
Research Article
Austin J Microbiol. 2024; 9(2): 1051.
The Essential Oil of Cymbopogon Citratus Can Significantly Inhibit the Mycelia Growth, Conidia Germination of Mycotoxigenic Aspergillus Isolated from Smoked Fish (Ethmalosa Fimbriata) and Protect Against Mold Contamination
Bijoux Sandra Kamgue Komyum¹; Sammuel Raymond Tchabong²; Victor Davy Moussango¹; Francioli Koro Koro¹, Modeste Lambert Sameza¹*; Pierre Michel Dongmo Jazet¹
1Department of Biochemistry, Faculty of Science, University of Douala, Cameroon
2Department of processing and Quality Control of Aquatic Products, Institute of Fisheries and Aquatic Sciences, University of Douala, Cameroon
*Corresponding author: Modeste Lambert Sameza Department of Biochemistry, Faculty of Sciences, University of Douala, P.O. Box 24 157, Douala, Cameroon. Email: samezamste@yahoo.com
Received: March 29, 2024 Accepted: April 30, 2024 Published: May 07, 2024
Abstract
Ethmalosa fimbriata is a fish recognized for its high nutritional value and its beneficial effect on the health of consumers. In order to ensure a permanent supply throughout the year, this fish is dried or smoked and store. Despite that, Ethmalosa fimbriata is often colonized by molds especially mycotoxigenic Aspergillus spp. To reduce contamination, peoples commonly use chemicals that however have a negative environment impact and may be harmful to human and animal health. The aim of this study was to evaluate the bio activity of Cymbopogon citratus essential oil against mycotoxigenic Aspergillus from smoked Ethmalosa fimbriata and to protect this fish against mold. Fish samples were purchased in Douala markets, fungi isolated by dilution plate method and identified on the basis of their macroscopic and microscopic characteristics. Oil was extracted by Hydro-distillation and analyzed by gas chromatography coupled with mass spectrometry. The antifungal activity was evaluated by the agar incorporation method and microdillution. As results, the oil yield obtained was of 0.45% with 32 compounds among which geranial (39.66%), neral (30.77%) and a-fenchene (14.14%) were major components. Three mycotoxigenic fungi were isolated and identified as Aspergillus parasiticus, Aspergillus glaucus and Aspergillus flavus. C. citratus essential oil completely inhibited the mycelial growth at 595 ppm, 1195 ppm and 1295 ppm respectively, for these mycotoxigenic Aspergillus. Moreover, the total conidia germination of these fungi was achieved at 100 ppm. Whether it be preventive or curative test, fish on which essential oil was applied did not show any visible signs of contamination after 18 days of incubation compare to control fish on which visible mycelia grow and conidia were observed just after 3 days of incubation. Any attempt to reisolate fungi from treated fish failed especially for preventive test. These results show that essential oil of Cymbopogon citratus could be used to formulate a mycobiocide for smoked Ethmalosa fimbriata preservation.
Keywords: Cymbopogon citratus; Essential oil; Aspergillus; Antifungal activity; Mycotoxins; Smoked fish
Abbreviations: C.c: Cymbopogon citratus; CEA: Coconut Extract Agar; DMSO: Di Methyl Sulphur Oxide; EO: Essential Oil; GPS: Global Positioning System; GS-MS: Gas Spectrometry-Mas Spectrometry; MFC: Minimal Fungicidal Concentration; MIC: Minimal Inhibitory Concentration; PDA: Potatoes Dextrose Agar; PDB: Potatoes Dextrose Broth; SDA: Sabouraud Dextrose Agar; UV: Ultra-Violet
Introduction
Ethmalosa fimbriata is a fish known as Bonga. It is an important source of animal proteins, vitamins, minerals and essential fatty acids [1]. The farming of this fish is a common occupation of peoples living in the coastal areas and along major river banks in Cameroon where fish consumption was around 20 kg/habitant/year [2-4]. Despite the high importance, the post-fishing losses are estimated at 27% and represented 3,4 billion/year [5]. In fact, like other fishes Ethmalosa fimbriata is a rapid perishable product and to reduce losses, peoples use traditional conservation processes. In Cameroon for instance, 75 to 80% of collected fish is smoked or dried [6]. Despite these treatments, smoked dried fish is frequently altered by many fungi such as members of the genus Aspergillus especially when stored in an unsuitable environment [7]. Previous survey carried out in some local markets in Cameroon revealed that Aspergillus species where the most associated fungi with fish deterioration [8]. These fungi are well known to decrease nutritional and commercial value of fish [9] and to their capacity to produce mycotoxins who can cause multiple health problems to human and animals, including digestive cancers, immunologic and allergic responses [10]. To reduce smoked fish contamination, peoples commonly use chemical synthesized organic acids like methyl bromide and phosphine [11]. However, these chemicals have a negative environment impact and may be harmful to human and animal health [12]. Therefore, additional ecofriendly control methods of this spoiling mold are essential. In this respect, plants and their essential oils have been evaluated as natural sources of compounds for food preservatives due to their antimicrobial and antioxidant effects [13]. Cameroon has a very rich flora with many aromatic plants which possess various biological activities [14]. Among them Cymbopogon citratus is used for many purposes in African medicine [15]. In certain households in Cameroon, leaves of these plants are used to protect stored food products including smoked fish. The aim of this study was to evaluate the antifungal activity of Cymbopogon citratus essential oil against mycotoxigenic Aspergillus from smoked Ethmalosa fimbriata and to protect this fish against mold.
