
Research Article
Austin J Microbiol. 2024; 9(2): 1054.
Otodectes Cynotis Infestation in A Cat Complicated by Secondary Staphylococcus Aureus Infection: Antibiotic Sensitivity Analysis
Rajesh Kumar Verma1*; Sonu Jaiswal2; Saurabh1; Alok Singh1; Kabeer Alam3; Utkarsh Verma4
1Assiatant Professor, Department of Veterinary Clinical Complex, College of Veterinary Science and Animal Husbandry, India
2Professor & Head, Department of Veterinary Clinical Complex, College of Veterinary Science and Animal Husbandry, India
3Ph.D. Scholar, Department of Gynaecology and Obstetrics, College of Veterinary Science and Animal Husbandry, India
4Scholar, BVSc & AH, Internship, College of Veterinary Science and Animal Husbandry, India
*Corresponding author: Rajesh Kumar Verma, Assiatant Professor, Department of Veterinary Clinical Complex, College of Veterinary Science and Animal Husbandry, Kumarganj, Ayodhya (Uttar Pradesh) 224229, India. Email: drrajesh16@yahoo.com
Received: September 13, 2024 Accepted: October 02, 2024 Published: October 09, 2024
Abstract
A domestic cat showed signs of ear scratching, head shaking, and waxy ear discharge for more than 45 days. The cat had been previously treated by a veterinarian, but no improvement was seen. It was then brought to the Department of Veterinary Clinical Complex at the College of Veterinary Science and Animal Husbandry, Acharya Narendra Deva University of Agriculture and Technology, Kumarganj, Ayodhya (U.P.), India. The cat was diagnosed with an ear mite infestation caused by Otodectes cynotis. A secondary bacterial infection with Staphylococcus aureus was confirmed through culture, Gram staining, and biochemical tests. Antibiotic sensitivity testing showed that the S. aureus strain was resistant to many antibiotics but sensitive to vancomycin and streptomycin, with intermediate sensitivity to gentamicin and amikacin. This report emphasizes the importance of diagnosing secondary bacterial infections in cases of ear mite infestations and the need for proper antibiotic sensitivity testing to guide effective treatment of secondary infections in otitis externa.
Keywords: Otodectes cynotis; Staphylococcus aureus; Ear mite infestation; Antibiotic sensitivity; Otitis externa; Cat; Multidrug resistance
Introduction
Ear mites, particularly Otodectes cynotis, are a common cause of otitis externa in cats, leading to clinical signs such as ear scratching, head shaking, and waxy discharge [10]. While mites primarily cause irritation and inflammation in the external ear, secondary bacterial infections often complicate the clinical presentation. Opportunistic bacteria such as Staphylococcus aureus frequently colonize the inflamed tissue, exacerbating the infection [8]. The case described here involves the diagnosis of an Otodectes cynotis infestation in a cat, followed by the identification of a secondary Staphylococcus aureus infection. Diagnostic methods included microscopic identification of the mites, bacterial culture, Gram staining, and catalase testing. Antibiotic Sensitivity Testing (AST) was also conducted to guide effective antimicrobial therapy.
Materials and Methods
Sample Collection
A domestic short-haired cat presented with signs of otitis externa, including severe ear scratching, head shaking, and dark, waxy discharge from the ears. A sterile ear swab was collected and used for diagnostic analysis.
Otodectes cynotis Identification
The ear swab was placed in 10% potassium hydroxide (KOH) for one hour to dissolve waxy debris. The sample was then examined under a light microscope at 10x magnification, and mites were identified based on their morphological features described by Baker et al. [1], including elongated bodies and short legs.
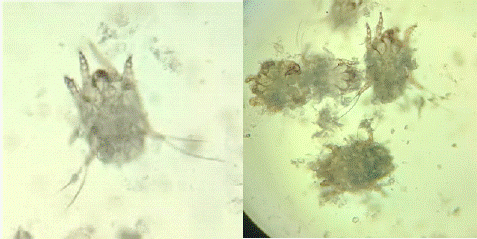
Figure 1: Otodectes cynotis mites.
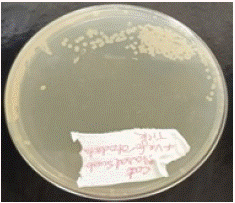
Figure 2: Growth on Nutrient Agar Plate.
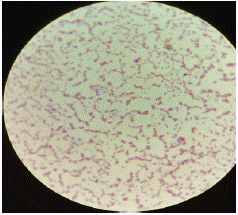
Figure 3: Gram’s stain.

Figure 4: Catalase.
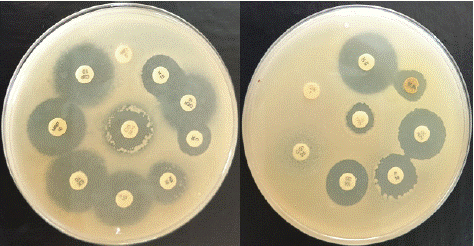
Figure 5: Antibiotic Sensitivity test.
Bacterial Culture from Ear Swab
The same ear swab was cultured in nutrient broth for 24 hours at 37°C to promote bacterial growth. After incubation, the broth culture was streaked onto nutrient agar plates and incubated aerobically at 37°C for 24 hours. This method allows for the isolation and identification of bacterial colonies from the sample [2].
Bacterial Identification
• Gram Staining: Colonies grown on nutrient agar were subjected to Gram staining using standard protocols [2]. The slides were stained with crystal violet, iodine, decolorized with alcohol, and counterstained with safranin. The morphology and Gram reaction of the bacteria were observed under a microscope at 100x magnification.
