
Review Article
Austin J Microbiol. 2023; 8(1): 1042.
Review on Pasteurellosis: Causes, Pathogenesis, Diagnosis and Current Status in Ethiopia
Abdi Ahmed Umer*; Ebisa Mezgebu
Animal Health Institute (AHI), Microbiology Research Laboratory, P.O. Box 04, Sebeta, Ethiopia
*Corresponding author: Abdi Ahmed Umer Animal Health Institute (AHI), Microbiology Research Laboratory, P.O. Box 04, Sebeta, Ethiopia. Email: abdivet2014@gmail.com
Received: April 27, 2023 Accepted: May 31, 2023 Published: June 07, 2023
Abstract
Infectious diseases are one of the key constraints like pneumonic pasteurellosis is most recurrent respiratory infections that affect ruminants. This disease is caused by transportation stress, bacteria, viruses and climatic changes. A variety of etiologic agent form this multi-factorial disease such as Pasteurella multocida (P. multocida) (P. multocida gallicida, P. multocida multocida) and P. multocida septica. may also be divided into five capsular serogroups (A-E) and sixteen somatic serotypes [1-16]. Mannheimia haemolytica (M. haemolytica) formerly named P. haemolytica has two biotypes A and T depending on arabinose and trehalose fermentation. The colonies produced by M. haemolytica are odorless, moist, smooth, grayish, and translucent measuring approximately 1-3mm in diameter on blood agar plates while the colonies of P. multocida are round, grayish, shiny and non-haemolytic. Pneumonic pasteurellosis diagnosis is based on the clinical symptoms, necropsy, and bacteria isolation, Biolog, molecular and by the recent developed bacterial diagnostic technique called Matrix-Assisted-Laser-Desorption/Ionization-Time-of-Flight Mass Spectrometry (MALDI TOF MS), a fast, reliable and cost-effective method. Pasteurellosis is complex multifactorial disease and difficult to control but good management and prevention is advisable.
Keywords: Mannheimia haemolytica; Pasteurella multocida; Pasteurellosis; Diagnosis.
Introduction
Livestock have been domesticated for their meat and milk all over the world. Ethiopia boasts Africa's largest national livestock population [20]. However, due to a various technical, financial and health issues, production of livestock resource is marginalized in Africa. Infectious diseases are one of the health elements that have an impact on animal output [36]. Pneumonic pasteurellosis is one of the most common respiratory diseases that affect ruminant. Pasteurella sp. are gram negative rods or coccobacilli, non-motile, non-spore forming, facultative anaerobic, oxidase and catalase positive, bipolar bacteria that belong to the family Pasteurellaceae [53]. A variety of etiologic agents create this multi-factorial disease of pasteurellosis which is caused by the bacteria Mannheimia haemolytica (M. haemolytica) and Pasteurella multocida (P. multocida) [46].
M. haemolytica and Pasteurella P. multocida are commensal bacteria found in the tonsils and nasopharynx of healthy animals [1]. Many are identified as opportunistic or primary infections in animals. Transmission of agents occurs by inhalation of infected droplet, coughed up or exhaled from infected animals, carriers in which the infection persists in the upper respiratorytract [35]. The disease is caused by transportation stress, bacteria, viruses and climatic changes. Pneumonic pasteurellosis diagnosis is based on the clinical signs, postmortem lesions, isolation and molecular characterization of the bacteria and by currently emerged bacterial and fungi diagnostic method Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI TOF MS) is a reliable alternative to detect bacteria and fungi Protein [69].
The method is based on analysis of bacterial proteins, mainly ribosomal which are particularly abundant in the bacterial cells [19]. MALDI-TOF MS represents a universal, fast and cost-effective and it is an open system that can be complemented with own reference data. A various genera and species of Pasteurellaceae was analyzed by MALDI-TOF MS [38]. Pasteurellosis is complex multifactorial disease and difficult to control but good management, control and preventive measures are desirable [63].
In recent years MALDI-TOF MS has revolutionized routine identification of bacteria diagnosis in many countries. However, in Ethiopia this Rapid and reliable machine (MALDI TOF) was not available before 2020 years and up to date, there is no report on the use of MALDI TOF mass spectrometry diagnosis of Pasteurellosis.
Therefore, this review is aimed to make inclusive overview of causes, pathogenesis, and diagnosis of Pasteurellosis, disease with MALDI-TOF MS and other methods and its status in Ethiopia.
Etiology of Pasteurellosis
The Mannheimia and Pasteurella are small, non-motile, non-spore forming, gram- negative rods or coccobacilli and facultative anaerobic bacteria that belong to the family Pasteurellaceae. They are oxidase and catalase positive and reduce nitrates and carbohydrates fermentatively and Bipolarity of Pasteurella and Mannheimia can be seen in Giemsa-stained smears [47]. There are several species of P. multocida and M. haemolytica are most clinically relevant to animals. P.multocida is divided into three different subspecies: P.multocida gallicida, P. multocida multocida, and P. multocida septica. P.multocida subspecies may also be divided into five capsular serogroups (A-E) and sixteen somatic serotypes [1-16]. B2 and E2 cause hemorrhagic septicemia in addition to the possible pneumonia, enteritis, or septicemia caused by the remainder of the capsular serogroups and somatic serotypes [48]. M. haemolytica formerly named (P. haemolytica) has two biotypes A and T depending on arabinose and trehalose fermentation. Biotype A is further subdivided into 13 serotypes (A1, A2, A5, A6, A7, A8, A9, A11, A12, A13, A14, A16 and A17) that cause pneumonic pasteurellosis (shiping fever) in cattle, sheep and goats (Table 1). P. haemolytica is carried in the nasopharynx and tonsils of apparently healthy sheep and goat. Lambs acquire infection soon after birth, presumably by contact [34]. The carriage rate is low in normal healthy flocks and an assortment of serotypes is present. In flocks suffering by outbreaks, the carriage rate is high, and a few specific serotypes dominate. Thus, a high carriage rate is indicative of prevalent infection in the vicinity. This carriage status has been found to display seasonal variations [10].
