
Research Article
Austin J Microbiol. 2024; 9(1): 1048.
Biofilm Formation and Disinfection on The Surface of Fermented Fish (Pla-Ra) Containers
Anggita Ratri Pusporini¹; Patimakorn Klaiprasitti¹*; Wannee Samappito²; Chanika Tianwitawat¹*
1Department of Food Technology, Faculty of Technology, Khon Kaen University, Thailand
2Department of Food Technology and Nutrition, Faculty of Technology, Mahasarakham University, Thailand
*Corresponding author: Patimakorn Klaiprasitti & Chanika Tianwitawat Department of Food Technology, Faculty of Technology, Khon Kaen University, Thailand. Email: chanika.tian@gmail.com
Received: January 17, 2024 Accepted: February 27, 2024 Published: March 05, 2024
Abstract
Pla-ra is one of Thailand’s most common lactic-fermented fish products. Fermented food was considered safe globally, but outbreaks of foodborne diseases have emerged. Biofilms produced on food processing equipment and other food-contact surfaces serve as a persistent source of contamination. Clostridium perfringens, Escherichia coli, Listeria innocua, and Staphylococcus aureus were isolated from the two types of containers most commonly used for fermenting pla-ra, made of clay and PE, from three representative pla-ra manufacturers in Khon Kaen province. The purpose of this study was to determine the ability of each of the pathogenic bacteria to form biofilms using a microplate assay and to find out how effective the disinfectants (chlorine and PAA) were at reducing the level of bacterial contamination through the artificial contamination method.Keywords: biofilm; formation; disinfection; fermented fish (pla-ra).
Introduction
In terms of fermented foods, fish is one of the most common products used as a fermented product. Southeast and East Asian countries are the leaders in this production [14]. In Thailand, a fermented fish product called pla-ra is the most popular lactic fermented fish product consumed as a condiment while eating papaya salad [26]. Even though known as a fermented product that is globally safe, Rattanasuk et al. (2015) revealed that from representative 20 samples of pla-ra in Roi Et province, Escherichia coli, Staphylococcus aureus, and Vibrio cholerae were found in the product by the rate 15 (75%), 20 (100%), and 3 (15%) of total samples, respectively. Some pla-ra products have also contained Clostridium perfringens and Salmonella spp [24]. Fish itself, however, is very perishable. It provides microorganisms with strong nutrient abundance coupled with a high water activity (aw) and moderate pH [23]. Besides, pla-ra making is classified as spontaneous fermentation, in which microorganisms on the raw materials are utilized for the direct fermentation process. It is difficult to control the environmental parameters that can lead to poor quality products [14]. Diverse microorganisms not only present in the fish and other ingredients used for making pla-ra, but also may exist in the food contact surface used during fermentation [13,28]. Among all foods, fermented products require the most extended time contact of the food with the fermenting equipment's surface, which can increase the potential of cross-contamination [13,17]. Multispecies bacteria can grow on food matrixes and along with food industry infrastructures. This growth may give rise to biofilm [9]. The biofilm development process is initiated with single cells attaching to a surface or each other, then followed by the formation of clustered cells or micro-colonies [2]. Over time, the micro-colonies are surrounded by a protective layer of protein-rich substances referred to as Extracellular Polymeric Substances (EPS) [28]. Previous research has suggested that almost all bacteria can form a biofilm and that once the transition from planktonic cells to their biofilm state is initiated, this becomes the optimum form for the existence of the bacterial cell [10,13,20].
