
Research Article
J Mol Biol & Mol Imaging. 2014;1(2): 6.
Alterations of Dopaminergic Synapse and Mitochondrial Structure by Parkinson’s Disease Toxins
Meelim J Lee1§, Robert A Colvin2 and Daewoo Lee2*
1Athens High School, The Plains, OH, USA
2Neuroscience Program and Interdisciplinary Graduate Program in Molecular & Cellular Biology, Department of Biological Sciences, Ohio University, Athens, OH, USA
*Corresponding author: Daewoo Lee, Neuroscience Program and Interdisciplinary Graduate Program in Molecular & Cellular Biology, Department of Biological Sciences, Ohio University, Athens, OH, USA
§Current address Department of Bioengineering, Stanford University, Stanford, CA, USA
Received: October 02, 2014; Accepted: November 15, 2014; Published: November 23, 2014
Abstract
Immunostaining with anti-tyrosine hydroxylase (TH) revealed that a small subset (~2.5%) of primary neurons (from rat embryonic E13-14 midbrains) is dopaminergic (DA). In this study, we tested whether DA neurons in culture are selectively degenerated by well-known PD toxins such as rotenone (5-100nM) and MPP+ (10µM). Both toxins significantly decreased the number of DA neurons and neurite length after 24-hour incubation. Interestingly, our results showed that mitochondria in DA neurites were also degenerated by these toxins. Since mitochondria play a critical role in proper synapse signaling and PD is directly linked to mitochondria dysfunction, we wanted to study altered properties of DA synaptic mitochondria in the early stage of PD. With triple staining of anti- TH, a fixable synaptic marker FM1-43fx and a mitochondrial dye MitoTracker Orange (MTO), we identified DA synaptic mitochondria. Our data demonstrated that DA synaptic mitochondria are degenerated by MPP+ and rotenone, and that this effect likely occurs prior to the degeneration of DA cell bodies. Therefore, our cellular PD model can be used to study presymptomatic alterations underlying development of PD pathology. Our study will help to understand mechanisms underlying selective loss of DA neurons, which is possibly due to the degeneration of DA synaptic mitochondria.
Keywords: primary dopamine neurons; MPP+; Rotenone; Dopamine synapse; Mitochondria
Abbreviations
DA: Dopaminergic; DIV: Day(s) in vitro; FM1-43fx: A Fixable Synaptic Marker; FOV: Field Of View; MPP+: 1-Methyl-4- PhenylPyridinium; MTO: MitoTracker Orange; NB: Neurobasal; PD: Parkinson’s disease; TH: Tyrosine Hydroxylase
Introduction
Dopamine (DA) is an important neurotransmitter mediating a variety of higher brain function (e.g. cognition, motor function; [1]). Indeed, selective loss of DA neurons in the substantia nigra is a key pathological feature of Parkinson’s disease (PD) which causes DA deficits and thus disturbs motor function [2, 3, 4]. However, preceding the selective loss of DA neurons, there must be changes in the sub-cellular structure of these neurons. It is known that neurite or synaptic loss precedes degeneration of DA cell bodies [5], strongly indicating that there is substantial damage to DA neurons and synapses at the sub-cellular level before PD symptoms are clinically detected. Therefore, characterization of these early changes is crucial not only to understand mechanisms underlying initiation of DA degeneration but also to provide clues on how PD pathology initiates and develops. Additionally, it contributes to development of early detection methods and therapeutic strategies for PD. However, the presymptomatic characteristics are not currently well understood at molecular or cellular levels.
Recent genetic discoveries firmly established that familial forms of PD are directly associated with mutations of certain genes [3, 6]. So far, at least 6 genes (a-Syn, parkin, UCHL-1, DJ-1, PINK1, LRRK2) have been identified and they are directly associated with the pathogenesis of PD [7, 8, 3]. In contrast, the majority of PD cases (~90 - 95%) are thought to occur sporadically without clear genetic linkage. Thus, neurotoxin-induced loss of DA neurons is widely used to model non-genetic, environmental onset of PD [9, 3].
The neurotoxins 1-methyl-4-phenylpyridinium (MPP+) and rotenone have been used in a wide range of animal PD models (e.g. worm, Drosophila, rat, primate) since they show selective loss of DA neurons and motor impairment. Therefore, we believe these two toxins to be excellent chemical tools for PD research examining early changes in sub-cellular structure of DA neurons. In this study, we examined early degenerative changes in sub-cellular structure (e.g. synapse, neurite length, synaptic mitochondria) of DA neurons mediated by PD toxins.
