
Research Article
Austin J Microbiol. 2024; 9(1): 1046.
High Fosfomycin Susceptibility in Escherichia Coli Recovered from Urine in Brazil
Fernanda Fernandes dos Santos1; Uile Paranhos5#, Tiago Barcelos Valiatti1,2; André Valêncio1; Yohanna Carvalho dos Santos Aoun Chikhani1; Carlos Alberto Franchi Júnior3; Adilson Aderito da Silva4; Ághata Cardoso da Silva Ribeiro1#
1Escola Paulista de Medicina, Universidade Federal de São Paulo – UNIFESP, São Paulo – SP, Brazil
2Faculdade de Educação de Jaru - Fimca-Jaru, Jaru – RO, Brazil
3Instituição Moura Lacerda, Ribeirão Preto – SP, Brazil
4Centro de Ciências Sociais e Aplicadas, Universidade Presbiteriana Mackenzie, São Paulo - SP, Brazil
*Corresponding author: Ághata Cardoso da Silva Ribeiro Escola Paulista de Medicina, Universidade Federal de São Paulo – UNIFESP, São Paulo – SP, Brazil. Email: aghata.cardoso@unifesp.br
Received: December 12, 2023 Accepted: January 27, 2024 Published: February 03, 2024
Abstract
Urinary Tract Infections (UTIs) are widespread globally, with a notably higher incidence in women. In 2018, the European Medicines Agency (EMA) endorsed the use of fluoroquinolones for uncomplicated UTI treatment. Following this recommendation, several international agencies adopted similar guidelines. Consequently, older antimicrobial agents like fosfomycin emerged as primary treatment options for UTIs. In this context, our study aimed to evaluate the susceptibility of Escherichia coli strains from urine samples to various recommended UTI antibiotics. These strains were collected between January 2017 and July 2020 in São Paulo, Brazil. We utilized the disk-diffusion method for antimicrobial susceptibility testing, interpreting the results according to BRCAST/EUCAST guidelines. Out of the 86,957 urine cultures undertaken during this timeframe, 10,041 yielded E. coli isolates. Of these, 8,655 were tested against fosfomycin, with 99.0% (8,572 strains) found to be susceptible. Additionally, susceptibility rates for other drugs were as follows: nitrofurantoin (95.8%), amoxicillin/clavulanic acid (83.9%), ciprofloxacin (65.1%), norfloxacin (65.6%), and levofloxacin (67.7%). Notably, of the 571 ESBL-positive strains, 94.0% were susceptible to fosfomycin. It’s important to mention a slight decline in fosfomycin susceptibility observed during this period. This finding underscores the importance of continuous monitoring for fosfomycin resistance and rational usage of the drug.
Keywords: Urinary tract infection; Fosfomycin; Fluoroquinolones; Escherichia coli.
Introduction
Urinary Tract Infections (UTIs) represent one of the most prevalent infections globally, impacting approximately 150 million individuals annually [4,20]. These infections can be anatomically categorized into lower (bladder, ureters, and urethra) and upper (kidneys) tracts. Additionally, UTIs can be defined based on symptomatic presence (symptomatic vs. asymptomatic) and the severity of the disease (complicated vs. uncomplicated). Symptomatic UTIs typically manifest with malaise, dysuria, increased urinary frequency, lumbar discomfort, and fever [2,18]. Women are more predisposed to UTIs, a susceptibility attributed to specific anatomical features of the genito-urinary tract. Factors like a shorter urethra and its proximity to the anus enhance the likelihood of urinary tract colonization by gastrointestinal microorganisms. Research indicates that 20-48% of women will experience a UTI at least once in their lives [10]. Of this cohort, 24% are anticipated to contract another UTI within the subsequent six months, with 2-5% presenting recurrent infections [3]. UTIs significantly affect morbidity; symptoms persist for an average of 6.1 days, leading to approximately 2.4 days of restricted activity and 0.4 days bedridden [18].