Biofilm cells produce proteinaceous substances that allow synergic growth and protection from possible harsh environments it may encounter [2,15,20,28]. By such complex regulation systems, biofilm confers many advantages to the microbial cells in a food industry environment, such as physical resistance against desiccation, mechanical resistance such as liquid streams in pipelines, and chemical protection against antimicrobials and disinfectants used in the industry [9,13]. The age of biofilm, stress responses, or dormant cells are some of the factors that have been related to the increased resistance [19,28]. Of particular importance to the food industry is that some biofilm-forming species in food factory environments are human pathogens. These pathogens can develop biofilm structures on different artificial substrates common in the food industry, such as stainless steel, polyethylene, polypropylene, clay, wood, glass, rubber, and so on [3,9,13]
High diversity of the affected environments and the variety of colonizing bacterial species complicates biofilm eradication in the food industry and increase the risk of food contamination [28]. Especially in terms of the fermentation process, longer contact time is required and usually resulted in the transfer of more bacteria from surface to food. Even in only 300 s, bacterial transmission from food contact surface has happened [17]. Food-borne diseases associated with bacterial biofilms on food matrixes or factory equipment may arise via intoxications or infections [9,19]. Toxins, for example, can be secreted by biofilm found within food processing plants. From there, they can contaminate a food matrix, causing an individual or multiple intoxications [9]. Biofilm was involved 65% of all microbial diseases, according to NIH and the Centers for Disease Control and Prevention (CDC). Besides, most biofilm formation studies have revealed that they were resistant to commonly used sanitizers and disinfectants [13].
Many researchers have investigated regarding the microbiota, lactic acid bacteria, chemical and sensory analysis in the pla-ra product [14,24-26], however, there is no further study analyzed the potential of cross-contamination comes from biofilm that exists in pla-ra making containers. Besides, the fact that biofilm is commonly resistant to disinfectants urge this study to be conducted. The objective of the current study was to assess the ability of four pathogenic bacterial species from pla-ra making containers to form biofilm on 96 wells-plate and on the clay and polyethylene coupons, together with the biocide tolerance of the developed biofilms against two common food industry chemical disinfectants. Pla-ra substrate was also used to support the sessile development during the condition of artificial contamination on the coupons.
Materials and Methods
Bacterial Strains and Preparation of the Inocula
Four isolates consist of Clostridium perfringens, Escherichia coli, Listeria innocua, and Staphylococcus aureus were used in this study. All bacteria were collected from pla-ra making containers made from clay and polyethylene material from 3 different pla-ra manufacturers in Khon Kaen province, Thailand. The species identity was confirmed by 16s rRNA gene sequencing analysis (Kimura, 1980; Wang et al., 2008; Razzaq, 2013). Isolates maintained in Tryptone Soy Broth (TSB; Himedia Laboratories, LLC, India) with 15% glycerol stock were revived for incubation at 37°C for 24 hours (precultures) [8,23]. These temperatures were chosen to be closely to the optimum for quickly and successfully resuscitate the bacteria [23]. Working cultures were prepared by adding a 10-μl aliquot of each preculture to 10 ml of TSB and incubating for another 24 h at the appropriate temperature mentioned above. Cells from final workingcultures in stationary phase were harvested and re-suspended in sterile TSB. The bacterial suspensions of the four isolates were alsocombined and further diluted to yield mixed cultureinocula of approximately 107–8 CFU/mL, to be used for the subsequent artificial contamination study [10,23].
Categorization of Isolates Based on Biofilm-Forming Capacity
The heterogeneity in the biomass of the samples requires definition of a cut off value that would divide the samples in non-adherent, weak, moderate, and strong adherent. For this reason, all samples were tested in triplicate and calculated the OD average using negative controls (medium without inoculum).
The cut off value was defined for each species. The following criteria were used for biofilm gradation in clinical isolates (Singh et al., 2017) [4].
ODcut = ODavg of negative control + 3 × standard deviation (SD) of ODs of negative control.
OD = ODcut = Non-Biofilm-Former (NBF)
ODcut < OD = 2 × ODcut = Weak Biofilm-Former (WBF)
2 × ODcut < OD = 4 × ODcut = Moderate Biofilm-Former (MBF)
OD >4 × ODcut = Strong biofilm-former.
Biofilm Formation Assay
Wells of 96-wells sterile polystyrene plates were each filled with 90 μL of another sterilized TSB and inoculated with 10 μL of the prior working cultures to develop biofilms on the surfaces of the microtiter plates [7,8]. Negative control wells containing only TSB were included in the assay. Each pathogenic bacterial strains was incubated at 37oC for 0, 6, 12, 24, 48 and 72 h. Removal of the culture medium from the microtiter plates were done three times by inverting the plates and shaking out the liquid and then gently submerged in a small tub of distilled water to wash off any remaining unbound cells or medium components. After air-drying in a laminar flow, wells were stained with 50 μL of 0.5% Crystal Violet (CV) for 5 min. Excess stain was removed by the same prior treatment for washing the plates five times with distilled water. Dye bound to adherent cells was de-stained by pipetting 50 μL of 95% ethanol. The concentration of crystal violet was determined by measuring the optical density at 595 nm (CV-OD595 value) using a spectrophotometer (SPECTROstar Nano, BMG Labtech, Germany) [7,21].