Hosts
Name of the Disease
Serotypes
Cattle
Hemorrhagic septicemia (HS) Occasionally, HS like septicemia disease Bovine pneumonic pasteurellosis
P. multocida serotypes B2 and E2 P. multocida serotype B3, 4
M. haemolytica A1; P multocida ABuffalo
HS
P. multocida serotypes B2 and E2
Shoats
Pneumonic pasteurellosis Septicemia pasteurellosis
M. haemolytica A; B. trehalosi
Pigs
Sporadic outbreaks of HS Atrophic rhinitis Pneumonia
P. multocida serotype B2 Toxigenic strains of P multocida type D, occasionally, type A; P. multocida type A
Poultry
Fowl cholera
P. multocida type A (type F in turkeys) and type D are less common.
Table 1: Summary of common diseases caused by Pasteurella serotypes in animals.
Reaction
M. haemolytica
P. multocida
Haemolysis
+
-
Motility
-
-
Indole formation
-
+
Litmus milk
Acid
Neutral
Lactose
+
-
Oxidase
+
+
Catalase
+
+
MacConkey agar
+
-
+=indicates present, -=indicates not present.
Table 2: Biochemical characteristics of M. haemolytica and P. multocida [32].
Taxonomy and Classification
The Mannheimia and Pasteurella are grouped taxonomically under
Super kingdom Bacteria
Phylum Proteobacteria
Class Gammaproteobacteria
Order Pasteurellales
Family Pasteurellaceae
Genera Mannheimia and Pasteurella
Species M. hemmolytica and Pasteurella multicoca
Source.
Based on number of characteristics including pathogenicity, antigenic nature and biochemical activity, P. haemolytica can be differentiated into two biotypes, biotype A and T. Biotype A ferments arabinose whereas biotype T ferments trehalose; however, based on molecular, biological techniques and analysis of phenotypic data, biotype T was reclassified as P. trehalosi and biotype A as M. haemolytica and M. glucosida.
Based on quantitative evaluation of phenotypic and genomic characteristics. They are classified trehalose-negative P. haemolytica multipart into five new species M.haemolytica, M.glucosidal, M.ruminalis, M.granulomatis and M.varigena. Based on extractable surface antigens, 17 serotypes of M.haemolytica and P. trehalosi are recognized. Serotype 3, 4, 10, and 15 are classified as P. trehalosi [61].
Microbiological Properties
Morphology and staining characteristics: Pasteurella and Mannheimia appear as short ovoid rods measuring 1μm in length and 0.5-0.8μm in width and most of them are capsulated. The organisms tend to bipolar staining when stained with Giemsa staining. Old culture usually revealed Gram-negative rods of various sizes. Cells are arranged in chains and filamentous forms are occasionally observed. The bipolarity feature is lost due to continuous culturing [62]. Mannheimia are gram-negative rods or coccobacilli, nonmotile, non-spore-forming, facultatively anaerobic, oxidase-positive, and fermentative, and they naturally inhabit in the domestic and wild animals upper respiratory and digestive tract mucous membrane [70]. These organisms grow best on blood agar and produce a narrow zone of hemolysis, and they also grow on MacConkey agar [62].
Growth requirements: The organisms are aerobic or facultative anaerobic. The optimum temperature for growth is 37°C at pH 7.2 to 7.4. Although non enriched media support their growth, the Pasteurella and Mannheimia species grow best in the presence of blood. Sheep blood is used for the demonstration of hemolysis. These microorganisms grow well in medium containing amino acids, a mixture of salts, vitamins, sugars like galactose and glucose. Mannheimia species requires a higher concentration of iron for production of cytotoxin than is needed for growth [64].
Cultural characteristics and Biochemical characteristics: The colonies produced by M. haemolytica are odorless, moist, smooth, grayish, and translucent measuring approximately 1-3mm in diameter on blood agar plates while the colonies of P. multocida are round, grayish, shiny and non-hemolytic. Some colonies of pathogenic strains of P. multocida are mucoid due to the production of thick hyaluronic acid capsules. The colonies have a subtle but characteristic odor. The M. haemolytica grow on blood agar in the form of smaller colonies with slight thickening in the center and circular surrounded by a narrow zone of β-hemolysis. M. haemolytica is very distinct from P. multocida by their growth on MacConkey agar as pink to red colonies [31].
Biochemical characterization of P. multocida strains were catalase and oxidase positive, did not have hemolytic properties, lysed in bile, did not form hydrogen sulfide, reduced nitrates to nitrites, and Voges-Proskauer reaction negative. Urea, sorbitol- positive and dulcitol-negative [49]. Although Mannheimia spp. have limited fermentative ability of carbohydrates, they utilize number of carbohydrates with acid production but not gas [1].
Chemical and physical properties: Pasteurella is not resistant to adverse agents and can easily be killed with common chemical and physical agents. Exposure of suspension to disinfectants such as to 0.5% phenol for 15 minutes, to heat at 55°C, to ultraviolet light and colonies on solid media to sun light are lethal, and are susceptible to commonly used antibiotics [9].
Virulence
Pathogenic bacteria produce virulence factors that enhance their ability to escape host defense mechanisms and increase the ability of the organisms to colonize and invade deeper tissues. Members of Mannheimia and Pasteurella spp produce several substances that are associated with the pathogenicity of these groups of microorganisms. These include the capsule that plays a great role in adherence and invasion, Outer Membrane Proteins (OMP) that are important in eliciting the protective immune response, adhesins implicated in colonization, the neuraminidase that reduces the viscosity of respiratory mucus and allows closer bacterial apposition to the cell surface [25].