Materials and Methods
Plant Material and Fish Samples
Fresh leaves of Cymbopogon citratus were collected early at morning time in a farm located in Yassa (GPS coordinates: 4.03151, 97214). They were identified at the National Herbarium of the institute of agricultural sciences for development under the number SK 2158/13.
Smoked samples of Ethmalosa fimbriata, used in this study were purchased on August 2021 in four different Douala markets: Dakar, Deido, Central market and Sandaga. These markets were chosen base on their high flow of peoples and of fish distribution. Samples were transported in sterile plastic bags to the laboratory where fishes were ground into powder.
Essential Oil Extraction
Fresh leaves of Cymbopogon citratus essential oil was obtained by hydro distillation during four hours using Clevenger type apparatus. Essential oil recovered was dried over anhydrous Na2SO4, sealed vials and stored in refrigerator at 4°C until use. The yield of extracted essential oil was calculated by the formula: yield (%) = [essential oil obtained (g) / vegetal material used (g)]×100.
GC-MS Analysis of Essential Oil
GC-MS analyses were carried out using a Hewlett Packard 5570 GC-MS system operating in the electron impact mode at 70 eV, equipped with a HP 1 fused silica column (30 m x 0.25 mm, film thickness 0.25 μm) and interfaced with a quadrupole detector. The initial temperature of the column was 70°C, followed by heating to 200°C with a 10°C/min. rate. Carrier gas was He (flow rate 0.6 ml/min). The identification of the components was based on the comparison of their mass spectra with those of Wiley, NIST, Libraries and those described by Adams [16], as well as by comparison of their retention indices with literature values.
Fungal Isolation and Morphological Identification
Fungi were isolated by using dilution plating method. Briefly, 10 g of smoked fish powder were added to 90 ml of sterile peptone water (0.1%) and thoroughly mixed. Further, 10-fold serial dilutions up to 10 were made. One ml volume of each dilution was separately poured in Petri dishes containing 15 ml of Potato Dextrose Agar (PDA) – Chloramphenicol; Difco, USA. Plates were incubated at 28±2°C and monitored daily for any mycelia grow [17,18]. Fungi were then purified by successive transfers. The suspected Aspergillus isolates were subcultured and transferred on to differential media; malt extract agar, czapek yeast extract agar and glycerol agar for species identification using macro morphological characteristics. Fungal slides were prepared from pure cultures on PDA media for microscopic characteristics [19].
Screening of Isolates for Aflatoxins Production
The method of Ouattara-Sourabie [20] was used to screen the ability of fungal isolates to produce aflatoxins. For intense fungal isolates were plated on Coconut Extract Agar (CEA) and incubated at 28°C ±2°C during 7 days. After incubation period, fluorescence under Ultraviolet (UV) radiation was assessed using wood lamp at wavelength of 365 nm. The fluorescence and characteristic color of fluorescence were used to evaluate the ability of each isolate to produce aflatoxins. Aflatoxin diffusion areas shows a blue radiation around the isolate under UV light.