Artificial Contamination
The two mL of previous overnight working cultures(107–8 CFU/mL) of mono-species and mixed-species were transferred to each sterile clay and polyethylene coupons surfaces and evenly spread in perpendicular directions with a sterile cotton swab. To study the effect of pla-rasolution which accumulated during fermentation, the model substrate for the condition of with and without pla-ra solution were prepared. Pla-ra was bought from the same manufacturers took place for the isolation. The substrate was prepared as 50% pla-ra suspensions by mixing 50 mL of the pla-ra solution and 50 mL of sterile distilled water [23]. All the coupons were incubated under controlled temperature (37°C) in an incubator for 6, 12, 24, 48, or 72 hours based on the result of the highest biofilm production for each pathogenic bacteria [7,10,23].
Disinfection Treatment
Two common disinfectants were used in this study: (i) chlorine based disinfectant (Sodium Hypochlorite) (Vittayasom Sriracha Co., Ltd., Thailand), and (ii) Peracetic acid based disinfectant (Calgonit DS 658) (Calvatis Asia Pacific Co., Ltd, Thailand) Both disinfectants were used in concentrations advised by the manufacturer’s instructions: 200 mg/L (ppm) for chlorine suspension on food contact surface (22.72 g in 1000 mL sterile distilled water) and 400 mg/L (ppm) for the PAA suspension (500 mL of solution in 1250 mL of sterile distilled water). Artificial contamination was performed on both clay and polyethylene coupons surfaces with incubation under controlled temperature at 37 °C. All coupons were soaked into chlorine solution and PAA solution for 10 min based on the disinfection procedure. After the exposure time attained, pre-soaked cotton swab in TSB was used to detach the bacterial cells on the surface and was transferred to 10 mL of sterile TSB followed by homogenizing step for 10 sec on vortex mixer (Labnet International, Inc., USA).
Bacterial Quantification
Enumeration of the pathogenic bacteria in TSB after the artificial contamination and disinfection treatment was performed by growing the inoculates on each specific agar plates using sterile spreaders. The plates were incubated for 24-48 h at 37°C and the specific colonies were counted. The killing effect of each disinfectants was calculated by the reduction between the initial incubated coupons (artificial contamination treatment) and the coupons exposed to disinfectant streatment (Log CFU/cm2) [23].
Results and Discussion
The Estimation of Biofilm Formation of Pathogenic Bacteria by Microtiter Plate Biofilm Assay
All of the pathogenic bacteria (Clostridium perfringens, Escherichia coli, Listeria innocua, and Staphylococcus aureus) were tested for the biofilm formation, performed by 96-well microtiter plate assay. Two types of containers used for fermentation in most pla-ra manufacturers were clay as the common and traditional one, then Polyethylene (PE). The OD values of biofilm formation and each bacteria ability of each pathogenic bacterium to produce biofilms are shown in Table 1 and Table 2.
Incubation time
Optical Density of Biofilm
C. perfringens
E. coli
L.innocua
S. aureus
0
0.089a±0.04
0.132a±0.04
0.111a±0.03
0.121a±0.01
6
0.205a±0.02
0.155a±0.05
0.164a±0.04
0.195ab±0.02
12
0.655b±004
0.580a±0.02
0.561a±0.02
0.407cd±0.02
24
0.522ab±0.03
0.963a±0.04
0.985a±0.03
0.477d±0.02
48
0.357ab±0.04
1.053a±0.05
0.966a±0.02
0.390cd±0.01
72
0.358ab±0.03
0.913a±0.04
0.883a±0.04
0.298bc±0.04
Table 1: Optical density of biofilm formation of pathogenic bacteria on clay surface from 3 different manufacturers.