Lipopolysaccharide causes immune-mediated hypersensitivity that can exacerbate inflammation and tissue damage [29] and the Leukotoxin that produces a lot of biological effects: at higher concentrations, the toxin creates pores in the cell membrane that leads to swelling and lyses [33]. The virulence factors are responsible for promoting adhesion, colonization, and proliferation of the organism which play vital role in pathogenesis [45], the alteration of the organism from commensal into a pathogenic [28].
Transmission and Pathogenesis
P. multocida and M. haemolytica species are highly vulnerable to environmental influences and a close contact is a main factor in the spread of the disease Particularly, when animals are closely confined in inadequately ventilated trains or held for long periods in holding pens and feed lots, the disease may spread very quickly and affect high proportion of the herd within short hours [42]. It also acquired infection through inhalation of infected nasal secretions, droplet, coughed up or exhaled from infected animals or recovered carriers in which the infection persists in the upper respiratory tract. Animals at pasture are able to move freely and the rate of spread may be slower. P.multocida have public health importance, humans infection is often transmitted by animal bites, scratches or licks from cats or dogs [53].
Mannheimia and Pasteurella play a major role as a secondary pathogen in the final progression of severe pleuropneumonias in animals. Their pathogenesis involves many predisposing agents such as bacteria, viruses, lungworms, environment changes (excessive temperature, sudden change of feed, dust) or stress associated during weaning, dehorning and shipping [47]. These factors seems to alter the upper respiratory tract epithelium allowing M. haemolytica and Pasteurella to colonize, escaping clearance, and to move from the nasopharynx to the lungs, leading to a broncho-alveolar type of pneumonia which is accompanied by high morbidity and mortality [13]. Mannheimia species are common commensals of the nasopharynx in many domestic and wild animals [56]. These species can cause infection when the animals’ immunity becomes compromised [6]. M.haemolytica induces most severe fibrinous pleuropneumonia characterized by extensive leukocyte infiltration in alveoli, intra-alveolar hemorrhage, deposition of fibrin, and consolidation of the lungs [7] (Figure 1). Pasteurellosis caused by Mannheimia haemolytica are transmitted from sick to healthy animals by direct contact and aerosol. Particularly, when animals are closely confined in inadequately ventilated held for long periods. These infectious disease are transferred by direct contact with body fluids (such as saliva and nasal secretions, coughed up or exhaled from infected animals or recovered carriers), contaminated feeders and troughs [70].
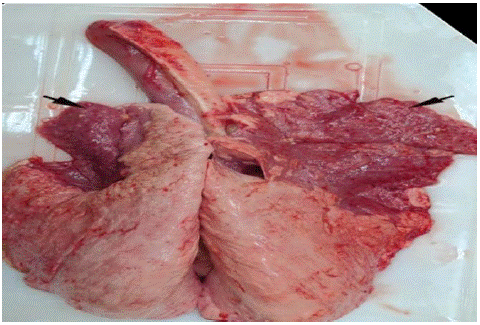
Figure 1: Dark red consolidation of cranio-ventral lobes (arrows) [8].
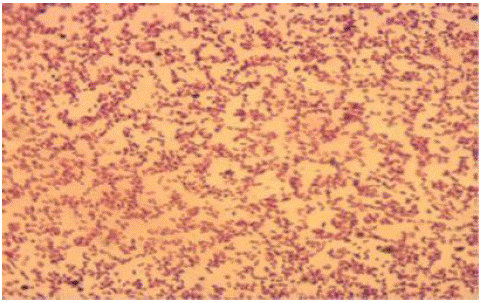
Figure 2: Gram stained isolate of P.multocida.
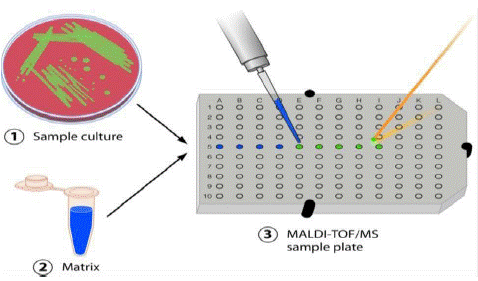
Figure 3: Sample preparation - for MALDI TOF MS [17].
Symptomatology and Diagnostic of Pasteurellosis
Clinical Signs: Pneumonia pasteurellosis is an important disease that affect animal often appear depressed, with a mucopurulent nasal and ocular, exhibit inappetance, weight loss and temperatures rises to 40.4°C to 42°C. Most cases happen within two weeks after transportation and the development of disease can be rapid with death without showing above clinical signs of disease [66]. The most common manifestation of P. multicoda in humans is a local wound infection, usually following an animal bite or scratch which can develop into a serious soft tissue infection that can be further complicated by abscesses, septic arthritis and osteomyelitis also cause meningitis, ocular infections [54]. The early clinical signs are anorexia, high fever, and frothing, coughing and rapid shallow breathing accompanied by profuse mucopurulent nasal and ocular discharges. In the later stage of infection severe cough predominates where dyspnea with an exhalation grunt [18].
Differential Diagnosis: The differentiation of pasteurellosis from other respiratory diseases is based on the high mortality and rapid progression to death and in pneumonic pasteurellosis is dark red/purple areas, firm to the touch, are evident mainly in the anterior and cardiac lobes of the lung [10] and respiratory diseases which are confused with pneumonic pasteurellosis caused by Mycoplasma species, Bordetella parapertussis, and Streptococcus zooepidemicus, lung abscesses caused by Staphylococcus aureus. The differential diagnosis should also be done with the viral pneumonia caused by ovine adenovirus, and Peste des Petits Ruminants (PPR), parasitic pneumonia caused by lung worms like Dictyocaulus filarial , mycotic pneumonia caused by Aspergillus species, upper respiratory tract disease [10].