Bacteria, predominantly Escherichia coli, are the primary etiological agents behind UTIs (accounting for nearly 80% of cases) [6,7,14]. For years, fluoroquinolones were the preferred treatment for UTIs. However, growing concerns over bacterial resistance and documented adverse reactions led to recommendations against its use for uncomplicated UTIs, prompting the emergence of alternative therapeutic guidelines [1,5]. Fosfomycin-trometamol now stands as the primary recommendation for treating uncomplicated UTIs [3]. Fosfomycin impedes bacterial cell wall synthesis by irreversibly binding to the MurA enzyme, critical for generating bacterial cell wall components [9,12]. Given the evolving UTI treatment landscape and the surge in fosfomycin prescriptions, it's noteworthy that resistance rates to this drug in E. coli remain notably low [13,17,19]. Given these developments, it's imperative to conduct epidemiological studies detailing E. coli susceptibility to fosfomycin. This research seeks to elucidate the susceptibility patterns of E. coli strains obtained from urinary cultures associated with UTIs in São Paulo from January 2017 to July 2020.
Materials and Methods
Collection of Isolates
Urine samples were obtained from outpatients at a clinical laboratory located in the northwest region of São Paulo state. Between January 2017 and July 2020, a total of 86,957 urine cultures were carried out using CPS chromogenic agar. Subsequent bacterial identification was achieved through biochemical testing methods.
Antimicrobial Susceptibility Testing
The antimicrobial susceptibility of the isolates was determined using the Kirby-Bauer disk-diffusion method, according to the guidelines set by BRCAST (BRCAST, 2020). In brief, an inoculum standardized to 0.5 McFarland was swabbed onto the surface of Mueller-Hinton (MH) agar. Disks of different antimicrobial agents were then applied. The presence of the Extended Spectrum Beta-Lactamases (ESBL) phenotype was verified via the disk-approximation test. This utilized disks of ceftazidime, ceftriaxone, cefepime, cefotaxime, and amoxicillin/clavulanic acid. The formation of a "ghost-zone" was indicative of a confirmed ESBL phenotype (EUCAST, 2017).
Statistical Analysis
To evaluate the trend in antimicrobial susceptibility of the isolates over the 43-month period, time series analysis employing a linear regression model was used. The normality of each model was tested. Furthermore, a multinomial logistic regression was executed to compare the susceptibility rates among isolates for various agents: fosfomycin, ciprofloxacin, levofloxacin, norfloxacin, nitrofurantoin, and amoxicillin/clavulanic acid. All statistical analyses were conducted using Jamovi 1.8 software. For these analyses, a 95% confidence interval was established, and p-values of 0.05 or lower were deemed statistically significant.
Results
Out of the 86,957 urine cultures conducted, 10,041 E. coli clinical isolates were identified. Of these, 8,655 were assessed for susceptibility to fosfomycin, with 99.0% (8,572 isolates) showing susceptibility. The susceptibility rates for other tested antimicrobials, such as nitrofurantoin, amoxicillin/clavulanic acid, ciprofloxacin, norfloxacin, and levofloxacin, were 95.8%, 83.9%, 65.1%, 65.6%, and 65.7%, respectively, as visualized in Figure 1. Table 1 enumerates the susceptibility percentages for all the antimicrobials examined. Intriguingly, out of the 571 E. coli isolates identified as ESBL-positive, a notable 94.0% were susceptible to fosfomycin.
Antimicrobial agent
S
S %
I
I %
R
R%
NT
Amoxicillin/clavulanic acid
8.166
83.9
522
5.4
1049
10.8%
304
Ampicillin
4.895
49.5
139
1.4
4851
49.1%
156
Aztreonan
8.030
90.8
3
0
806
9.1%
1202
Cefazolin
7.272
82.3
331
3,7
1231
13.9%
1207
Cefotaxime
8.910
89.2
3
0
1080
10.8%
48
Ceftriaxone
8.891
89.1
2
0
1087
10.9%
61
Ceftazidime
8.515
89.7
3
0
976
10.3%
547
Cefepime
8.919
90.3
0
0
951
9.6%
164
Amikacin
8.880
89.2
844
8.5
235
2.4%
82
Tobramicin
7.669
84.0
367
4.0
658
7.2%
914
Gentamicin
8.308
92.6
444
4.9
939
10.5%
1066
Levofloxacin
6.499
65.7
59
0.6
3332
33.7%
151
Ciprofloxacin
6.481
65.1
59
0.6
3409
34.3%
92
Norfloxacin
6.530
65.6
59
0.6
3370
33.8%
82
Nitrofurantoin
9.563
95.8
134
1.3
286
2.9%
58
Sulfametoxazole/trimetroprim
6.642
66.4
61
0.6
3299
33.0%
39
Tetracyclin
5.402
60.2
55
0.6
3506
39.1%
1075
Fosfomycin
8.572
99.0
0
0
83
1.0%
1386
S, susceptible; R, resistant; I, susceptible increasing the exposure; NT, not tested.