Incubation time
Optical Density of Biofilm
C. perfringens
E. coli
L.innocua
S. aureus
0
0.117a±0.02
0.146a±0.02
0.129a±0.04
0.170a±0.02
6
0.329a±0.03
0.404a±0.01
0.366a±0.01
0.243ab±0.03
12
0.367a±0.02
0.529a±0.02
0.550a±0.03
0.282bc±0.03
24
0.365a±0.03
0.628a±0.04
0.572a±0.03
0.278bc±0.02
48
0.372a±0.03
0.768a±0.02
0.668a±0.01
0.491d±0.03
72
0.247a±0.02
0.823a±0.01
0.714a±0.02
0.369c±0.03
Table 2: Optical density of biofilm formation of pathogenic bacteria on polyethylene surface from 3 different manufacturers.
Based on Charlebois et al. (2014) study, Clostridium perfringens biofilm growth appeared to be strong from the 6 hours, after that begins to decline after 24 hours of incubation. Whereas Vidal et al. (2015) research reported that the spectrum of gene expression that stimulates biofilm growth has continued to grow insignificantly for the next 24 hours. Several antibiotics, administered as feed additives, are approved for treating intestinal infections, such as necrotic enteritis caused by Clostridium perfringens. Clostridium perfringens is an opportunistic bacterial pathogen that can cause food poisoning in humans and various enterotoxemia in animal species because of its ability to produce many different toxins and extracellular enzymes. These bacteria are obligate anaerobes but are found widely in soil and animal guts because of their ability to form spores.
For Escherichia coli biofilm, it was recognized that the biofilm development on the clay surface started to decrease at 72 h. While on the PE surface, the graph continues to show an increase in biofilm production up to 72 hours. Besides, in another study, Ma et al. (2019) found that the most prominent growth in E. coli biofilms was seen until 24 hours, whereas the findings found by Nakao et al. (2018) and Schiebel et al. (2017) declared that the growth of E. coli biofilms continued to increase until 48 hours and began to decrease in the following hours. Escherichia coli are Gram-positive bacteria isolated from fresh produce processing facilities. The process of formation of E. coli bacteria on the surface is usually found on microtiter plates, silicone rubber, and glass surfaces.
As for Listeria innocua, Figure 3 indicated that the Listeria innocua biofilm on the clay surface had the highest production at 24 hours, after which it began to decline but not considerably. The development of biofilm on the PE surface showed growing results until 72 hours. Lezzoum-Atek et al. (2019) also observed that L. innocua biofilm continued to grow at the first 72 hours, up to 6 days of incubation. Koo et al. (2014) obtained similar results where Listeria monocytogenes and Listeria innocua were each grown on steel and aluminum surfaces. Biofilm production was increased up to 72 hours for both bacteria. Listeria innocua is a non-pathogenic Listeria species found in similar environments to L. monocytogenes. L. monocytogenes is a Gram-positive facultative anaerobe that grown under static conditions such as in various natural environments (soil, water, and vegetation), in food processing environments and in Ready-To-Eat food products (RTE). Generally composed of homogeneous layers of cells and/or microcolonies, with biofilm cells displaying a morphology similar to that of planktonic cells.
As for Staphylococcus aureus, the most prominent biofilm growth was seen at 24 hours incubation and thereafter biofilm creation declined significantly on the clay surfaces. While on the PE surface, biofilm production continued to rise to 48 hours and then started to decline afterwards. Based on Figure 4, previous research of Rodriguez-Lazaro et al. (2018) and Peng et al. (2018) observed the growth of S. aureus biofilm on polystyrene microplates and showed that biofilm development increased over 48 hours and then subsequently decreased. Additionally, Periasamy et al. (2012) also observed S. aureus biofilm in rat catheters and the highest production occurred in the first 24 hours. Studies have demonstrated that almost all biofilm-producing bacteria mature within 24-72 hours (Fallatah et al., 2019; Lezzoum-Atek et al., 2019; Chua et al., 2014).
Staphylococcus aureus biofilms grown under both static and continuous flow conditions consist of a dense layer of cells with an elaborate matrix harboring various types of polymers. Biofilm formation and maturation in S. aureus is dependent on the interplay between various regulators including those encoded by sara, agr, ica, and sigB. While the process of forming S. aureus bacteria on the surface is usually found on 96-well polystyrene microtiter plates [1].
