
Research Article
Austin J Microbiol. 2024; 9(3): 1055.
In Vitro Antibacterial Activity of Monofloral Bee Pollen from Western Oromia, Ethiopia
Ofijan Tesfaye*
Oromia Agricultural Research Institute, Haro Sebu Agricultural Research Center, Haro Sebu, Kellem Wollega, Oromia, Ethiopia
*Corresponding author: Ofijan Tesfaye, Oromia Agricultural Research Institute, Haro Sebu Agricultural Research Center, Haro Sebu, Kellem Wollega, Oromia, Ethiopia. Email: apistesfaye@gmail.com
Received: October 14, 2024; Accepted: October 31, 2024 Published: November 07, 2024
Abstract
Pollen is a natural product collected by bees from flowering plants for brood rearing. It has been used as a medicine and food supplement. However, its biological and nutritional composition primarily depends on floral origin. The aim of the present study was therefore to determine the antioxidant and antibacterial activities of methanolic (99.9%) extract of pollens among the floral origin. The Total Phenolic Compound Content (TPCC) and Total Flavonoid Compound Content (TFCC) were measured following the standard method. In vitro antibacterial activity was evaluated using the agar well diffusion method against five bacterial strains. The findings showed that the pollen from Eucalyptus plants had the highest TPCC (62.4 ± 0.5 mg GAE) and TFCC (49.6 ± 0.2 mg QE)/100g of pollen), while the pollen from Bidens plants had the lowest TPCC (27.5 ± 0.8 mgGAE) and TFCC (18.8 ± 0.7 mgQE/100g of pollen). Bee pollen exhibited varying levels of antibacterial activity, with Bidens spp. showing 6.6 ± 0.6 mm against Escherichia coli (ATCC-25922) and Acinetobacter baumannii (ATCC- 17978) and Eucalyptus spp. showing 23.3 ± 0.6 mm against Staphylococcus aureus (ATCC-25923). Additionally, it was shown that the antioxidant content and the antibacterial activity were positively correlated. Among all the examined strains, Eucalyptus pollen proved to be the most effective, whereas Bidens pollen showed the least effectiveness. The results showed the pollen that had more antioxidant content exhibited more inhibition diameter against the tested bacterial strain. It is concluded that pollen could be used as an alternative therapy against diseases caused by bacterial pathogens and free radical compounds while its efficiency influenced by floral origin.
Keywords: Antibacterial activity; Antioxidant activity; Bee pollen; Flavonoid content; Phenolic content
Introduction
Infectious diseases caused by various pathogens are a leading global cause of illness and death [1]. These fast-spreading microbial diseases continue to challenge various health sectors and show resistance to drug treatments [2]. The recent WHO Global Antimicrobial Resistance and Use Surveillance System (GLASS) report indicates a rise in antibacterial resistance, particularly in low- and middleincome countries, resulting in significant mortality and morbidity [3]. Around 30% of infants with sepsis perish due to bacterial infections that do not respond to initial antibiotics [4]. Therefore, exploring alternative remedies from natural health products is crucial. A rich supply of protein and minerals, pollen is a highly flexible natural molecule that bees collect for its vast reservoir of bioactive chemicals, which have substantial chemical and medicinal potential [5]. Because of their many bioactive compounds and powerful therapeutic qualities, pollen and bee products have long been regarded as wellliked natural treatments and appreciated for their nutritional content and wide range of medical uses [6]. Bee pollen is an intricate mixture of plant pollens that bees collect. Its elemental makeup varies significantly depending on the type of flower it comes from, where it is located, the type of soil it is made of, and the temperature [7]. Pollen's antibacterial qualities can be ascribed to bioactive substances such flavonoids, phenolic compounds, and other phytochemicals [8].
The antibacterial and antioxidant characteristics of bee pollen are determined by a variety of factors, including plant species, growth circumstances (soil, climate, and location), harvesting time, and extraction technique [9]. For example, rape bee pollen methanol extract was very efficient against Salmonella enterica, whereas poppy plant pollen from Slovakia demonstrated great efficacy against S. aureus by ethanol extraction [10]. Likewise, Bacillus subtilis, E. coli, Klebsiella spp., Listeria monocytogenes, Pseudomonas aeruginosa, and S. aureus were shown to be inhibited in growth by bee-pollen extracts obtained from plants belonging to the Papaveraceae, Brassicaceae, and Asteraceae families [11].