Antifungal Assay
The antifungal activity of the essential oil was evaluated by the agar incorporation method [21]. The test was carried out in 90 mm Petri dishes containing PDA-chloramphenicol medium. The oil was first diluted with Di Methyl Sulphur Oxide (DMSO) (ratio1:9). The essential oil was added aseptically into the medium at an appropriate volume to produce various concentrations ranging from 500 to 2000 ppm. PDA-chloramphenicol medium supplemented only with DMSO was used as negative control. After solidification, the media were inoculated with 5 mm discs obtained from the edge of 3 days old mycelia culture of Aspergillus spp. Each treatment consisted of triplicate plates incubated at 28°C ±2°C for seven days in the dark. Mycelia growth was monitored by measuring the growth diameter following two perpendicular lines going through the center of the dish. The inhibition percentage of mycelia growth was calculated according to the formula: %I = [(Dc - Dt) / Dc] ×100, where Dc is the diameter of microbial colony in the control and Dt the diameter of the colony in the treated plate. The fungicidal or fungistatic activity was determined by transferring the discs from the Petri dishes with no apparent growth into non-supplemented medium.
Inhibition of Conidia Germination
The effect of the EO on the conidia germination was tested using a liquid dilution method in Potatoes Dextrose Broth (PDB). Firstly, to obtain conidia suspension the surface of 7 days old Aspergillus PDA plate was washed with 2 mL of sterile distilled water containing few drops of Tween 80. This solution was centrifuged at 2000 rpm for 5 min and the spore suspension (supernatant) recovered and adjusted to 105 spores/mL using the Malassez cell. For the test, oil was added separately into microcupules containing 100 μl PDB in serial dilution following a geometrical progression with a common ratio of two in order to obtain the final concentrations of 2000, 1000, 500, 250, 125 and 62.5 ppm for the Cymbopogon citratus essential oil. After that aliquot (100 μl) of the spore suspension of each Aspergillus was introduced in microcupules [22]. Tests were carried out in triplicates and incubated during five days at 28 ± 2°C. The minimal sporicidal concentration was observed visually and under optical microscopic (40×).
In Situ Test
Smocked Ethmalosa fimbriata collected was disinfected with ethanol 70% for 3 min, rinsed with sterilized distilled water cut into pieces and distributed in sterile Petri dish in triplicate for each test. For preventive test, 1mL of EO (MIC) solution was first spray on fish and spore suspension (100 μl) four hours later while for curative test conidia suspension was spray and EO added four hours later. Petri dish containing fish pieces were stored at 28± 2°C and monitored daily to detect visual contamination. After 18 days, an attempt to re-isolate Aspergillus from infected fish tissue was done as previously described.
Statistical Analyses
Experiments were performed in triplicate, and data obtained as mean ± standard deviation were analyzed by ANOVA test using IBM SPSS Statistics 20.1 (Chicago, IL, USA), version 10.0. Means are separated by the Tukey’s multiple range test when ANOVA was significant (p<0.05).
Results
Hydrodistillation of fresh leave of Cymbopogon citratus shown a yellowish essential oil with a yield of 0.45%. GC-MS mass spectra of the oil is shown on Figure 1. Analytical results shown thirty-two components. The major components were geranial (39.66%), neral (30.77%) and a-fenchene (14.14%). Many Aspergillus fungi were isolated from smocked Ethmalosa fimbriata samples. Three of them could produce mycotoxins on coconut extract agar plate base on the observations under UV light microscope (Figure 3). They were identified as Aspergillus parasiticus, Aspergillus glaucus and Aspergillus flavus on the basis of their macroscopic and microscopic characteristics (Table 1 and Figure 2). The antifungal activity shows that Cymbopogon citratus essential oil caused a dose dependent inhibition of mycelial growth on the three tested mycotoxigenic Aspergillus (Figure 4). The minimal inhibitory concentrations of their radial growth were (ppm) 1295, 1195, 595 respectively, for Aspergillus parasiticus, Aspergillus glaucus, Aspergillus flavus. These MIC were also the fungicidal concentrations. In the same line the conidia of all the tested Aspergillus were inhibited with the same MIC and MIF 100 ppm. Base on that concentration the preservative test was done with direct application of essential oil by spraying method. Whether it be preventive or curative test, fish on which essential oil was applied did not show any visible signs of contamination after 18 days of incubation compare to control fish on which visible mycelia grow and conidia were observed just after 3 days of incubation (Figure 5). Moreover, attempt to reisolate fungi from treated fish failed especially for preventive test.
Species
Surface
Color
Shape
Vesicle serration
A. flavus
Spherical
Pale brown
Ellipsoid
Uniseriate
A. parasiticus
Finely
Yellowish
Pyriform
Uniseriate
A. glaucus
Spherical
Green orange
Small
Biseriate
Table 1: Macroscopic and microscopic characters of Aspergillus species.