Furthermore, the same pattern result was attained in isolated bacteria from polyethylene surfaces. All bacterial biofilm OD increased after 24 hours until 72 hours. Some are decreased after 48 hours though the statistical analysis indicated no significant difference. However, the OD value for each isolated bacterium from polyethylene is distinctly lower compared to OD value from clay surface. There is strong evidence supporting the conclusion that bacterial adherence and biofilm formation increase with the roughness of the implant surface (Noble et al., 2018). A study conducted by Karygianni et al. (2013) found that Enterococcus faecalis, Staphylococcus aureus, and Candida albicans adhered more to a rougher implant surface relative to a smoother surface. Furthermore, Braem et al. (2014) also demonstrated that a porous surface coating was more susceptible to biofilm formation than a smoother titanium-based surface after exposure to S. aureus and S. epidermidis. Interestingly, all bacteria isolated from the surface of the polyethylene container consistently showed higher biofilm development at 48 hours and were categorized as moderate biofilm-forming, except for S. aureus at 24 hours which was classified as weak biofilm-forming.
Meanwhile, the highest biofilm-forming clay surface reported was E. coli at 48 hours, other bacteria isolated from the surface of the clay container showed significantly higher biofilm development at 24 hours and was categorized as moderate biofilm forming, and for weak biofilm formers it is found in C. perfringens at 48 hours. On top of that, while classified based on the ability of each pathogenic bacterium to produce biofilms, the strongest biofilm formers in both clay and polyethylene surfaces were reported to be Listeria innocua and E. coli. Lezzoum-Atek et al. (2019) also found that Listeria species and E. coli could adhere and grow well to polystyrene and form biofilms under different conditions.
It is well documented that each food and food contact surface consists of a specific niche and contains a wide variety of microorganisms. For the formation of bacteria Clostridium prefringens on the surface usually grows in the environment such as soil and animal intestines because of its ability to form spores. For the formation of Escherichia coli bacteria on the surface, it is usually found on microtiter plates, silicone rubber, baby carrots, and on glass surfaces. For the formation of the bacteria Listeria innocua on surfaces it is commonly found in a variety of natural environments (including soil, water, and vegetation), in food processing environments, and in Ready-To-Eat food (RTE) products. As for the formation of Staphylococcus aureus bacteria on the surface, it is usually found on polystyrene micro plates, polypropylene coupons, and on glass fiber filters [13].
Artificial Contamination of Pathogenic Bacteria Biofilm and Resistance to Disinfectants on Clay and Polyethylene Surfaces
To determine the effect of disinfectants on the bacterial survival for each pathogenic bacterium, mimicking condition were performed by purposely contaminating clay and polyethylene container surfaces with one-night incubated cultures of each pathogenic bacterium. Both clay and PE surfaces were cut into pieces (10×10 cm2) and added with 2 ml bacterial culture for 24 hours of incubation time to produce biofilm formation. Thereafter, all of the coupons were applied with two types of disinfectants for 10 minutes.
Disinfectants used in this experiment were chorine-based disinfectant (sodium hypochlorite; 200 ppm) and PAA-based disinfectant (Calgonit DS 658; 400 ppm). As to the results in Figure 7 and Figure 8, it showed that killing activity between chlorine and PAA were not significant different. Both disinfectant in each clay and the polyethylene coupon can reduce the 5 to 6 log reduction for all the pathogenic bacteria. It can be seen in the figure below which is the result of the initial contamination and disinfection treatment that is shown in Figures 3 and Figure 4 for clay and polyethylene coupon surfaces. Chlorine is the most widely employed disinfectant to treat wastewater before it is discharged into receiving water bodies worldwide. Chlorine is widely known for being low cost, utilized in common basic technology, and has proven efficiency in inactivating a great variety of pathogenic microorganisms. However, the awareness of harmful by-products and the formation of chlorination-resistant bacteria strains has caused wastewater plants to consider other options. The main alternatives to chlorination are ozonation, ultraviolet light, and peracetic acid (PAA).