Ethiopia leads in honey production in Africa and ranks 10th globally [12]. The country boasts over 10 million bee colonies and more than 800 identified honey-source plants [13,14], showcasing rich plant diversity that yields various biological compounds. Despite this, there is scarce data on the antioxidant contents and antibacterial properties of pollen gathered by Apis mellifera L. from Ethiopian flora. Hence, this study aims to assess the total phenol and flavonoid levels and antibacterial effects of bee pollen extracted with methanol from five major bee plants.
Materials and Methods
Study Area
The study was carried out at the Haro Sebu Agricultural Research Center on the station, situated 550 kilometers from Addis Ababa in the Western Oromia region, in the Kellem Wollega Zone of the Dale Sedi district. The Illubabor Zone borders it on the south; Dale Wabara borders it on the west; Mirab Welega Zone borders it on the north; and Lalo Kile borders it on the east. Haro Sebu is the district's administrative hub. The location of the pollen collection site was 1,495 meters above sea level. The research region was home to a variety of tropical plants, including cultivated crops, weeds, forest trees, and wild coffee (Coffea arabica).
Bee Pollen Collection and Plant Identification
The sample was taken using pollen traps with a 16% pollen catching effectiveness from September 2021 to August 2022. After being taken from the honeybees' rear legs, the pollen samples were scraped off and placed in a tray. After being taken off a tray and put in a fresh paper bag, the pollen pellets were allowed to dry at room temperature for a full day. They were categorized according to color and recognized down to the genus or species level after drying.
Following their collection, weighing, and overnight drying at room temperature, pollen pellets were separated by size and color (Figure 1A-C). Melissopalynological analysis, a routine process, was followed to mount representative pellets of each hue on slides for microscopic inspection. The pellets were then cleaned with ether and coated with glycerin jelly [15]. The slides were inspected using a light microscope with a 400-x magnification after being covered with a cover slip. A light microscope (Zeiss, 2010) connected to a computer program was used to analyze the morphology of pollen, as seen in Figure 1D, and Figure 2 depicted the morphology of each plant's pollen grain. Based on the pollen atlas created specifically for this purpose from Ethiopian bee plants, the observed shape of the pollen grains was confirmed [13].

Figure 1: A) Unsorted (color mixed pollen pellet) and weighing, B) Sorting
based on color, C) Slide preparation for
each color, D) Microscopically identifying pollen grain morphology.

Figure 2: Microscopic pollen grain morphology of the selected major bee
plants.
Methanolic Pollen Extraction (MPE)
The pollen sample was extracted using methanol solvent in accordance with Addi et al. [16] methodology. Using a temperaturecontrolled shaker incubator, two grams of dried pollen powder and 25 mL of methanol were macerated at 25°C for 60 minutes. After filtering the mixture, the leftover residue was extracted twice more using 25 mL of methanol each time. At 40°C, the mixed methanolic extracts evaporated, leaving a dry residue behind. To be used later, this residue was dissolved in 50 mg/mL of methanol and kept at 4°C.
Total Phenolic Compound Content (TPCC)
To find the TPCC in bee pollen, the Folin-Ciocalteau colorimetric technique [17] was employed. For 2 hours, a combination of 2.0 mL 4% sodium carbonate, 2.5 mL diluted Folin-Ciocalteau reagent (1:10), and 0.5 mL MPE was incubated in the dark at room temperature. Then, at 740 nm, absorbance was measured. Using gallic acid (0-200 mg/mL) as a reference, TPCC was measured in mgs of Gallic acid equivalent (GE) per g of bee pollen dry weight. The calibration formula (y = 17.941x + 0.2778; R2 = 0.9948) derived from the calibration curve was used (Figure 3).