Isolation and Identification: Pasteurella can be isolated by culturing different sample (lung tissue or nasal swab, blood) in pre-enriched in 5ml of tryptose Soya broth specimen incubated for 24 hours at 37°C. The collected culture is streaked on to blood agar base containing 5% sheep blood and incubated aerobically at 37°C for 24 hours. Then typical colonies subjected to gram’ s staining and cellular morphology under light microscope and gram negative, coccobacilli bacteria will further sub cultured on blood agar containing 5% sheep blood and MacConkey agar for isolation and characterization. Existence of hemolysis, the kind of haemolysis, the general appearance of colonies will be examined and followed by biochemical, MALDI TOF MS and molecular tests for identification and characterization of bacteria [45].
Postmortem: P. multicoda causes fibrino-purulent bronchopneumonia without the multifocal coagulation hemolytic necrosis, M.haemolytica were also characteristics of fibrinous lobar pneumonia. Pasteurellosis is manifested by the association involving the lungs, catarrhal bronchitis, bronchiolitis a fibrinous pleurisy the cut surface consists of several colors due to hemorrhage, necrosis and red and grey consolidation, coagulation necrosis of pneumonic lungs [4].
Serological Diagnosis: The presence of P.multocida and M. haemolytica is carried out using different serological methods. These methods consist of a rapid slide agglutination test, an (IHA) immune hemagglutination test for capsular typing, Indirect Enzyme Linked Immunosorbent Assay (I-ELISA) [2].
Molecular methods: Conventional and real time Polymerase Chain Reaction (PCR): Molecular methods of bacterial identification have been proved valuable to overcoming some limitations of the conventional biochemical and serological methods and has better sensitivity and rapidity [40]. Molecular identification is further advance accuracy of characterization in pure and/or mixed cultures, speed of detection, determination of taxonomic position, and indulgent of intra-species genetic relationships [12].
MALDI TOF mass spectrometry: based isolation method
Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) has recently emerged as a powerful technique for identification of bacteria altering the workflow of well-established diagnostics laboratory [15]. MALDI-TOF MS is a useful, fast, cost-effective and accurate tool for routine laboratory diagnostic and has been used to identify bacteria. A typical consists of growth of the bacteria, pure colony and placement on a target plate, addition of matrix, and analysis with MALDI-TOF MS [26]. Matrix is a small organic molecule used to facilitate ionization process by absorption of UV light. Moreover, it is an open system that can be complemented with own reference data. The Biotyper 3.0 database from the Bruker MALDI-TOF MS system contains a few, selected representatives of the family Pasteurellaceae.
Pasteurella and M.haemolytica rapid and accurate identification from ruminants these bacteria are essential for effective prevention and treatment of infected animals. Phenotypic tests are commonly used in diagnostic laboratory to identify P.multicoda and M.haemolytica based on biochemical methods [32]. The protein spectrum obtained for the strain analyzed is compared with reference spectra, and then, the bacterium is automatically identified. Mass spectrometry is currently used successfully for rapid identification of a variety of Pasteurella species [14]. Recent study shows that, 100% identification agreement between MALDI TOF and the biochemical method. However, in comparison with conventional phenotyping methods, MALDI-TOF MS is much faster, and labor and reagent costs are lower [5]. The suitability of MALDI-TOF MS for rapid identification of many species of bacteria isolated from animals, including M. haemolytica, has only been confirmed in a few publications [52]. The identification of Pasteurella by MALDI-TOF MS using the [27] and the growth is isolated where from plated culture media and applied directly onto the MALDI test plate including Bacterial Test standard (BTS) as a control. Pasteurella samples are then overlaid with matrix dried and Load the MALDI target plate into the mass spectrometer [38].
The plate is subsequently loaded into the MALDI-TOF MS instrument and analyzed by software associated with the respective system, allowing rapid identification of the Pasteurella bacteria [17]. The identification criteria of MALDI Biotyper™ system log (score values) higher than 2.0 indicates species identification, while log (score values) between 1.7 and 2.0 are sufficient for identification of the genus. For values below 1.7, no isolate identification is possible [43].
A substantial cost savings can be achieved by implementing MALDI-TOF MS as the primary method of identification in the diagnostic laboratory. While the initial cost of the instrument is high, the cost savings on reagents and labor can offset the expenditure [68]. Significantly decreases the turnaround time. The sample preparation is simple and the sample requirement is minimal. A single colony is sufficient to generate spectra of sufficient quality. Cost effective, automated, robust, interlaboratory reproducibility, broad applicability (all types of bacteria including anaerobes, fungi) [50].
MALDI-TOF MS in the diagnostic laboratory can accurately identify most closely related species. However, there are some exceptions. The inability to discriminate between related species can be due to the inherent similarity of the bacteria themselves. For example, MALDI-TOF MS is currently unable to differentiate E. coli from Shigella [57]. This is likely because these may not be two species, but one, as has been suggested by taxonomists. In the future, the addition of proteomic based approaches to the typical MALDI-TOF MS system may improve the discriminatory power of this method and make it possible to identify organisms at the strain or serotype level. Another reason why similar species may be incorrectly identified is a lack of sufficient spectra in the database [65].
Identification of new isolates is possible only if the spectral database contains peptide mass fingerprints of the type strains of specific genera/species/subspecies/strain. No susceptibility information is provided, not useful for direct testing of samples (except urine). Some organisms require repeat analysis and additional processing (extraction). The acceptable score cutoffs vary between studies and some closely related organisms are not differentiated [61]. The important challenges in species identification arise from incomplete databases, close relatedness of species of interest, and spectral quality, which is currently vaguely defined [21].
Control and Prevention
Management
Pasteurellosis represents diseases, amongst which is influenced by a wide variety of environmental and management risk factors. Thus, the reduction or even elimination of such predisposing factors is of major importance [51]. Most cases are acute or per acute, resulting in death within 8-24 hours after the onset of disease. Management factors such climatic changes and avoiding crowding stress, mixing of different flocks, movement, and deprivation of feed and water, exposure to aerosol infection from other sheep and goats providing shelter especially during extreme weather condition reduce the outbreaks of the disease [42].