Table 1: Susceptibility rates of E.coli to different antimicrobial agents.
Antimicrobial agent
b
p-value
Fosfomycin
-1.460
<0.001
Amoxicillin/clavulanic acid
- 1.030
<0.001
Nitrofurantoin
- 0.752
0.048
Ciprofloxacin
- 0.586
0.041
Norfloxacin
- 0.614
0.030
Levofloxacin
- 0.600
0.032
Table 2: Time series analysis of susceptibility rates to fosfomycin, amoxicillin/clavulanic acid, nitrofurantoin, ciprofloxacin, norfloxacin and levofloxacin.
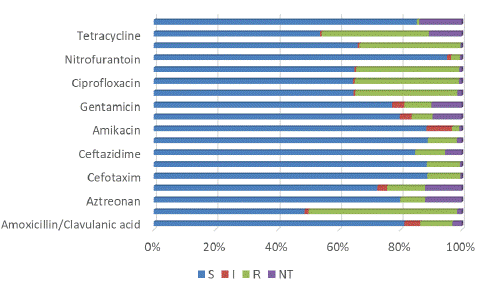
Figure 1:
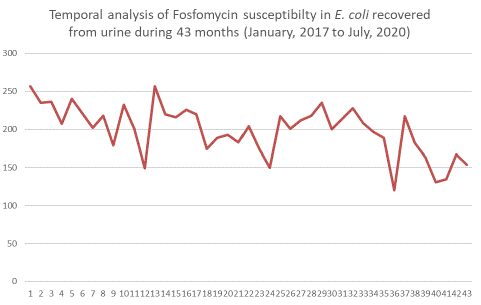
Figure 2:
Utilizing a linear regression within a time series analysis model, we noted a downward trend in the susceptibility rates for all the examined antimicrobials, including fosfomycin. A multinomial logistic regression was carried out to contrast the susceptibility rates of fosfomycin with other antimicrobials. The data suggest that the likelihood of isolates being resistant compared to susceptible was significantly lower for fosfomycin than for the other antimicrobials. Relative to fosfomycin, the probability of resistance was 54.31 times greater for ciprofloxacin (logOR=3.99; p-value<0.001), 53.29 times for norfloxacin (logOR=3.98; p-value<0.001), 52.94 times for levofloxacin (logOR=3.97; p-value<0.001), 13.26 times for amoxicillin/clavulanic acid (logOR=2.59; p-value<0.001), and 3.08 times for nitrofurantoin (logOR=1.13; p-value<0.001). These findings are comprehensively laid out in Table 3.
Predictor (Antimicrobial agente comparator -fosfomycin)
Estimate (logOR)
p-value
Odds Ratio (OR)
95% Confidence Interval
Lower
Upper
Resistant - Susceptible
Amoxacillin/clavulanic acid – Fosfomycin
2.59
< .001
13.26572
10.58741
16.6216
Ciprofloxacin – Fosfomycin
3.99
< .001
54.31795
43.58669
67.6913
Levofloxacin – Fosfomycin
3.97
< .001
52.94524
42.48282
65.9843
Nitrofuratoin – Fosfomycin
1.13
< .001
3.08843
2.41470
3.9501
Norfloxacin – Fosfomycin
3.98
< .001
53.2945
42.7646
66.4172
OR: Odds Ratio; p-value = < 0.05
Table 3: Probability of occurring resistant to susceptible isolates of each antimicrobial comparing to the same ratio obtained for fosfomycin.