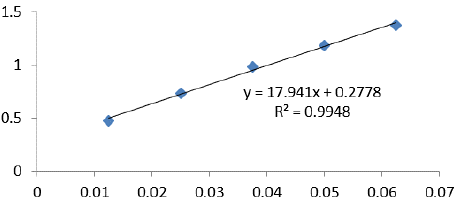
Figure 3: Calibration curve for phenol compound content.
Total Flavonoid Compound Content (TFCC)
TFCC was analyzed in accordance with the method outlined by Zou et al. [17]. For this, 4.3 mL of 99.9% methanol, 0.1 mL of 10% Al (NO3)3, and 0.1 mL of 1 M potassium acetate were combined with 0.5 mL of the MPE (1:10). Using a spectrophotometer (UV-Vis Mini 1240, Shimadzu Co.), the absorbance was measured at 415 nm after 40 minutes at room temperature. A standard of quercetin (0-200 mg/ mL) was employed for the calibration curve. Based on the mean of triplicate results, the TFCC was reported as milligrams of Quercetin equivalent (QE) per gram of dry weight bee pollen. The calibration curve (Figure 4) yielded the calibration equation (y = 9.4688x + 0.2578; R2 = 0.9962).
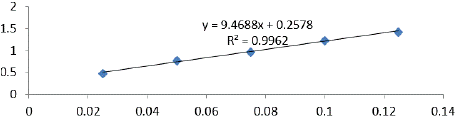
Figure 4: Calibration curve for flavonoid compound content.
Test Bacteria Used and Inoculum Preparation
The Ethiopian Public Health Institute (EPHI) provided the standard bacterial strains, which include S. aureus (ATCC-25923), Klebsiella pneumonia (ATCC 43816), A. baumannii (ATCC-17978), Enterobacter cloacae (ATCC-13047), E. coli (ATCC-25922), and Pseudomonas aeruginosa (ATCC-27853).
The preparation of the inoculum was done in accordance with the Clinical and Laboratory Standards Institute (CLSI) recommendations [18]. In order to do this, 2-3 colonies were chosen from a culture cultured on their selective media for 24 hours, and they were then put in 5 mL of saline solution (0.85%). After 15 seconds of vortexing, the turbidity of these suspended inoculums was measured by adding saline or colony solution to the microbial stock solution. A visual comparison was made between this and a standardized 0.5 McFarland (108 CFU/mL) on white paper with black lines that contrasted [19].
Agar well Diffusion Assay
The agar well diffusion test was performed [20]. Mueller Hinton Agar was poured on the petridishes and using a sterile cork borer, wells of 6 mm in diameter and 4 mm in depth were made in the agar medium. Then, the solidified medium was uniformly seeded on using a sterile cotton swab from an inoculated saline solution containing bacteria strains. After that, the plates were placed on the bench to absorb extra liquids. Afterwards, a micropipette was used to add 60 μL of MEP samples at a 75% concentration (3g of MEP combined with 1 mL of dimethyl sulfoxamide) to the plate wells. Additionally, dimethylsulfoxamide; a negative control, and ciprofloxacin (10 μg/60 μL); a positive control, were added to the wells. For twenty-four hours, the plates were incubated at 37°C. The diameters of the inhibition zones around well were measured in millimeters using a ruler, and the results were recorded and replicated three times.
Data Analysis
Means ± standard deviations of the recorded data were calculated using SAS Software. Determination of the significant differences between MEP was done using one-way ANOVA. Inhibition zones of the tested MEP were used for mean separation. Multiple pairwise comparison between the mean values was done using the Least Significant Difference (LSD).
Results and Discussions
Plant Identification
Figure 5 illustrates the different percentages of the bee pollen plant species that contribute to the pollen output. Five key bee plants namely: Bidens spp., G. scabra, C. macrostychus, Eucalyptus spp., and Z. mays were selected based on their notable production and sample availability for examination. Z. mays had the lowest pollen output at 7.7%, whereas Bidens spp. had the greatest at 35%.
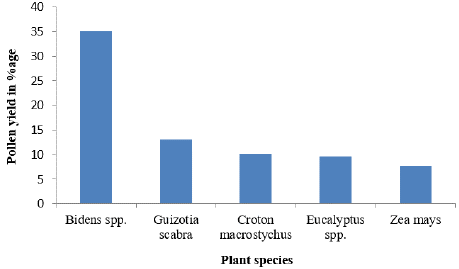
Figure 5: The percentage pollen yield/hive of the tested plants.