Materials and Methods
Rat DA neuronal cultures and PD toxin treatment
Primary neuronal cultures were prepared from embryonic rat ventral midbrain (E13-14) and grown in neurobasal (NB) culture media supplemented with B-27 as previously described [10, 11]. Timed pregnant Sprague Dawley rats were ordered from a commercial supplier (Harlan Laboratories). Primary neuronal cultures were maintained in 37°C incubator with 5% CO2 supply. 6-8 day old neurons were treated with PD toxins, MPP+ (10µM) or rotenone (5, 20 and 100nM) for 24 hours. Finally, cultured neurons were fixed and stained with antibodies (e.g. anti-TH) and/or fluorescent dyes (e.g. FM1-43fx, MitoTracker Orange - see below). PD toxins were purchased from Sigma and all other reagents used here were purchased from Invitrogen. For all our experiments, MPP+ was prepared and disposed according to the guideline reviewed in [12]. Rotenone was similarly handled.
Quantification of DA neurites
Anti-TH antibody (Chemicon) was used to identify DA neurites in addition to the cell body. Stained neurites were observed under a fluorescent microscope (Olympus IX71). Images were taken using a Spot CCD digital camera (Diagnostic Instruments). Using a neuron/ neurite tracing software Neurolucida (BMF software), we manually traced neurites and cell bodies to create “skeletons,” which allowed the Neurolucida software to calculate neurite length (Figure 2B).
Quantification of DA mitochondria
DA mitochondria were stained using fixable mitochondriaspecific fluorescent dyes (i.e. MitoTracker Orange (MTO), Invitrogen). Cultures were incubated in 50nM MTO for 1 hour at 37°C in NB media. MTO labels all mitochondria in neuronal cultures; thus signals positive for both anti-TH and MTO staining were considered DA mitochondria and thus quantified using a Javabased image processing software program ImageJ. Morphological properties of DA mitochondria (e.g., size, number, elongation, etc) were examined as described previously [13].
Quantification of DA synapses
FM1-43fx is a fixable styryl dye known to identify functional synapses, particularly presynaptic terminals. This lipophilic dye (50nM) is taken-up by presynaptic endocytosis after neurons are depolarized by exposure to 50mM KCl for 5 min [14]. Since this dye recognizes all functional synapses, rat neuronal cultures were subsequently stained with anti-TH antibody. Regions double-stained with FM1-43fx (a fixable form of FM1-43, Invitrogen) and anti-TH antibody were identified as functional DA synapses (presynaptic punctae).
DA synaptic mitochondria
Triple staining was required in order to identify mitochondria specific to DA synapses. Therefore, DA neuronal cultures were stained with anti-TH, FM1-43fx and MTO. All three signals (i.e. FM1- 43fx, MTO, anti-TH) were distinguishable using epifluorescence microscopy. The overlapping fluorescent signals were considered DA synaptic mitochondria and quantified using ImageJ.
Results
Dopaminergic (DA) neurodgeneration by PD toxins
Using the primary rat midbrain neuronal cultures containing dopaminergic (DA) neurons (Figure 1), we first examined whether DA neurons were degenerated by exposure to PD toxins (MPP+ and rotenone). DA neurons were identified using a DA marker antityrosine hydroxylase (TH) antibody. The percent of DA neurons in the culture was 2.5+/-2.4% (n=18). This percent was significantly reduced when primary neuronal cultures (6-8 days old) were incubated for 24 hours with either 10µM MPP+ or 100nM rotenone (Figure 1D). The effect of 20nM rotenone was intermediate while there was no change in the number of DA neurons when exposed to 5nM rotenone (Figure 1D). In order to examine whether DA neurons are selectively degenerated, we determined the total number of neurons in each field of view (FOV) in the presence or absence of toxin exposure. Individual cells in the neuronal culture were quantified using a nuclear dye DAPI [15, 16]. Figure 1E shows that there was no observable difference in the total number of cells in culture with or without exposure, confirming the selective degeneration of DA neurons by these two PD toxins. Our results demonstrate that we successfully developed a cellular PD model using these toxins. Therefore, this model was used to further study early sub-cellular changes in DA neurons.