Treatment
Antimicrobials are still the tools of choice for control of infections due to Pasteurella and Mannheimia spp. [22]. The priority of treatment against pasteurellosis is directed towards saving the lives of animals and depends on early detection of the disease and administration of appropriate and effective antibiotics. The antibiotics such as Oxytetracycline, trimethoprim, sulfonamides, penicillin, timilcosin, streptomycin and florfenicol are the most commonly used drugs to treat pasteurellosis [37]. It is well known that these organisms are having the tendency of becoming resistance to antibiotics the antimicrobial sensitivity of the Pasteurella and Mannheimia isolates should be tested and a suitable antibiotic should be chosen on the basis of the in vitro sensitivity test. The use of long-acting antibiotics in the face of an outbreak is common approach to prevent pasteurellosis in small ruminants [53].
Vaccines and Vaccination
The development of effective vaccine used for the P. multocida and M. haemolytica is very important in control strategy to reduce the incidence and burden of the disease and to minimize antimicrobial use. Currently, several vaccine types exist against pasteurellosis globally. Problems with vaccination arise where there is more than one serotype circulating, due to the lack of cross-protection [3]. At present, only a monovalent P. multocida serotype A vaccine is commercially available in Ethiopia, although P. multocida serotypes A and D and 11 serotypes belonging to M. haemolytica have long been detected [23]. Consequently, repeated outbreaks are reported in Ethiopia even among vaccinated sheep and goats, which practitioners and communities ascribe to vaccine failure [10]. Serotypes have different levels of virulence, host-species adaptability with possible inter-species transmissibility, antigenicity, immunogenicity, drug resistance and a lack of inter-serotype cross-reactivity [71]. Routine serotyping is not practised in Ethiopia due to the lack of detailed information, time, expense involved, and the lack of commercially available antisera, so that the serotypes of the circulating and outbreak-causing isolates remain unknown [10]. Many predisposing factors are normal environmental changes or routine management practices that cannot be avoided. Outbreaks are sporadic and unpredictable. Thus vaccines should seek to achieve round the year protection in areas with a high prevalence [30].
Vaccines against pneumonic pasteurellosis involved will help to reduce the severity of the disease, since it is the secondary bacterial phase of the disease that contributes to both its severity and fatality (Sarah et al., 2011). Killed vaccines from locally isolated P. multocida types A and D and M. haemolytica in oil adjuvant are widely used for prevention of pneumonic pasteurellosis of ruminants. Such vaccines were found to be effective in prevention of the natural disease caused by homologous strains [44]. A trial at National Veterinary Institute (NVI), showed that vaccination with a formalin killed vaccine prepared from combined A2 and A7 M. haemolytica serotypes grown under iron restriction, deliberated relatively good protective efficacy in sheep than either of M. haemolytica A7 or A2 monovalent vaccines [44].
Status of Pasteurellosis in Ethiopia
Epidemiology of Pasteurellosis
Pasteurellosis caused by M. hemolytica is one of the most economically substantial infectious diseases affecting small ruminants, with an international distribution (Table 3). However, Iceland was the first country to report it, followed by Australia, the United Kingdom, Ethiopia, Norway, South Africa, Somalia, and the United States [10]. The disease is reported most frequently in Asia and Africa countries where sheep or goat breeding is widespread. It is also prevalent in USA and Canada where cattle breeding is common. In Europe, pasteurellosis widespread in many countries where sheep and cattle are present such as the Netherlands, Germany, Italy and France [35].
Country
Region
Host species
M.h
P.m
Authors
Iraq
Baghdad
Goat
4.46
-
(Ahmed et al., 2017)
Morocco
Sheep
42
15
(Ghizlane Sebbar et al., 2018)
Goat
100
0
Ethiopia
Afar (Mille)
Sheep
-
-
(Shiferaw et al., 2006)
Goats
-
-
Oromia (Hararghe)
Sheep
87.5
12.5
[45]
Oromia
Sheep
12
8
[66]
Amhara
Sheep
32.62
-
(Ali et al., 2022)
Table 3: Distribution of M. haemolytica and P. multicoda in different geographical locations.
Mannheimia haemolytica are a common commensal bacterium found in the upper respiratory tracts of sheep and goats and they effect of all ages group. Outbreak of pasteurellosis is often associated with climate changes and occur in spring and summer, but can happen sporadically at any time of the year [35]. The worst epidemics occur during the rainy season, in poor body condition, stress and wet conditions seem to contribute to the spread of the disease. The majority of M.haemolytica infections are mostly endogenous, caused by the normally resident bacteria on the upper respiratory tract, although exogenous infections can also occur by direct contact with sick animals or through infected aerosols [46].
Numerous studies have determined the extent (Table 4) and the relative distribution of P. multicoda and M. haemolytica species. Different serotypes of M. haemolytica, P. multocida and P. trehalosi, such as A1, A2, A5, A6, A7, A8, A9, A11, A12, A13, A14, A, D and T3, T4, T10 and T15 which A1, A2, A8, A7, T3 and T4 are the dominant serotypes pasteurellosis identify from small ruminants (Jarso et al., 2016). M. haemolytica serotype A1 causes pneumonic pasteurellosis in cattle; M. haemolytica serotype A2 and causes pneumonic pasteurellosis in sheep and goats and B. trehalosi serotypes cause septicemic pasteurellosis in sheep and goats [36].