Numerous scholars have identified these plants as important Ethiopian bee plants [16,21]. After June to August, which is the major wet season, herbaceous plants such as Guzotia and Bidens blossomed abundantly, offering nectar and pollen to bees. Single-flower honey is produced by G. scabra, which grows well in a variety of environments including open grasslands, woodland borders, and farmed areas [22].
Total Phenolic Compound Content (TPCC) and Total Flavonoid Compound Content (TFCC)
Figure 6 and 7 showed the TPCC and TFCC for various pollen kinds, respectively. The results showed that, of the pollen types tested, Eucalyptus had the highest TPCC (62.4 ± 0.5 mg GAE) and TFCC (49.6 ± 0.2 mg QE)/100g of pollen), while the pollen from Bidens had the lowest TPCC (27.5 ± 0.8 mg GAE) and TFCC (18.8 ± 0.7 mg QE/100g of pollen). The present study's TPCC is lower than the ranges reported in Eastern Croatia (7.08 to 15.27 mg GAE/g) [24], Poland (12.93 to 82.43 mg GAE/g) [25], and Italy (5.78 to 20.15 mg GAE/g) [23]. The TPC in pollen grains of Castanea sativa varied from 64.02 ± 0.26 to 103.8 ± 6.72 mg GAE/g, according to another research [26].
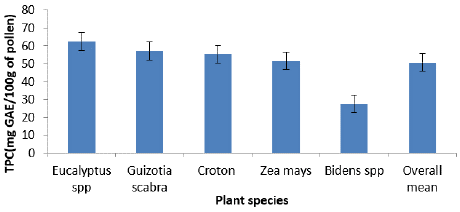
Figure 6: Average total phenolic compound content among plant species.
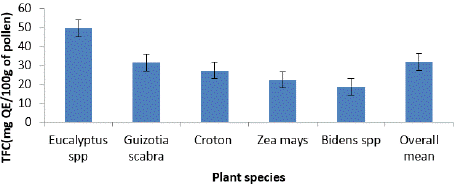
Figure 7: Average total flavonoid compound content among plant species.
The TFCC in this research (18.8 ± 0.7 to 49.6 ± 0.2 mg QE/100g of pollen) was lower than that from Northwest Algeria (TFC, 8.92 mg QE/g) [27], the Baltic region (TF, 6.1 to 11.6 mg QE/g) [28], Thailand (7.53± 0.30 to 56.40± 4.85 mg QE/g) [29]. In line with prior research [30], variations in the botanical and geographic origins of bee pollen samples may account for the diversity in phenolic compounds and flavonoids. Furthermore, as noted by previous research [31], the variations in phytochemical composition found in various bee pollen samples, especially those originating from the same plant, may be due to a variety of factors, including climate and beekeeping techniques, in addition to their botanical provenance.
Antibacterial Activity Among Pollen Types
Table 1 displays the inhibitory diameters of the methanol (99.9%) extracts of monofloral bee pollens that were evaluated as natural antibacterial agents against common human bacterial strains in the current investigation. Using the well diffusion technique, the antibacterial activity of bee pollen against S. aureus varied from 23.3 ± 0.6 mm for pollen from Eucalyptus spp. to 6.6 ± 0.6 mm for pollen from Bidens spp. against E. coli and A. baumannii. In comparison to other pollen kinds, bee pollen from Eucalyptus and Guizotia displayed statistically equivalent inhibitory diameters (p>0.05) and shown better efficacy against S. aureus and K. pneumonia. Furthermore, against every pathogenic strain examined, Eucalyptus pollen proved to be much more efficient (p<0.0001) than Bidens pollen. When compared to the positive control (an antibiotic; Ciprofloxacin), all pollen types shown statistically significant differences (p<0.0001) and were less effective; in contrast, the negative control, dimethylsulfoxamide, exhibited no inhibition against the tested strains. The test organism that was shown to be most vulnerable to all forms of pollen was S. aureus.