Discussion
Fosfomycin has recently re-emerged as a viable therapeutic alternative for various infections, particularly uncomplicated UTIs. As we grapple with the escalating rates of antimicrobial resistance, the use of older antimicrobial agents has become an alternative option for treatment (Gardiner et al., 2019). Therefore, it's essential to conduct surveillance studies to gauge resistance levels and determine the continued viability of these agents in clinical settings. This study provided insights into the resistance patterns of E. coli isolates from São Paulo against multiple antimicrobials, with fosfomycin displaying a resistance rate of merely 1%.
UTIs are a prevalent community infections. For years, fluoroquinolones were the primary treatment choice. However, following the European Medicines Agency's 2019 advisory against their use for uncomplicated UTIs due to severe adverse effects and escalating bacterial resistance [5], there has been a decline in their prescription. This paved the way for alternatives like fosfomycin and nitrofurantoin [1]. A collective Brazilian recommendation, encompassing multiple medical societies, suggested fosfomycin-trometamol and nitrofurantoin as the primary treatment for cystitis, with cefuroxime or amoxicillin-clavulanate as secondary options [3].
In our study, we noted an impressive fosfomycin susceptibility rate of 99.0% the highest among all tested agents. Even among ESBL isolates, a 94.0% susceptibility to fosfomycin was observed. Comparable findings have been reported in Brazil and Switzerland, underscoring fosfomycin's potential as a frontline UTI treatment, especially given the minimal resistance observed even in ESBL-positive isolates [15,17].
However, it's worth noting a gradual decline in fosfomycin susceptibility (β=-1.46; p-value <0.001) in our data. This decline, albeit slower than other agents, reaffirm findings by Martínez et al. (2020), who linked increased fosfomycin consumption to rising resistance. The increased fosfomycin use, drove by changing UTI treatment guidelines [3,5], likely precipitated this trend. This diminishing susceptibility trend is evident across all antimicrobials in our study, even with different β-value, underlining the mounting challenge of antimicrobial resistance. This emphasizes the pressing need for vigilant surveillance and rational antimicrobial use. Comparatively, the likelihood of resistance in fosfomycin-treated isolates was the lowest among all agents evaluated, underscoring its current efficacy. Furthermore, single-dose prescriptions improve patient adherence, and a recent meta-analysis affirmed the equivalency of single-dose fosfomycin-trometamol with other UTI treatments concerning microbial and clinical outcomes in women [22].
Conclusion
This study underscores the pivotal role of fosfomycin in treating uncomplicated UTIs, particularly given the persistently low in vitro bacterial resistance to this drug. While various studies have attested to fosfomycin's clinical efficacy in managing uncomplicated UTIs, our findings specifically emphasize its superior standing in terms of resistance, displaying the lowest resistance rates among the evaluated agents. The gradual decline in fosfomycin susceptibility among E. coli isolates, coupled with limited Brazilian data on the topic, accentuates the urgent need for continuous surveillance of its susceptibility trends and rational application in the Brazilian medical landscape.
Author Statements
Acknowledgment
We would like to acknowledge the H. laboratory for suppling this study with the microbiological data and MsC. Paranhos, U. which dedicated time to the work conduction and supported it with collaborations.
References
- Cai T, Tamanini I, Tascini C, Köves B, Bonkat G, Gacci M, et al. Fosfomycin trometamol versus Comparator antibiotics for the Treatment of Acute Uncomplicated Urinary Tract Infections in Women: A Systematic Review and Meta-Analysis. J Urol. 2020; 203: 570-8.
- Dautt-Leyva JG, Canizalez-Román A, Acosta Alfaro LFA, Gonzalez-Ibarra F, Murillo-Llanes J. Maternal and perinatal complications in pregnant women with urinary tract infection caused by Escherichia coli. J Obstet Gynaecol Res. 2018; 44: 1384-90.
- de Rossi P, Cimerman S, Truzzi JC, Cunha CAD, Mattar R, Martino MDV, et al. Joint report of SBI (Brazilian Society of Infectious Diseases), FEBRASGO (Brazilian Federation of Gynecology and Obstetrics Associations), SBU (Brazilian Society of Urology) and SBPC/ML (Brazilian Society of Clinical Pathology/Laboratory Medicine): recommendations for the clinical management of lower urinary tract infections in pregnant and non-pregnant women. Braz J Infect Dis. 2020; 24: 110-9.