Prevalence of M. haemolytica (%)
Prevalence of P. multocida (%)
References
Study Area
48.0
2.0
Mekonnen et al,(2000)
Arsi
20.0
10.0
Ayelet et al.(2004)
Debre Berhan
19.0
15.0
Deressa et al.(2004)
Debre Berhan
5.6
25.0
Melese et al,(2005)
Debre Zeit
11.1
1.8
Belay et al,(2007)
South Wollo
87.4
12.5
[45]
Haramaya district
46.4
14.3
Abera et al.(2014)
Bedele district
28.0
2.2
Demissie et al.(2014)
Bishoftu
79.5
20.5
Alemneh et al.(2016)
Gonder
11.2
8·85
[10]
Tigray
Table 4: Occurrence of P. multocida and M. haemolytica in Ethiopia [42].
Conclusion and Recommendations
Livestock plays an important role in the economy and livelihood of community. These animals serve as a foremost source of cash income for household expense as well as domestic consumption. However, efficient utilization of this resource is impaired by different technical and non-technical factors. Infectious diseases are one of the health elements that influence livestock production. Pneumonic pasteurellosis is one of the most significant and recurrent devastating respiratory diseases in ruminants, caused by bacteria, viruses, lung worm, stress, and environmental change. This disease is caused by different etiological agent either by M. haemolytica or P. multocida, and it causes great economic losses. M. haemolytica is the most common cause, whereas P. multocida is rare. Young animals are mostly susceptible to pasteurellosis. In diagnosis of bacteria, the implementation of MALDI-TOF MS bacteria, the implementation of MALDI-TOF MS has changed the approach of Pasteurella identification in last 10 years. MALDI TOF MS rapid, accurate and cost-effective diagnostic method for pasteurollosis and other bacteria. Good husbandry practices are the best ways for prevention and control of P. multocida and M. haemolytica in reducing stressors and stress associated disease in animals.
Therefore, based on the above conclusion, the following recommendations are forwarded:
¾Diagnosis of Pasteurella should be adopted to know real cause of pasteurollosis.
¾MALDI TOF MS Diagnostic technique should be adopted in Ethiopia for isolation and identification of P. multocida and M. haemolytica
¾Sustainable pasteurollosis survey should be implemented which is important in disease control and prevention strategy and to reduce respiratory disease.
References
- Abbas G, Ali MN, Javid MA, Hussain Qamar S, Wasim Abbas S, et al. Mannheimia haemolytica infection in small ruminants: a review. Adv Zool Bot. 2019; 7: 1-10.
- Abed AH, El-Seedy FR, Hassan HM, Nabih AM, Khalifa E, et al. Serotyping, genotyping and virulence genes characterization of Pasteurella multocida and Mannheimia haemolytica isolates recovered from pneumonic cattle calves in north upper Egypt. Vet Sci. 2020; 7: 1-13.
- Ahmad AM. Efforts towards the development of recombinant vaccines against Pasteurella multocida. Sci World J. 2014; 9: 1-7.
- Aiswarya V, Mathakiya RA, Bhanderi BB, Roy A. Characterization of Pasteurella multocida isolates of buffalo origin from Gujarat state of India by outer membrane protein profile analysis. Buffalo Bull. 2017; 36: 313-22.
- Almuzara M, Cárdenas V, Barberis C, Ramirez MS, Famiglietti A, Vay C. Performance of MALDI-TOF mass spectrometry for the identification of the HACEK group and other fastidious Gram-negative rods. Open Microbiol J. 2019; 13: 216-21.
- Assefa GA, Kelkay MZ. Goat pasteurellosis: serological analysis of circulating Pasteurella serotypes in Tanqua Aberegelle and kola Tembien Districts, Northern Ethiopia. BMC Res Notes. 2018; 11: 485.
- Aulik NA, Hellenbrand KM, Czuprynski CJ. Mannheimia haemolytica and its leukotoxin cause macrophage extracellular trap formation by bovine macrophages. Infect Immun. 2012; 80: 1923-33.
- Azizi S, Korani FS, Oryan A. Pneumonia in slaughtered sheep in south-western Iran: pathological characteristics and aerobic bacterial aetiology. Vet Ital. 2013; 49: 109-18.
- Patten BE, Spencer TL, Johnson RB, DH, LL 1992. Pasteurellosis. Animals: a review. The Australian Centre for International Agricultural Research (ACIAR). 1992; 43: 43-256.
- Berhe K, Weldeselassie G, Bettridge J, Christley RM, Abdi RD. Small ruminant pasteurellosis in Tigray region, Ethiopia: marked serotype diversity may affect vaccine efficacy. Epidemiol Infect. 2017; 145: 1326-38.
- Bizzini A, Jaton K, Romo D, Bille J, Prod’hom G, Greub G. Matrix-assisted laser desorption ionization – time of flight mass spectrometry as an alternative to 16S rRNA gene sequencing for identification of difficult-to-identify bacterial strains. J Clin Microbiol. 2011; 49: 693-6.
- Blackall PJ, Miflin JK. Identification and typing of Pasteurella multocida: a review. Avian Pathol. 2000; 29: 271-87.
- Brogden KA, Lehmkuhl HD, Cutlip RC. Pasteurella haemolytica complicated respiratory infections in sheep and goats. Vet Res. 1998; 29: 233-54.
- Cameron M, Perry J, Middleton JR, Chaffer M, Lewis J, Keefe GP. Short communication: evaluation of MALDI-TOF mass spectrometry and a custom reference spectra expanded database for the identification of bovine-associated coagulase-negative staphylococci. J Dairy Sci. 2018; 101: 590-5.
- De Carolis E, Vella A, Vaccaro L, Torelli R, Spanu T, Fiori B et al. Review Article Application of MALDI-TOF mass spectrometry in clinical diagnostic microbiology.
- Cherkaoui A, Hibbs J, Tangomo M, Girard M, Francois P, Schrenzel J. Comparison of Two matrix-assisted laser desorption ionization – time of flight mass spectrometry Methods with Conventional phenotypic Identification for Routine Identification of Bacteria to the Species Level. 2014; 48: 1169-75.