Bacterial strain
Pollen types (mean ± standard deviation of the inhibition diameter zone; mm)
Eucalyptus spp.
Guizotia spp.
Croton macrostychus
Zea mays
Bidens spp.
Ciprofloxacin
DMSM
LSD
p-value
CV
R2
SA (ATCC-25923)
23.3±
0.6b22.0±
1b16.6±
1.5c14.0±
1.0d12.3±
0.6e33.0±
1.0aNIZ
1.56
<.0001
4.84
.99
KN (ATCC-43816)
18.3±
1.2b17.0±
1b15.0±
1c12.0±
1.0d9.0±
1.0e27.6±
0.6aNIZ
1.56
<.0001
5.87
0.98
AB (ATCC-17978)
16.6±
0.6b15.3±
0.6c13.3±
0.6d9.6±
0.6e6.6±
0.6f25.3±
0.6aNIZ
0.86
<.0001
3.67
0.99
ECL(ATCC-13047)
17.3±
0.6b±16.0±
1.0b±13.6±
1.5c9.3±
1.2d7.3±
0.6e26.3±
0.6aNIZ
1.66
<.0001
6.81
0.98
PA (ATCC-27853)
14.3±
0.6b13.0±
1c10.3±
0.6d9.3±
0.6d8.0±
1.0e26.6±
0.6aNIZ
1.28
<.0001
5.7
.99
EC (ATCC-25922)
14.6± 1.1b
13.3±
0.6b11.3±
0.6c8.0±
1.0d6.6±
0.6de23.0±
1.0aNIZ
1.43
<.0001
6.80
0.98
Values in the same row followed by the same letter are not significantly different by Tukey’s multiple range tests (p < 0.05). SA (S. aureus), KN (K. pneumonia), AB (A. baumannii), ECL (E. cloacae), EC (E. coli), PA (P. Aeruginosa), DMSM (Dimethylsulfoxamide), LSD (Least Significant Difference), CV (Coefficient of Variation), ATCC (American Type Culture Collection) and NIZ (No Inhibition Zone)
Table 1: Inhibition zone (mean ± standard deviation) among pollen types against the strains.
The results emphasize the potential of pollen to inhibit human bacterial growth and highlight the influence of floral origin on its biological activities. Ethiopia has not carried out any study of this kind to compare with the results of the current finding but compared with research done at somewhere. Additionally, bee flora is not always available in all nations. For instance, Citrus aurantium pollen extracted with 50% ethanol demonstrated a less effective inhibition diameter (6 ± 0.01) against A. baumannii, E. cloacae, E. coli, K. pneumonia, and P. aeruginosa when compared to different plant types, whereas Ruta graveolens pollen showed a higher effectiveness (22.33 ± 1.20) against S. aureus. A pollen extract from Morocco was less effective against Gram-negative while Gram-positive (S. aureus) was susceptible [32]. Pollen extracts from Quercus ilex and Punica granatum demonstrated substantial antibacterial activity against S. aureus, with inhibition zones of 19.33 ± 0.33 mm and 22.33 ± 1.20 mm, respectively [33]. Nonetheless, E. coli (14 ± 0.57 mm), A. baumannii (13 ± 0.88 mm), and S. aureus (14 ± 0.57 mm) were all successfully combated by Ruta graveolens pollen extract.
Phenolic and flavonoid chemicals found in bee pollen have been linked to a range of medical benefits, including antibacterial, antiinflammatory, antioxidant, and antitumor effects [34]. The antibacterial qualities of bee pollen are attributed to its bioactive constituents, which include flavonoids, plant phenolics, glucose oxidase, and secondary metabolites [35]. It has been proposed that polyphenols provide a novel approach to counteract bacterial resistance [36]. It has been shown that pollen grains from various plant species and geographical regions vary in their antibacterial effectiveness [37,38]. The content and properties of pollen are influenced by a variety of factors, including the kind of plant, growth circumstances such as soil and climate, harvesting season, extraction solvent, and laboratory techniques [39]. Differences in the composition of bacterial walls may account for the differing susceptibilities of gram-positive and gram-negative bacteria to certain kinds of pollen [40]. Compared to gram-negative bacteria, gram-positive bacteria have a cytoplasmic membrane that is richer in anionic phospholipids, which may be the reason why certain gram-negative bacteria are naturally resistant to antibiotics [41]. For instance, daptomycin, a lipopeptide antibiotic, works well against gram-positive bacteria but not gram-negative ones because it cannot cross the outer membrane, which is an essential step in its antibacterial activity [42].