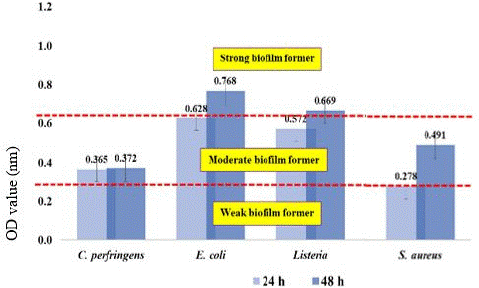
Figure 1: Classification of the ability of bacteria to form biofilms as weak (OD595<0.323), moderate (OD595=0.324 - 0.648), and strong biofilm forming (OD595>0.648) isolated from the surface of the PE container.
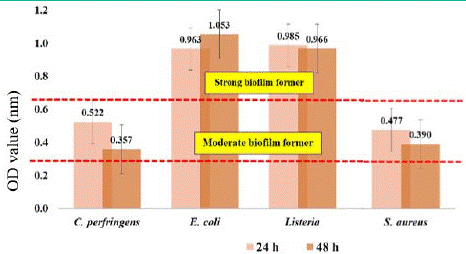
Figure 2: Classification of the ability of bacteria to form biofilms as weak (OD595<0.323), moderate (OD595=0.324 - 0.648), and strong biofilm forming (OD595>0.648) isolated from the surface of clay containers.
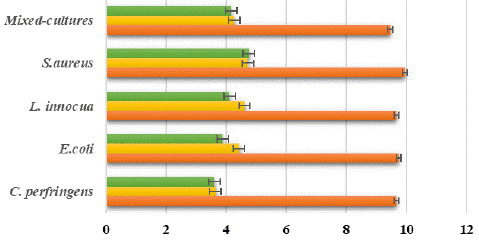
Figure 3: Log reduction bacteria after exposure to a disinfectant (chlorine and PAA based) for 10 minutes on the surface of the clay coupon.
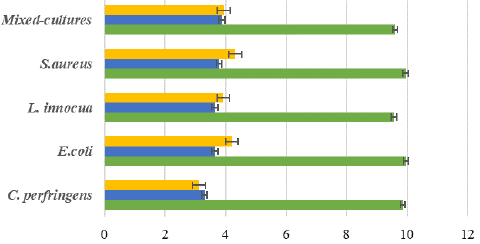
Figure 4: Log reduction bacteria after exposure to a disinfectant (chlorine-based and PAA) for 10 minutes on the surface of the polyethylene coupon.
PAA is an oxidizing agent that can dissolve in water into hydrogen peroxide and acetic acid, which can further decompose into water, oxygen, and carbon dioxide. PAA by products are non-toxic, while only negligible or low levels of aldehydes (Banach et al., 2015; Van Haute et al., 2015) and modest levels of carboxylic acids have been reported to form (Dominguez Henao et al., 2018). It has a unique oxygen or oxygen bond arrangement that rapidly releases oxygen to destroy bacteria and oxidize unwanted odors (hydrogen sulfide) and compounds. When PAA is applied to a process, it rapidly degrades to harmless products, acetic acid (a component found in table vinegar), and water. Commercially available PAA consists of a quaternary mixture of acetic acid, hydrogen peroxide, peracetic acid and water. It has strong oxidizing properties and is active against enteric bacteria and to a lesser extent against viruses, bacterial spores, and protozoan cysts. One of the main advantages of PAA is the possibility of easy retrofitting of sodium hypochlorite disinfection equipment, which is common in existing Waste Water Treatment Plants (WWTPs), thereby avoiding costly and structural interventions. In addition, PAA can produce sustainable, safe and effective decomposition against a wide spectrum of microorganisms. These benefits in particular can support the spread of PAA disinfection technology in various industries.
Additionally, in order to differentiate and evaluate the effect food residues which possibly remained on the food containers, 50% pla-ra substrate was also added to the coupons. With the addition of pla-ra substrate on each contaminated coupon, it showed lower reduction of bacterial survival in all pathogenic bacteria. All the bacteria profiling reduction shown for only 2 log reduction both in clay and PE coupons. This proved many priors research conducted with the effect of food residues in biofilm formation (Paz-Mendez et al., 2017).