- Dubbs SB, Sommerkamp SK. Evaluation and management of urinary tract infection in the emergency department. Emerg Med Clin N Am. 2019; 37: 707-23.
- European Medicines Agency (EMA). Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics; 2018. Available from: https://www.ema.europa.eu/en/news/disabling-potentially-permanent-side-effects-lead-suspension-restrictions-quinolone-fluoroquinolone. 2023.
- Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015; 13: 269-84.
- Foxman B. Urinary tract infection syndromes. Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin N Am. 2014; 28: 1-13.
- Gardiner BJ, Stewardson AJ, Abbott IJ, Peleg AY. Nitrofurantoin and fosfomycin for resistant urinary tract infections: old drugs for emerging problems. Aust Prescr. 2019; 42: 14-9.
- Golla VK, Sans-Serramitjana E, Pothula KR, Benier L, Bafna JA, Winterhalter M, et al. Fosfomycin permeation through the outer membrane porin OmpF. Biophys J. 2019; 116: 258-69.
- Heilberg IP, Schor N. Abordagem diagnóstica e terapêutica na infecção do trato urinário: ITU [Diagnosis and clinical management of urinary tract infection]. Rev Assoc Med Bras (1992). 2003; 49: 109-16.
- Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012; 366: 1028-37.
- Kahan FM, Kahan JS, Cassidy PJ, Kropp H. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci. 1974; 235: 364-86.
- Keepers TR, Gomez M, Celeri C, Krause KM, Biek D, Critchley I. Fosfomycin and comparator activity against select Enterobacteriaceae, Pseudomonas, and enterococcus urinary tract infection isolates from the United States in 2012. Infect Dis Ther. 2017; 6: 233-43.
- Korbel L, Howell M, Spencer JD. The clinical diagnosis and management of urinary tract infections in children and adolescents. Paediatr Int Child Health. 2017; 37: 273-9.
- Pinheiro MS, Santos áPd, Rocha JLA, Santos Neto AG. Efetividade da fosfomicina contra Uropatógenos isolados em Uroculturas. Saúde Ambiente. 2019; 7.
- Martínez EP, van Rosmalen J, Bustillos R, Natsch S, Mouton JW, Verbon A. Trends, seasonality and the association between outpatient antibiotic use and antimicrobial resistance among urinary bacteria in the Netherlands. J Antimicrob Chemother. 2020; 75: 2314-25.
- Mueller L, Cimen C, Poirel L, Descombes MC, Nordmann P. Prevalence of fosfomycin resistance among ESBL-producing Escherichia coli isolates in the community, Switzerland. Eur J Clin Microbiol Infect Dis. 2019; 38: 945-9.
- Narciso A, Nunes F, Amores T, Lito L, Melo-Cristino J, Duarte A. Persistence of uropathogenic Escherichia coli strains in the host for long periods of time: relationship between phylogenetic groups and virulence factors. Eur J Clin Microbiol Infect Dis. 2012; 31: 1211-7.
- Shortridge D, Rhomberg PR, Bradford P, Ellis-Grosse EJ, Flamm RK. Activity against Gram-negative isolates from Eastern Europe collected by the SENTRY Antimicrobial Surveillance Program. ASM Microbe. 2018.
- Tamadonfar KO, Omattage NS, Spaulding CN, Hultgren SJ. Reaching the end of the line: urinary tract infections. Microbiol Spectr. 2019; 7.
- The jamovi project; 2020. Jamovi. version 1.8 [computer software]. Available from: https://www.jamovi.org.
- Wang T, Wu G, Wang J, Cui Y, Ma J, Zhu Z, et al. Comparison of single-dose fosfomycin tromethamine and other antibiotics for lower uncomplicated urinary tract infection in women and asymptomatic bacteriuria in pregnant women: A systematic review and meta-analysis. Int J Antimicrob Agents. 2020; 56: 106018.