N0
Compound
Retention Index
Percentage (%)
Method of identification
1
a-pinene
941
1.63
RI, GC-MS
2
a-fenchene
947
14.14
RI, GC-MS
3
Camphene
949
0.05
RI, GC-MS
4
a- phellandrene
994
0.53
RI, GC-MS
5
a-terpinene
1006
0.33
RI, GC-MS
6
Δ-3-carene
1011
0.04
RI, GC-MS
7
γ-terpinene
1050
0.08
RI, GC-MS
8
Thiophene
1056
0.74
RI, GC-MS
9
Octanol
1065
0.07
RI, GC-MS
10
Hydrate transsabinene
1073
0.03
RI, GC-MS
11
Thujanol
1100
0.06
RI, GC-MS
12
Thujone
1103
0.29
RI, GC-MS
13
Campholenal
1110
0.34
RI, GC-MS
14
Oxyd limonene
1121
1.15
RI, GC-MS
15
Terpinen-1-ol
1139
1.94
RI, GC-MS
16
Borneol
1165
0.06
RI, GC-MS
17
Myrtenol
1185
0.30
RI, GC-MS
18
Neral
1202
30.77
RI, GC-MS
19
Geraniol
1212
5.08
RI, GC-MS
20
Geranial
1232
39.66
RI, GC-MS
21
β-citronellol
1238
0.07
RI, GC-MS
22
Cinamaldehyde
1244
0.08
RI, GC-MS
23
Thymol
1293
0.07
RI, GC-MS
24
Acetate of terpinyl
1306
0.16
RI, GC-MS
25
Acetate of myrtenyl
1312
0.60
RI, GC-MS
26
Menthol
1328
0.07
RI, GC-MS
27
Acetate of cytronellyl
1335
1.31
RI, GC-MS
28
a-copaene
1378
0.09
RI, GC-MS
29
β-cubebene
1390
0.07
RI, GC-MS
30
a-guaiene
1444
0.06
RI, GC-MS
31
a-humulene
1477
0.06
RI, GC-MS
32
Germacreme-B
1576
0.09
RI, GC-MS
Monoterpene hydrocarbons
17.60
Oxygenated Monoterpenes
82.03
Sesquiterpene hydrocarbons
0.37
Table 2: Chemical composition of Cymbopogon citratus essential oil.
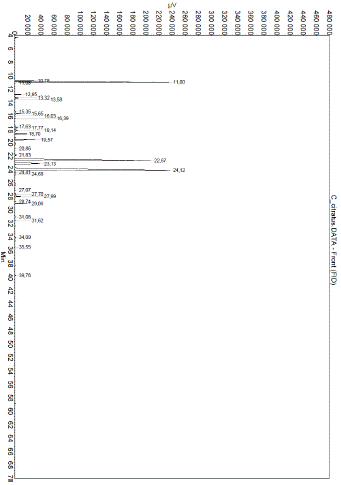
Figure 1: GC-MS mass spectra of Cymbopogon citratus essential oil.
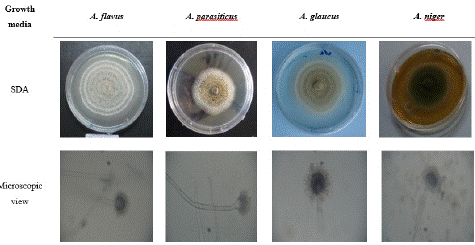
Figure 2: Macroscopic and microscopic (×40) characteristics of Aspergillus species on sabouraud plate.
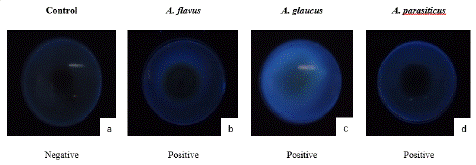
Figure 3: Mycotoxin producing isolates identification under UV light (a: control; b: Aspergillus flavus; c: Aspergillus glaucus; d: Aspergillus parasiticus).
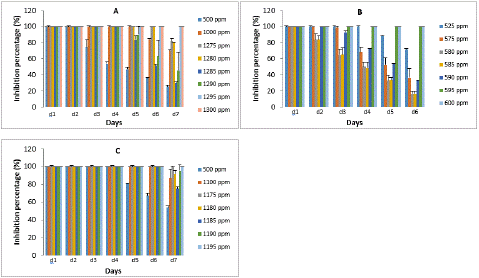
Figure 4: Inhibition percentage of essential oil on fungal: (A) Aspergillus parasiticus, (B) Aspergillus flavus and (C) Aspergillus glaucus.