Pearson Correlation Coefficient
One useful method for determining correlations between different research factors is the correlation test. The relationships between antioxidant levels and antibacterial activity are shown in Table 2. Due to the high concentration of bioactive components in the pollens, phenolics and flavonoid contents were shown to significantly positively correlate. A significant positive correlation was discovered between the antioxidant content and the antibacterial activities against different strains of bacteria, as well as between the flavonoid content and the antibacterial activities displayed by the methanol extract of various pollens against all strains of bacteria. This finding is consistent with other studies showing an antibacterial activityantioxidant content relationship [43,44].
Phenol
Flavonoid
Phenol
1
Flavonoid
0.73407**
1
SA (ATCC-25923)
0.78501***
0.87375***
KN (ATCC-43816)
0.88581***
0.84034***
AB (ATCC-17978)
0.90266***
0.85298***
ECL(ATCC-13047)
0.84354***
0.84411***
PA-27853
0.79420***
0.88958***
ECOLI25922
0.82483***
0.87037***
Statistical significance, **p < 0.01; ***p < 0.001
Table 2: Pearson correlation coefficient.
Conclusion
This is the first study to look at how five different botanical sources of bee pollen affect different strains of human bacteria. The findings show that these bee pollen extracts have potent antibacterial qualities as well as a high concentration of natural antioxidants. In particular, the greatest concentrations of antioxidant chemicals and antibacterial activity were found in pollen derived from Eucalyptus and G.scabra flowers. S. aureus were more vulnerable than gram-negative bacteria. In the methanol extracts of different pollen types against all bacterial strains, a significant association was found between the flavonoid content and antibacterial activities, the phenol content and antibacterial activities, and the flavonoid content and phenol content. A thorough examination of the biological and chemical components of the pollen samples was not carried out due to a lack of resources. Hiwever, this research will further our knowledge of the antimicrobial and antioxidant characteristics of pollen derived from various plant sources.
Author Statements
Author Contributions
Ofijan Tesfaye: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Writing – original draft and Writing – review & editing.
References
- Qadri H, Shah AH, Ahmad SM, Alshehri B, Almilaibary A, Mir MA. Natural products and their semi-synthetic derivatives against antimicrobial-resistant human pathogenic bacteria and fungi. Saudi Journal of Biological Sciences. 2022; 29: 103376.
- Mir MA. Human pathogenic microbes: Diseases and concerns. Academic Press. 2022.
- Vickers R, Robinson N, Best E, Echols R, Tillotson G, Wilcox M. A randomised phase 1 study to investigate safety, pharmacokinetics and impact on gut microbiota following single and multiple oral doses in healthy male subjects of SMT19969, a novel agent for Clostridium difficile infections. BMC Infect Dis. 2015; 15: 91.
- Vickers RJ, Tillotson GS, Nathan R, Hazan S, Pullman J, Lucasti C, et al. Efficacy and safety of ridinilazole compared with vancomycin for the treatment of Clostridium difficile infection: a phase 2, randomised, double-blind, activecontrolled, non-inferiority study. Lancet Infect Dis. 2017; 17: 735–44.
- Sawicki T, Starowicz M, Klebukowska L, Hanus P. The profile of polyphenolic compounds, contents of total phenolics and flavonoids, and antioxidant and antimicrobial properties of bee products. Molecules 2022; 27: 1301.
- Martinello M, Mutinelli F. Antioxidant activity in bee products: A review. Antioxidants. 2021; 10: 71.
- Giampieri F, Quiles JL, Cianciosi D, Forbes-Hernández TY, Orantes-Bermejo FJ, Alvarez-Suarez JM, et al. Bee products: An emblematic example of underutilized sources of bioactive compounds. J Agric Food Chem. 2022; 70: 6833–6848.