- Clark AE, Kaleta EJ, Arora A, Wolk DM. Matrix-assisted laser desorption ionization – time of flight mass spectrometry: a fundamental shift in the routine practice of clinical. Clin Microbiol Rev. 2013; 26: 547-603.
- Cozens D, Sutherland E, Lauder M, Taylor G, Berry CC, Davies RL. Pathogenic Mannheimia haemolytica invades differentiated bovine airway epithelial cells. Infect Immun. 2019; 87: 13-5.
- Croxatto A, Prod G, Greub G. Diagnostic microbiology. 2012; 36: 380-407.
- CSA. Agricultural sampling survey. Report on livestock and livestock characteristics. 2020; 2: 1-215.
- Cuénod A, Foucault F, Pflüger V, Egli A. Factors associated with MALDI-TOF mass spectral quality of species identification in clinical routine diagnostics. Front Cell Infect Microbiol. 2021; 11: 646648.
- Cuevas I, Carbonero A, Cano D, García-Bocanegra I, Amaro Má, Borge C. Antimicrobial resistance of Pasteurella multocida type B isolates associated with acute septicemia in pigs and cattle in Spain. BMC Vet Res. 2020; 16: 222.
- Deressa A, Asfaw Y, Lubke B, Kyule MW, Tefera G, Zessin KH. Molecular detection of Pasteurella multocida and Mannheimia haemolytica in sheep respiratory infections in Ethiopia. Int J Appl Res Vet Med. 2010; 8: 101-8.
- Van Driessche L, Jade B, Deprez P, Haesebrouck F, Boyen F. Rapid identification of respiratory bacterial pathogens from bronchoalveolar lavage fluid in cattle by MALDI-TOF MS. Sci Rep. 2019; 9: 18381.
- Ekundayo SO, Odugbo MO, Olabode AO, Okewole PA. Phenotypic variability among strains of Pasteurella multocida isolated from avian, bovine, caprine, leporine and ovine origin. Afr J Biotechnol. 2008; 7: 1347-50.
- Emami K, Askari V, Ullrich M, Mohinudeen K, Anil AC, Khandeparker L et al. Characterization of bacteria in ballast water using MALDI-TOF mass spectrometry. PLoS One. 2012; 7: e38515.
- Emonet S, Shah HN, Cherkaoui A, Schrenzel J. Application and use of various mass spectrometry methods in clinical microbiology. Clin Microbiol Infect. 2010; 16: 1604-13.
- Franco MF, Gaeta NC, Alemán MAR, Mellville PA, Timenetsky J, et al. Bacteria isolated from the lower respiratory tract of sheep and their relationship to clinical signs of sheep respiratory disease. Pesq Vet Bras. 2019; 39: 796-801.
- Gharibi D, Hajikolaei MRH, Ghorbanpour M, Barzegar SK. Profil gena za virulenciju izolata bakterije Pasteurella multocida izdvojenih iz goveda I bivola. Vet Arhiv. 2017; 87: 677-90.
- Haig S-J. Adherence of Mannheimia haemolytica to ovine bronchial epithelial cells. Biosci Horiz. 2011; 4: 50-60.
- Harper M, Boyce JD, Adler B. Pasteurella multocida pathogenesis: 125 years after Pasteur. FEMS Microbiol Lett. 2006; 265: 1-10.
- Hawari D, Hassawi S. Isolation of Mannheimia Hemolytica and Pasteurella multocida. Pdf. 2008; 8: 1251-4.
- Highlander SK. Molecular genetic analysis of virulence in Mannheimia (pasteurella) haemolytica. Front Biosci. 2001; 6: D1128-50.
- Irsik M. Bovine respiratory disease associated with Mannheimia haemolytica or Pasteurella multocida. Veterinary Medicine-Large Animal Clinical Sciences Department. 2010; 1: 2-4.
- Jilo K, Belachew T, Birhanu W, Habte D, Yadeta W, Giro A. Health Pasteurellosis Status in Ethiopia: A Comprehensive Review. Journal of Tropical Diseases and Public. 2020; 8: 1-5.
- Kabeta T, Fikadu T, Zenebe T, Kebede G. Review on the pneumonic pasteurellosis of cattle. Acad J Anim Dis. 2015; 4: 177-84.
- Kehrenberg C, Schulze-tanzil G, Martel J, Schwarz S, Kehrenberg C, et al. Antimicrobial resistance in Pasteurella and Mannheimia: epidemiology and genetic basis To cite this version: HAL Id : hal-00902701 Review article Antimicrobial resistance in Pasteurella and Mannheimia : epidemiology and genetic basis. 2001.
- Kuhnert P, Bisgaard M, Korczak BM, Schwendener S, Christensen H, Frey J. Identification of animal Pasteurellaceae by MALDI-TOF mass spectrometry. J Microbiol Methods. 2012; 89: 1-7.
- Kuhnert P, Scholten E, Haefner S, Mayor D, Frey J. Basfia succiniciproducens gen. nov., sp. nov., a new member of the family Pasteurellaceae isolated from bovine rumen. Int J Syst Evol Microbiol. 2010; 60: 44-50.
- Kumar AA, Shivachandra SB, Biswas A, Singh VP, Singh VP, Srivastava SK. Prevalent serotypes of Pasteurella multocida isolated from different animal and avian species in India. Vet Res Commun. 2004; 28: 657-67.
- Kumar J, Dixit SK, Kumar R. Rapid detection of Mannheimia haemolytica in lung tissues of sheep and from bacterial culture. Vet World. 2015; 8: 1073-7.
- Legesse A, Abayneh T, Mamo G, Gelaye E, Tesfaw L, et al. Molecular characterization of Mannheimia haemolytica isolates associated with pneumonic cases of sheep in selected areas of Central Ethiopia. BMC Microbiol. 2018; 18: 205.
- Li Y, Shan M, Zhu Z, Mao X, Yan M, et al. Application of MALDI-TOF MS to rapid identification of anaerobic bacteria. BMC Infect Dis. 2019; 19: 941.