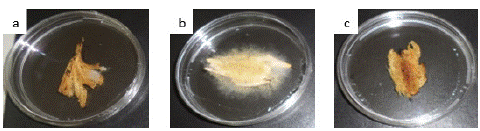
Figure 5: In situ preservative test of smocked Ethmalosa fimbriata at minimal inhibitory concentration. (a: not treated fish after 3 days; b: not treated fish after 8 days c: treated fish after 18 days).
Discussion
Essential oils are natural mixtures of hydrocarbons and oxygen such as alcohols, aldehydes, ketones, carboxylic acids, esters and lactones containing organic substances of plants. They contain three or four major components while the others exist as trace amounts [23].
They have a long history of application as antimicrobial agents in food preservation [24]. In this study the main components found in Cymbopogon citratus essential oil was géranial (39.66%), néral (30.77%) and a-fenchene (14.14%). However, many studies revealed quantitative and qualitative variations in the composition of this oil. Therefore, previous studies showed that neral (41.3%), myrcene (33.0%), and geraniol (10.4%), were the major compounds in oil extracted from Cymbopogon citratus of southern Benin [25]. The difference between the chemical compositions of essential oils could be explained by the extrinsic and intrinsic factors including age of the plant and agroecological factors such as locality, climatic and seasonal conditions [26]. In this study, three mycotoxicogenic Aspergillus species were isolated from smoked E. fimbriata namely Aspergillus flavus, Aspergillus parasiticus, and Aspergillus glaucus. Previous studies in southern Benin showed that many Aspergillus species were associated with the deterioration of smocked E. fimbriata including these species [27]. The efficacy of plant essential oils to inhibit growth of different post-harvest food pathogenic fungi has been demonstrated [28,29]. Our finding revealed the effectiveness of Cymbopogon citratus essential oil to inhibit the mycelia growth and conidia germination of Aspergillus spp.
This inhibition significantly varied with essential oil concentration as well as with the fungal species. Different changes in the antifungal activity against Aspergillus spp may be attributed to the lipolytic variation in the composition of cellular membrane of the target microorganisms and their genetic constitution. The efficiency of Cymbopogon citratus essential oil as antifungal agents, inhibitory and fungicidal activities against mycelial growth and conidia germination was previously reported in many studies and be attributed to the presence of some interactions between the major and the minor compounds. Indeed, the antifungal activities of Cymbopogon citratus oil could be associated with its major components such as geranial, neral, and a-fenchene as well as the minor one present in the oil. They may act together synergistically to induce disturbance in several enzymatic systems that are involved in the energy production and structural component synthesis. Once the phenolic compounds contained in the oil cross the cellular membrane, the membrane enzymes and proteins could cause an opposite flow of protons thereby affecting the cellular activity [31-31]. Moreover, the antifungal activity of Cymbopogon citratus may be in part attributed to the presence of an aromatic nucleus and a phenolic hydroxyl (OH) group that are known to be reactive and can form hydrogen bonds with the –SH groups of the active sites of the targeted enzymes, resulting in the deactivation of the enzymes in the fungi [32]. In situ tests revealed that essential oil from C. citratus inhibited the colonization of Ethmalosa fimbriata by Aspergillus species at a certain concentration after many days of storage. These results corroborate the finding from previous studies on the potential of Cymbopogon citratus essential oil to preserved food stuff against microorganisms. In this line Cymbopogon citratus essential oil was used to control contamination of stored rice and had the potential to be an alternative to synthetic fungicides in the food industry [33].
Conclusion
These findings revealed in vitro and in situ biological activities effects of Cymbopogon citratus essential oil against three mycotoxicogenic Aspergillus spp and their preservative potential to protect smocked Ethmalosa fimbriata against mold contamination. However, its use for formulation of organic fungicide needs more investigation on post-harvest fish process, essential oil optimization and impact of this essential oil on fish organoleptic properties.
References
- Manz JCK, Tuem SR, Ekwalla RJMN, Dongho FFD, Nchoutpouen MN, Nsoga JVF, et al. In vivo Antihyperlipidemic and Antioxidant Effect of Oil Extracted from Sardinella maderensis (Lowe, 1838) on Strain Wistar Rats. Journal of Food and Nutrition Sciences. 2024; 12: 61-71.
