening, identification and evaluation of Trichoderma spp. for biocontrol potential of common bean damping-off pathogens. Biocontrol Science and Technology. 2020; 30: 228-242.
- Maria GL, Sridhar KR, Raviraja NS. Antimicrobial and enzyme activity of mangrove endophytic fungi of southwest coast of India. Journal of Agricultural Technology. 2005; 1: 67-80.
- Ntah A Ayong M, Tchameni NS, Siebatcheu EC, Ambata A, Henri T, et al. Efficacy of Trichoderma harzianum (Edtm) and Trichoderma aureoviride (T4) as potential bio-control agent of taro leaf blight caused by Phytophthora colocasiae. International Journal of Applied Microbiology and Biotechnology Research. 2018; 6: 115-126.
- Khaledi N, Taheri P. Biocontrol mechanisms of Trichoderma harzianum against soybean charcoal rot caused by Macrophomina phaseolina. J o Plant Prot Res. 2016; 56: 21–31.
- Cherkupally R, Amballa H, Reddy BN. In vitro antagonistic activity of Trichoderma species against Fusarium oxysporum f. sp. melongenae. Int J Appl Agri Res. 2017; 12: 87–95.
- Tchameni NS, Cotârle? M, Ghinea OI, Bedine BMA, Sameza ML, et al. Involvement of lytic enzymes and secondary metabolites produced by Trichoderma spp. in the biological control of Pythium myriotylum. Int Microbiol. 2020; 23: 179-188.
- Kubicek, CP, Mach RL, Peterbauer CK, Lorito M. Trichoderma: From genes to biocontrol. J Plant Pathol. 2001; 83: 11–23.
- Tahía BA, Rincón M, Carmen LAC. Biocontrol mechanisms of Trichoderma strains. Int J Food Microbiol. 2004; 7: 249–260.
- Hermosa R, Viterbo A, Chet I, Monte E. Plant-benefi cial eff ects of Trichoderma and of its genes. Microbiol. 2012; 158: 17–25.
- Gadji AAG, Yapo OB, Abo K, Coulibaly K, Kebe BI, et al. In vitro assessment of biopesticide bacillus thuringiensis var. Kurstakihd-1effectiveness on Phytophthora palmivora, agent of cocoa black pod rot in côte d’ivoire. European Scientific Journal 2015; 1857.
- Rosado I, Rey M, Codon A, Gonavites J, Moreno-Mateos MA, et al. QID74 Cell wall protein of Trichoderma harzianum is involved in cell protection and adherence to hydrophobic surfaces. Fung Gen Biol. 2007; 44: 950-64.
- Bell DK, Wells HD, Markham CR. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathol. 1982; 72: 379-382.