- Sadeq O, Mechchate H, Es-safi I, Bouhrim M, Jawhari FZ, Ouassou H, et al. Phytochemical Screening, Antioxidant and Antibacterial Activities of Pollen Extracts from Micromeria fruticosa, Achillea fragrantissima, and Phoenix dactylifera. Plants. 2021; 10: 676.
- Rodríguez-Pólit C, Gonzalez-Pastor R, Heredia-Moya J, Carrera-Pacheco SE, Castillo-Solis F, Vallejo-Imbaquingo R, et al. Chemical Properties and Biological Activity of Bee Pollen. Molecules. 2023; 28: 7768.
- Fatrcová-Šramková K, Nôžková J, Kacániová M, Máriássyová M, Rovná K, Stricík M. Antioxidant and antimicrobial properties of monofloral bee pollen. J Environ Sci Health Part B. 2013; 48: 133–138.
- Futui W, Thongwai N. Antimicrobial and antioxidant activities, total phenolic and flavonoid contents of bee pollen crude extracts. Int J Biosci Biochem Bioinform. 2020; 10: 42-48.
- Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E. Antioxidant and Antimicrobial Effects of Phenolic Compounds Extracts of Northeast Portugal Honey. Food Chem Toxicol. 2008; 46: 3774–3779.
- Adgaba N. Atlas of Pollen Grains of Major Honey Bee Flora of Ethiopia, Holeta Bee Research Centre, Commercial Printing Enterprise; Addis Ababa, Ethiopia. 2007: 152.
- Kebede N, Subramanian P, Gebrekidan M. Physicochemical Analysis of Tigray Honey: An Attempt to Determine Major Quality Markers of Honey; Chemical Society of Ethiopia: Addis Ababa, Ethiopia. 2011
- Louveaux J, Vorwohl G, Maurizio A. Methods of melissopalynology. Bee World. 1978; 59: 139–157.
- Addi A, Ensermu K, Teshome S. Proximate composition and antioxidant power of bee collected pollen from moist Afromontan forests in southwest Ethiopia. Agr Sci Res J. 2017; 7: 83-95.
- Zou Y, Hu J, Huang W, Zhu L, Shao M, Dordoe C, et al. The botanical origin and antioxidant, anti-BACE1 and antiproliferative properties of bee pollen from different regions of South Korea. BMC Complementary Medicine and Therapies. 2020; 20: 1-4.
- Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard – Ninth Edition M07 – A9. National Committee for Clinical Laboratory Standards. 2012; 32: 1–64.
- Cavalieri SJ, Harbeck RJ, Mccarter YS, Ortez JH, Rankin ID, Sautter RL, et al. Manual of antimicrobial susceptibility testing. In M. B. Coyle (Ed.), American Society for Microbiology. American Society for Microbiology. 2005: 1-236.
- Campos MG, Anjos O, Chica M, Campoy P, Nozkova J, Almaraz-Abarca N, et al. Standard methods for pollen research. Journal of Apicultural Research. 2021; 60: 1-109.
- Tesfaye O, Mekonnen E. Floral Calendar of Honeybee Plants in Kellem and West Wollega Zone, Western Ethiopia. International Journal of Forestry Research. 2023; 2023.
- Addi A, Bareke T. Floral resources diversity and vegetation types important for honeybees in Ethiopia. Asian Journal of Forestry. 2019; 3.
- Barbieri D, Gabriele M, Summa M, Colosimo R, Leonardi D, Domenici V, et al. Antioxidant, nutraceutical properties, and fluorescence spectral profiles of bee pollen samples from different botanical origins. Antioxidants. 2020; 9: 1001.
- Bili´c Rajs B, Primorac L, Cvijeti´c Stokanovi´c M, Soldi´c A, Vukadin I, Flanjak I. Botanical origin and antioxidant capacity of bee pollen from Eastern Croatia. Food Health Dis Sci Prof J Nutr Diet. 2018; 7: 1–5.
- Leja M, Mareczek A, Wyzgolik G, Klepacz-Baniak J, Czekonska K. Antioxidative properties of bee pollen in selected plant species. Food Chem. 2007; 100: 237–240.