- Liyuwork T, Shiferaw J, Hundera S, Tesfaye S, Haileleul N. Efficacy of Mannheimia haemolytica A2, A7, and A2 and A7 combined expressing iron regulated outer membrane protein as a vaccine against intratracheal challenge exposure in sheep. Afr J Microbiol Res. 2014; 8: 1237-44.
- Marru HD, Anijajo TT, Hassen AA. A study on Ovine pneumonic pasteurellosis: isolation and Identification of Pasteurellae and their antibiogram susceptibility pattern in Haramaya District, Eastern Hararghe, Ethiopia. BMC Vet Res. 2013; 9: 239.
- Miller MW, Clay BR. DigitalCommons@University of Nebraska – Lincoln pasteurellosis transmission risks between domestic and wild sheep; 2008.
- Mohamed Ra, Abdelsalam EB. A Review on Pneumonic Pasteurellosis (Respiratory Mannheimiosis) With Emphasis on Pathogenesis, Virulence Mechanisms and Predisposing Factors. Bulg J Vet Med. 2008; 11: 139-60.
- OIE. Pasteurella spp. potential impacts of disease agent beyond clinical illness references articles; 2018.
- Orynbayev M, Sultankulova K, Sansyzbay A, Rystayeva R, Shorayeva K, et al. Biological characterization of Pasteurella multocida present in the Saiga population. BMC Microbiol. 2019; 19: 37.
- Osa M, Belo MC, Dela Merced Z, Villanueva AMG, Mauhay J, et al. Performance of MALDI – TOF mass spectrometry in the Philippines. Trop Med Infect Dis. 2021; 6: 1-9.
- Public health investigation. Identification of Pasteurella species and Morphologically Similar Organisms. UK Standards for Microbiology Investigations. 2015; B55: 1–21.
- Puchalski A, Urban-Chmiel R, Dec M, Stegierska D, Wernicki A. The use of MALDI-TOF mass spectrometry for rapid identification of Mannheimia haemolytica. J Vet Med Sci. 2016; 78: 1339-42.
- Radostits OM, Gay CC, Hinchcliff K, Constable PD. 10th ed Radostits. Usa. 2010. A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. 2010; 51: 541.
- Rawat N, Gilhare VR, Kushwaha KK, Hattimare DD, Khan FF, et al. Isolation and molecular characterization of Mannheimia haemolytica and Pasteurella multocida associated with pneumonia of goats in Chhattisgarh. Vet World. 2019; 12: 331-6.
- Report F. Sub-Project Title: development of matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) for serotyping Pasteurella multocida Development of matrix-assisted laser desorption ionization Time -of – flight Mass Spectromet. June; 2016.
- Roier S, Fenninger JC, Leitner DR, Rechberger GN, Reidl J, Schild S. Immunogenicity of Pasteurella multocida and Mannheimia haemolytica outer membrane vesicles. Int J Med Microbiol. 2013; 303: 247-56.
- Rychert J. Benefits and limitations of MALDI-TOF mass spectrometry for the identification of microorganisms. J Infectiology. 2019; 2: 1-5.
- Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database (Oxford). 2020; 2020: 1-21.
- Schulthess B, Bloemberg GV, Zbinden A, Mouttet F, Zbinden R, et al. Evaluation of the Bruker MALDI biotyper for identification of fastidious Gram-negative rods. J Clin Microbiol. 2016; 54: 543-8.
- Singh K, Ritchey JW, Confer AW. Mannheimia haemolytica: bacterial-host interactions in bovine Pneumonia. Vet Pathol. 2011; 48: 338-48.
- Singhal N, Kumar M, Kanaujia PK, Virdi JS. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol. 2015; 6: 1-16.
- Tabatabaei M, Abdollahi AF. Isolation and identification of Mannheimia haemolytica by culture and polymerase chain reaction from sheep’s pulmonary samples in Shiraz, Iran. Vet World. 2018; 11: 636-41.
- Tadesse B, Alamirew K, Ketema A, Kiflie W, Endashaw M. Ruminant pneumonic pasteurellosis: review on epidemiology, pathogenesis and virulence mechanism. Acad J Anim Dis. 2017; 6: 30-9.
- Taunde PA, Argenta FF, Bianchi RM, de Cecco BS, Vielmo A, et al. Mannheimia haemolytica pleuropneumonia in goats associated with shipping stress. Cien Rural. 2019; 49: 1-6.
- To KN, Cornwell E, Daniel R, Goonesekera S, Jauneikaite E, et al. Evaluation of matrix-assisted laser desorption ionisation time-of-flight mass spectrometry (MALDI-TOF MS) for the Identification of Group B Streptococcus. BMC Res Notes. 2019; 12: 85.
- Tolera T, Wirtu A, Kumsa B, Zerabruk E, Albene Y, Tadesse F et al. Identification of serotypes Pasteurella multocida and Mannheimia haemolytica from cattle and sheep in central Ethiopia; 2019; 1-17.
- Torres-sangiao E, Leal Rodriguez C, García-riestra C. Application and perspectives of maldi–tof mass spectrometry in clinical microbiology laboratories. Microorganisms. 2021; 9: 1-19.
- Tran A, Alby K, Kerr A, Jones M, Gilligan PH. Cost savings realized by implementation of routine microbiological identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2015; 53: 2473-9.
- WeIser GC, DeLong WJ, Paz JL, Shafii B, Price WJ, et al. Characterization of Pasteurella multocida associated with pneumonia in bighorn sheep. J Wildl Dis. 2003; 39: 536-44.
- Wilson BA, Ho M. Pasteurella multocida: from zoonosis to cellular microbiology. Clin Microbiol Rev. 2013; 26: 631-55.
- Zaremba LS, Smolenski WH. Optimal portfolio choice under a liability constraint. Ann Oper Res. 2000; 97: 131-41.