Organic charge, surface topography, temperature, relative humidity, pH, water hardness or other chemicals are all important environmental factors to consider. Organic matter creates a physical barrier shielding microorganisms from disinfectant contact. Debris and organic material can also neutralize many chlorines and iodine-containing disinfectants [5]. In addition, chlorine reacts with organic matter to form carcinogenic trihalomethanes (Brown et al., 2011) and chlorates (Gil et al., 2016), potentially hazardous substances that may form (e.g., trihalomethane and haloacetic acid) and their effects on public health. This explains why chlorine was less successful than PAA by adding pla-ra substrate onto the coupons during the incubation time. By the result in this section, it also indicated that the results were unsurpassed the safe level of pla-ra microbiological criteria based on Thai Agricultural Standard regulation.
Lastly, four pathogenic bacteria could be found from both surfaces of the pla-ra container. These all bacteria were tested for biofilm formation using a 96-well microtiter plate assay. The two types of containers used for fermentation are clay and polyethylene. The results showed that all the pathogenic bacteria could form biofilms, especially Listeria innocua and Escherichia coli, classified as solid biofilm formers (OD595>0.648). Chlorine-based disinfectants (sodium hypochlorite; 200 ppm) PAA-based disinfectants (Calgonit DS 658; 400 ppm) were used in the study. Organic matter creates a physical barrier shielding microorganisms from disinfectant contact; hence, it showed a lower reduction of bacterial survival with pla-ra substrate. However, both disinfectants can reduce the inoculated bacteria on the coupon surfaces up to 5 log CFU/10×10 cm2 by the end of the exposure time. In this experiment, the efficacy between chlorine-based solution (sodium hypochlorite) and PAA-based solution (Calgonit DS 658) showed no significant difference during the artificially contaminated conditions. However, PAA performed better to successfully remove the prior pathogenic bacteria due to its different mechanism compared to chlorine. Proper hygiene and consistent sanitation practices are required in the pla-ra industry to lower the possibility of an outbreak due to some pathogenic biofilm contamination.
Author Statements
Acknowledgment
On behalf of all authors, the corresponding author states that there is no conflict of interest. Moreover, this manuscript does not receive any fund.
References
- Abee T, Kovács AT, Kuipers OP, Veen SVD. Biofilm formation and dispersal in Gram-positive bacteria. Current Opinion in Biotechnology. 2010; 22: 133–308.
- Annous BA, Fratamico PM, Smith JL. Quorum Sensing in Biofilms: Why Bacteria Behave the Way They Do. Journal of Food Science. 2008; 74: 24–37.
- Bridier A, Sanchez-Vizuete P, Guilbaud M, Piard JC, Naïtali M, Briandet R. Biofilm- associated persistence of foodborne pathogens. Food Microbiology. 2015; 45: 167–78.
- Cepas V, López Y, Muñoz E, Rolo D, Ardanuy C, Martí S, et al. Relationship Between Biofilm Formation and Antimicrobial Resistance in Gram-Negative Bacteria. Microbial Drug Resistance. 2019; 25: 72–9.
- CFSPH (Center for Food Security and Public Health. Disinfection. 2008; 101.
- Charlebois A, Jacques M, Archambault M. Biofilm formation of Clostridium perfringens and its exposure to low-dose antimicrobials. Front Microbiology. 2014; 5: 183–205.
- Choi NY, Bae YM, Lee SY. Cell surface properties and biofilm formation of pathogenic bacteria. Food Science and Biotechnology. 2015; 24: 2257–64.
- Doijad SP, Barbuddhe SB, Garg S, Poharkar KV, Kalorey DR, Kurkure NV. Biofilm-Forming Abilities of Listeria monocytogenes Serotypes Isolated from Different Sources. PLoS ONE. 2015; 10: 137–51.
- Galié S, García-Gutiérrez C, Miguélez EM, Villar CJ, Lombó F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front Microbiology. 2018; 9: 898–904.
- Heir E, Møretrø T, Langsrud SSA. Listeria monocytogenes strains show large variations in competitive growth in mixed culture biofilms and suspensions with bacteria from food processing environments. International Journal of Food Microbiology. 2018; 275: 46–55.