- Tamgno BR, Tekou NH, Nyamsi TNL, Mouamfon M, Ngamo TLS. Insectes ravageurs des poissons fumés au cours du stockage et dégâts occasionnées dans la boucle Nord de la Réserve de Biosphère du Dja (Est-Cameroun). International Journal of Chemical and Biological Sciences. 2020; 14: 528-538.
- El-nabarawy E, Anwaar MEN, Shakal MA, Hegazy AHM, Batikl MM. Comparative clinicopathological study of Salmonellosis in integrated fish-duck farmin. Journal of world’s poultry research. 2020; 10: 184-194.
- Taiga. Développement de la pèche au Cameroun. Investir au Cameroun N°24/Mars. 2014.
- Hissein O, Abdoullahi, TF, Guira F, Zongo C, Abakar LI. Technologies, qualité et importance socioéconomique du poisson séché en Afrique. Revue Sciences, Technologie et Synthèse. 2018; 37: 49-63.
- Nsoga JVF, Milong MCS, Nchoutpouen NM, Manz KJC, Ekwalla MNRJ, et al. Characterization of Smoking Activity and Perception of Smoked Fish by Households in the City of Douala (Cameroon). International Journal of Nutrition and Food Sciences. 2021; 10: 159-166.
- Tamgno BR, Tekou NH, Nyamsi, TNL, Mouamfon M, Ngamo TLS. Contraintes de stockage du poisson fumé dans la boucle Nord de la Réserve de Biosphère du Dja. 26ème Conférence Annuelle des Biosciences, du 26 au 30 novembre 2019, Maroua, Cameroun, Poster 28, Sécurité alimentaire. Book of abstract of Cameroon Biosciences Society. 2019.
- Ziem O, Ekwe PG, Plidikoua A, Ntah AAM, Adande B, Sameza ML, et al. First Insight in Fungi Diversity and Mycotoxins Contaminating Smoked Fish Sold in Yaoundé Retails Markets in Cameroon. Journal of Bacteriology and Mycology. 2024; 11: 9.
- Hegazy EM, Mohamed GF, Abdellatef M. Physicochemical properties and mycotoxins contents of Tilapia fish fillets after solar drying and storage. Global Veterinaria. 2021; 7: 138-148.
- Nguegwouo E, Azoumboi AN, Wade A, Medoua GM, Fokou E. Validation of an ELISA test kit for the quantitative analyses of total Aflatoxins in spices marketed in Cameroon. Magna Scientia Advanced Research and Reviews. 2023; 07: 086-097.
- Yehouenou B, Ahoussi E, Sessou P, Alitonou GA, Toukourou F, Sohounhloue CKD. Chemical composition and antimicrobial activities of essential oil EO extracted of leaves of Lippia rugosa A. chev. against foods pathogenic and adulterated microorganisms. Afr J Microbiol Res. 2012; 6: 5496-5505.
- Taghavi M, Khosravi A, Mortaz E, Nikaein D, Athari SS. Role of pathogen-associated molecular patterns (PAMPS) in immune responses to fungal infections. European Journal of Pharmacology. 2017; 808: 8-13.
- Nagaraj B, Kwang-hyun B. Synergistic antioxidant and antibacterial advantages of essential oils for food packaging applications. Biomolecules. 2021; 11: 1267.
- Amvam ZPH, Biyiti L, Tchoumbougnang F, Menut C, Lamaty, Bouchet P. Aromatic plants of tropical central Africa Chemical composition and antifungal activity of thirteen essential oils from aromatic plants of Cameroon. Flav Frag J. 1998; 13: 1-8.
- Nakada-freitas PG, Santos CA, Magalhães TH, Bustamonte SS, Santos DC, Cardoso AII, et al. Effect of thyme, lemongrass and rosemary essential oils on Aspergillus flavus in cauliflower seeds. Horticultura Brasileira 2022; 40: 71-75.
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometry. Allured Pub Corp; Carol Stream, USA. 4th edition. 2007.
- Hissein O, Abdoullahi, Tapsoba F, Guira F, Zongo C, Abakar LI. Technologies, qualité et importance socioéconomique du poisson séché en Afrique. Revue Sciences, Technoogie et Synthèse. 2018; 37: 49-63.