- Avs¸ar C, Ozler H, Berber €I, Civek S. Phenolic composition, antimicrobial and antioxidant activity of Castanea sativa Mill. pollen grains from Black Sea region of Turkey. International Food Research Journal. 2016; 23: 1711–1716.
- Rebiai A, Lanez T. Chemical composition and antioxidant activity of Apis mellifera bee pollen from Northwest Algeria. J Fundam Appl Sci. 2012; 4: 155 –63.
- Kaškoniene V, Ruočkuviene G, Kaškonas P, Akuneca L, Maruška A. Chemometric Analysis of Bee Pollen Based on Volatile and Phenolic Compound Compositions and Antioxidant Properties. Food Anal Methods. 2015; 8: 1150–63.
- Futui W, Thongwai N. Antimicrobial and antioxidant activities, total phenolic and flavonoid contents of bee pollen crude extracts. Int J Biosci Biochem Bioinform. 2020; 10: 42–48.
- Carpes ST, de Alencar SM, ISR C, TLC O, Mourão GB, CWI H, et al. Polyphenols and palynological origin of bee pollen of Apis mellifera L. from Brazil. Characterization of polyphenols of bee pollen. J Food. 2013; 11: 150– 61.
- Campos MG, Bogdanov S, de Almeida-Muradian LB, Szczesna T, Mancebo Y, Frigerio C, et al. Pollen composition and standardization of analytical methods. J Apic Res Bee World. 2008; 47: 156–163.
- Bakour M, Laaroussi H, Ousaaid D, Oumokhtar B, Lyoussi B. Antioxidant and antibacterial effects of pollen extracts on human multidrug-resistant pathogenic bacteria. Journal of Food Quality. 2021; 2021: 1-1.
- Ramalhosa E, Delgado T, Estevinho LM, Pereira JA. Hazelnut (Corylus avellana L.) cultivars and antimicrobial activity. InNuts and seeds in health and disease prevention 2011: 627-636.
- Pascoal A, Rodrigues S, Teixeira A, Feás X, Estevinho LM. Biological activities of commercial bee pollens: Antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chemical Toxicology. 2014; 63: 233–239.
- Sawicki T, Starowicz M, Klebukowska L, Hanus P. The profile of polyphenolic compounds, contents of total phenolics and flavonoids, and antioxidant and antimicrobial properties of bee products. Molecules 2022; 27: 1301.
- Daglia M. Polyphenols as antimicrobial agents. Current opinion in biotechnology. 2012; 23: 174-81.
- Alici´c D, šubari´c D, Jaši´c M, Pašali´c H, Ackar Ð. Antioxidant properties of pollen. Hrana Zdr. Boles. Znan. Strucni Casopis Za Nutr. Dijetetiku. 2014; 3: 6–12.
- Fatrcová-šramková K, Nôžková J, Kačániová M, Máriássyová M, Rovná K, Stričík M. Antioxidant and antimicrobial properties of monofloral bee pollen. Journal of Environmental Science and Health, Part B. 2013; 48: 133-138.
- Kocot J, Kielczykowska M, Luchowska-Kocot D, Kurzepa J, Musik I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxidative Med Cell Longev. 2018; 2018: 7074209.
- Semeniuc CA, Pop CR, Rotar AM. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. Journal of food and drug analysis. 2017; 25: 403-8.
- Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harbor perspectives in biology. 2010; 2: a000414.
- Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nature reviews microbiology. 2015; 13: 42-51.
- Denisow B, Denisow Pietrzyk M. Biological and therapeutic properties of bee pollen: a review. Journal of the Science of Food and Agriculture. 2016; 96: 4303-4309.
- Dinkov DH. Correlation between antibacterial and antioxidant activity in oak honeydew and Acacia (Robinia pseudoacacia l.) bee honeys. InProceedings of the V. International Scientific and Practical Conference on “Current State and Perspectives of Food Industry and Catering Development. 2013.
- Todorovic V, Milenkovic M, Vidovic B, Todorovic Z, Sobajic S. Correlation between antimicrobial, antioxidant activity, and polyphenols of alkalized/ nonalkalized cocoa powders. Journal of Food Science. 2017; 82: 1020-7.