- Hydritecom. PERATIC ACID SOLUTIONS. 2021.
- Banach JL, Veen HB, Overbeek L, Zouwen P, MH Zwietering H der F-K. Effectiveness of a peracetic acid solution on Escherichia coli reduction during fresh-cut lettuce processing at the laboratory and industrial scales. International Journal of Food Microbiology. 2020; 321: 128–36.
- Jahid I, Ha S. The Paradox of Mixed-Species Biofilms in the Context of Food Safety. Comprehensive Reviews in Food Science and Food Safety. 2014; 13: 990–1011.
- Zang J, Xu Y, Xia W, Regenstein JM. Quality, functionality, and microbiology of fermented fish: a review. Critical Reviews in Food Science and Nutrition. 2020; 60: 1228–42.
- Kokare C, Chakraborty S, Khopade A, Mahadik K. Biofilm: Importance and applications. Indian Journal of Biotechnology. 2009; 8: 159–68.
- Kostova RS D, Goranov BG, Teneva DG, Tomova TG, Denkova ZR, Shopska V, et al. Bio-Preservation of Chocolate Mousse with Free and Immobilized Cells of Lactobacillus Plantarum D2 And Lemon (Citrus Lemon L.) Or Grapefruit (Citrus Paradisi L.) Zest Essential Oils. Acta Scientiarum Polonorum Technologia Alimentaria. 2021; 20: 5–16.
- Miranda RC, Schaffner DW. Longer contact times increase cross-contamination of Enterobacter aerogenes from surfaces to food. Journal of Applied and Environmental Microbiology. 2016; 82: 6490–6.
- Mohan V, Wibisono R, Hoop LD, Summers G, Fletcher GC. Identifying Suitable Listeria innocua Strains as Surrogates for Listeria monocytogenes for Horticultural Products. Front Microbiology. 2019; 10: 251–73.
- Møretrø T, Langsrud S. Residential bacteria on surfaces in the food industry and their implications for food safety and quality. Journal of Comprehensive Reviews in Food Science and Food Safety. 2017; 16: 1022–41.
- Neelakantan P, Romero M, Vera J, Daood U, Khan AU, Yan A, et al. Biofilms in Endodontics-Current Status and Future Directions. International Journal of Molecular Sciences. 2017; 18: 1714–48.
- O’Toole GA. Microtiter Dish Biofilm Formation Assay. Journal of Visualized Experiments. 2011; 47: 1–2.
- Obana N, Nakamura K, Nomura N. Temperature-regulated heterogeneous extracellular matrix gene expression defines biofilm morphology in Clostridium perfringens. Nature Journal: Biofilms and Microbiomes. 2020; 6: 29–42.
- Papaioannoua E, Giaouris ED, Berillisa P, Boziaris IS. Dynamics of biofilm formation by Listeria monocytogenes on stainless steel under mono-species and mixed-culture simulated fish processing conditions and chemical disinfection challenges. Internationfal Journal of Food Microbiology. 2018; 267: 9–19.
- Prakhongsil P, Phianphak W, Malakrong A, Komolamisra C. Decontamination of Clostridium perfringens and Salmonella spp. in Thai Fermented Fish (Pla-ra) by Gamma Radiation. International Nuclear Science and Technology Conference Abstract. 2014; 119.
- Rattanasuk S, Boonbao J, Sankumpa S, Suraslip T. Foodborne pathogens in fermented fish purchased in Selaphum, Roi Et. Vol. 1. International Conference of Science and Technology. 2015.
- Sangjindavong M, Chuapoehuk P, Runglerdkriangkrai J, Klaypradit W, Vareevanich D. Fermented fish product (pla-ra) from marine fish and preservation. Kasersart Journal. 2008; 42: 129–36.
- Shen X, Sheng L, Gao H, Hanrahan I, Suslow TV, Zhu MJ. Enhanced Efficacy of Peroxyacetic Acid Against Listeria monocytogenes on Fresh Apples at Elevated Temperature. Front Microbiology. 2019; 10: 1173–96.
- Srey S, Jahid IK, Ha S. Biofilm formation in food industries: a food safety concern. Journal of Food Control. 2013; 31: 572–85.