• Catalase Test: A colony was placed on a glass slide, and a drop of 3% hydrogen peroxide was added. The immediate formation of bubbles (oxygen release) indicated a positive catalase reaction, suggesting the presence of Staphylococcus aureus [9].
Antibiotic Sensitivity Testing
The antibiotic sensitivity test was performed using the Kirby-Bauer disc diffusion method on Mueller-Hinton agar plates [4]. Eighteen antibiotic discs (Hi-Media) were placed on the Mueller-Hinton media plate, and plates were incubated at 37°C for 24 hours. Zones of inhibition were measured, and the results were interpreted according to zone size interpretation chart of Hi-media.
Results
Otodectes Cynotis Identification
Microscopic examination of the ear swab after KOH treatment confirmed the presence of Otodectes cynotis mites. The mites were identified based on their characteristic elongated bodies, short and stout legs, and suckers on the first and second pairs of legs.
Bacterial Culture and Identification from Ear and Nasal Swabs
• Ear Swab Culture: Colonies grew on nutrient agar after incubation, and Gram staining revealed Gram-positive cocci arranged in clusters, characteristic of Staphylococcus aureus. The catalase test was positive, confirming the bacterial identity.
Antibiotic Sensitivity Testing (ABST)
(Table 1)
S.No.
Antibiotics
Concentration (μg)
Diameter of Zone of Inhibition (mm)
Interpretation
Ceftriaxone Sulbactam (CIS)
30/15
19
Resistant
Ciprofloxacin (CIP)
10
21
Resistant
Cotrimoxazole/Sulpha Trimethoprim (COT)
25
0
Resistant
Vancomycin (VA)
30
18
Sensitive
Methicillin (MET)
10
0
Resistant
Tetracycline (TE)
30
12
Resistant
Azithromycin (AZM)
30
20
Resistant
Gentamicin (GEN)
10
22
Intermediate
Amikacin (AK)
30
23
Intermediate
Penicillin (P)
10 units
10
Resistant
Enrofloxacin (EX)
10
21
Resistant
Chloramphenicol (C)
50
18
Resistant
Norfloxacin (NX)
10
18
Intermediate
Oxacillin (OX)
1
0
Resistant
Streptomycin (S)
10
23
Sensitive
Amoxicillin (AMX)
10
11
Resistant
Erythromycin (E)
15
15
Resistant
Meropenem (MRP)
10
20
Resistant
Table 1: The antibiotic sensitivity results are summarized.
Discussion
Otodectes cynotis is a prevalent cause of otitis externa in cats and can often result in secondary bacterial infections [10]. In this case, Staphylococcus aureus was identified as the secondary pathogen. Gram staining and catalase testing provided rapid confirmation of the bacterial species, which is consistent with literature on diagnostic techniques [9]. The presence of Staphylococcus aureus in both the ear and nasal swabs suggests systemic involvement or possible cross-contamination, highlighting the importance of sampling multiple sites when dealing with severe infections [3]. This finding underscores the role of thorough diagnostics in uncovering potential reservoirs of infection within the same host [6].
The antibiotic sensitivity test revealed a multidrug-resistant strain of Staphylococcus aureus, resistant to most antibiotics except vancomycin and streptomycin. This result aligns with recent studies demonstrating the emergence of Methicillin-Resistant Staphylococcus Aureus (MRSA) in veterinary medicine [5]. The sensitivity to vancomycin and streptomycin provided viable treatment options, while intermediate sensitivity to gentamicin and amikacin could support combination therapy.
The nasal swab culture showing Staphylococcus aureus growth further indicates that nasal colonization may contribute to the persistence of the infection, a well-documented phenomenon in humans with recurrent S. aureus infections [7]. Systemic antibiotic therapy was initiated based on the sensitivity profile, and the cat showed marked improvement in clinical signs following treatment.
This case underscores the importance of performing both ear and nasal swab cultures in cases of otitis with suspected secondary bacterial infections. The detection of nasal S. aureus colonization might have clinical implications for recurrent or difficult-to-treat infections.
Conclusion
This case highlights the diagnostic challenges and treatment complexities involved in managing Otodectes cynotis infestations complicated by secondary bacterial infections. The use of multiple diagnostic methods, including microscopic examination, bacterial culture, Gram staining, and antibiotic sensitivity testing, was crucial in identifying the correct pathogen and guiding effective treatment. Comprehensive testing, including nasal swabs, is recommended in severe or recurring cases to ensure that all potential sources of infection are addressed.
References
- Baker EW, Wharton GW. An Introduction to Acarology. Macmillan, New York. 1956.
- Barrow GI, Feltham RKA. Cowan and Steel’s Manual for the Identification of Medical Bacteria. Cambridge University Press. 2003.
- Bond R. Antimicrobial resistance in dermatology. Veterinary Dermatology. 2017; 28: 413-421.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100. 2020.
- Couto N, Jones C. Multidrug-resistant bacteria in veterinary medicine. Journal of Veterinary Medicine. 2020; 65: 451-460.
- Hendrix CM. Laboratory Procedures for Veterinary Technicians. Elsevier Health Sciences. 2016.
- Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clinical Microbiology Reviews. 1997; 10: 505-520.
- Paterson S. Bacterial skin disease in cats. Companion Animal. 2002; 7: 25-29.
- Quinn PJ, Markey BK, Carter ME, Donnelly WJC, Leonard FC. Veterinary Microbiology and Microbial Disease. Blackwell Science. 1994.
- Scott DW, Miller WH, Griffin CE. Small Animal Dermatology. 6th ed. W.B. Saunders. 2001.