- Adjou ES, Kouton S, Dahouenon AE, Soumanou MM. Effect of essential oil from fresh leaves of Ocimum gratissimum L. on mycoflora during storage of peanuts in Benin. Mycotoxin research 2013; 29: 29-38.
- Raper KB, Fennel DI. The perfect states of Aspergillus. Subramanian. Current science 1965; 41: 755-761.
- Ouattara-Sourabie. Caractérisation des souches d’Aspergillus isolées des graines d’arachides cultivées au burkina faso, afrique de l’ouest. International journal of biological and chemical sciences 2011; 5.
- Lahlou M. Methods to study the phytochemistry and bioactivity of essential oils. Phytotherapy Res. 2004; 18: 435-448.
- Oussou KR, Coffi K, Guessend N, Séri Y, Koukoua G, Dosso M, et al. Activités antibactériennes des huiles essentielles de trois plantes aromatiques de Côte-d’Ivoire. Comptes rendus chimie. 2004; 7: 1081-1086.
- Pandey A, Shalini T. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. Journal of Pharmacognosy and Phytochemistry. 2014; 2: 115-119.
- Soumanou MM, Adjou ES. Sweet fennel (Ocimum gratissimum L.) oil: botanical aspects and uses in food preservation. Essential oils in food preservation, flavour and safety. 2016; 765-773.
- Degnon RG, Atrevy B, Adjou ES, Ahoussi, E, Soumanou, MM. Occurrence of microbial loads in smoked fishes marketed in the Lakeside Village of Guezin (Southern Benin) and associated microbiological hazards. American Journal of Microbiological Research. 2018; 6: 187-190.
- Mekemzeu FP, Nguikwie KS, Nguimatsia F, Chouela MLS, Bawane GB, Guiadem MC, et al. Antioxidant, antiradical power of Syzygium aromaticum essential oil, and its antidermatophytic activity against Epidermophyton floccosum and Trichophyton soudanense. GSC Biological and Pharmaceutical Sciences. 2021; 15: 327-334.
- Degnon RG, Atrevy B, Adjou ES, Ahoussi, E, Soumanou, MM. Occurrence of microbial loads in smoked fishes marketed in the Lakeside Village of Guezin (Southern Benin) and associated microbiological hazards. American Journal of Microbiological Research. 2018; 6: 187-190.
- Kamsu FPN, Dikongue FJN, Ngouana V, Tchinda ES, Jiogue MB, Ambata HT, et al. Effectiveness of massep (Ocimum gratissimum L.) essential oil and its nanoemultion toward Sclerotium rolfsii, Phytophthora infestans and Alternaria solani, pathogens associated with tomato rot diseases. Biocatalysis and agricultural biotechnology. 2023; 47: 102591.
- Moussango D, Sameza ML, Tchameni SN, Ntah AAM, Bedine MAB, Youassi YO, et al. Antifungal effects of clove (Syzygium aromaticum) essential oil against Colletotrichum gloeosporioides, the fungus associated with papaya (Carica papaya L.) fruit anthracnose. Int. J. Appl. Microbiol. Biotechnol. Res. 2020; 8: 51-57.
- Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, et al. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J Appl Microbiol. 2000; 88: 170-175.
- Omidbeygi M, Barzegar M, Hamidi Z, Naghdibadi H. Antifungal activity of thyme, summer savory and clove essential oils against Aspergillus flavus in liquid medium and tomato paste. Food Control. 2007; 18: 1518-1523.
- Velluti A, Sanchis V, Ramos AJ, Egido J, Marin S. Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essentials oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. International journal of food microbiology. 2003; 89: 145-154.
- Yasodha P, Karpagam M, Senthil P, C. Gailce LJC, Masilamani1 P, et al. Documentation of Phosphine Resistance in Red Flour Beetle, Tribolium castaneum Herbst (Tenebrionidae, Coleoptera) and Rice Weevil, Sitophilus oryzae Linn (Curculionidae, Coleoptera) in Tamil Nadu, India. Int J Curr Microbiol App Sci. 2019; 8: 1426-1433
Citation: Komyum BSK, Tchabong SR, Moussango VD, Koro FK, Sameza ML, et al. The Essential Oil of Cymbopogon Citratus Can Significantly Inhibit the Mycelia Growth, Conidia Germination of Mycotoxigenic Aspergillus Isolated from Smoked Fish (Ethmalosa Fimbriata) and Protect Against Mold Contamination. Austin J Microbiol. 2024; 9(2): 1051.