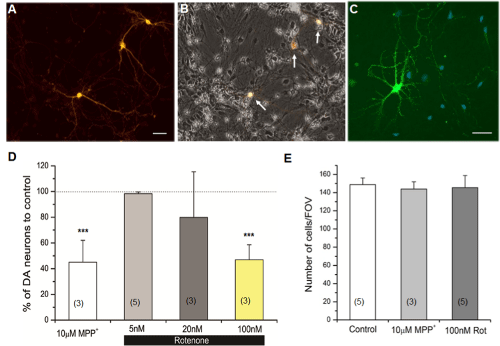
Figure 1: Parkinson’s disease (PD) toxins selectively degenerated dopaminergic (DA) neurons in rat primary neuronal cultures. (A) Three DA neurons immunostained with anti-tyrosine hydroxylase (TH) antibody. (B) A merged image of anti-TH in (A) and bright field views. A small subset of cultured neurons (indicated by arrows) is dopaminergic. (C) An example image of neuronal cultures co-stained with anti-TH (green) and a nuclear dye DAPI (blue). DAPI signal was used to quantify total neurons in the culture [16]. Scale bar = 40µm. (D) Degeneration of DA neurons by PD toxins, MPP+ (10µM) or rotenone (5-100nM). (E) A graph showing the total number of cells in control, in the presence of MPP+ (10µM) or rotenone (100nM). Cells were exposed to PD toxins for 24 hours before immunostaining. Neuronal cultures used in these experiments were 6-8 days in vitro (DIV) in neurobasal (NB) media. Number of individual experiments is shown in parentheses. Student’s t-test, ***p≤0.001.
Reduced length of DA neurites after 24 hour MPP+ or rotenone exposure
In addition to selective loss of DA neurons, we observed that the overall length of DA neurites was reduced in the presence of either MPP+ or rotenone (Figure 2). In order to quantify changes in neurite length, we traced all DA neurites using Neurolucida and quantified their length. The toxin treatments, especially rotenone at higher concentrations (e.g. 100nM), resulted in significantly shorter neurites (Figure 2C). In contrast, DA soma size was unaffected by toxin exposure (Figure 2B). This reduced neuritic length suggests likely changes to the sub-cellular structure of DA neurons such as synapses and mitochondria colocalized to neurites.
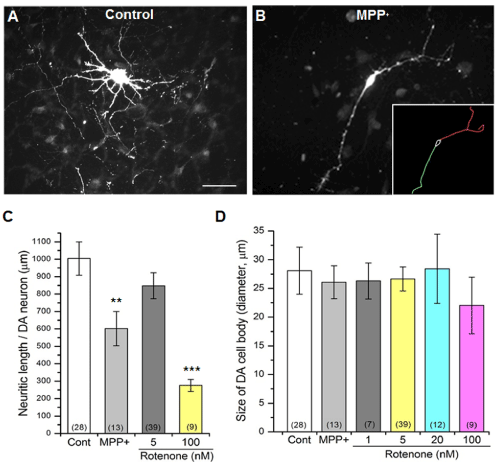
Figure 2: Reduced neurite length by either MPP+ or rotenone.
(A) A DA neuron shows well developed neurites which were stained with
anti-TH antibody.
(B) A DA neuron treated with 10µM MPP+ was used to trace neurites.
(Inset) Skeletons of traced neurites (red & green) and cell body (white) were
measured using Neurolucida (see Materials and Methods). Scale bar = 50µm.
(C) A graph showing the neurite length comparison between the control and
different concentrations of the rotenone and MPP+ treatments.
(D) Cell body size showed no significant decrease. The number of neurons
analyzed is shown in parentheses. All data from 4 separate experiments from
4 different rats. Student’s t-test, ** p≤0.01, ***p≤0.001.
Degeneration of mitochondria in DA neurites after 24 hour MPP+ or rotenone exposure
Since some PD causing factors (e.g. MPP+ , rotenone) are known to compromise mitochondrial function [17, 18], we wanted to examine structural properties of mitochondria in DA neurites, which are likely very important for DA signaling in the nervous system. When the toxin treatments (e.g. MPP+ , rotenone) inhibit complex I of the mitochondria, the resulting stress is expected to cause larger mitochondria to undergo mitochondrial fragmentation and mitophagy, yielding a reduced number of mitochondria that are smaller in size. In this study, therefore, we analyzed morphological changes in DA mitochondria by examining changes in mitochondria number, size and elongation using ImageJ as previously reported [13]. In order to quantify mitochondrial morphology specifically in DA neurites, primary neuronal cultures were stained with both anti-TH antibody and MitoTracker Orange (MTO) - a fixable mitochondrial dye (Figure 3A & B). Then, regions where the two fluorescent signals were overlapping indicated the mitochondria localized to DA neurites (Figure 3C). Elongation was calculated by taking the inverse of the value for circularity (1/circularity), which was calculated by ImageJ. This is best thought of as a measure of mitochondrial shape. Higher values are indicative of abstract shapes, and thus longer mitochondria, while a value of 1 (the minimum value) would be considered a perfect circle and, in this context, a fragmented mitochondria. The number and size of mitochondria were reduced by MPP+ and rotenone (5, 20 or 100nM; Figure 3D-F). However, mitochondrial elongation was not significantly affected by MPP+ or 100nM rotenone (Figure 3F). The reason for this observation is not clear yet but it might be due to strong mitophagy effects [19, 20] of MPP+ and higher rotenone concentrations. If so, fragmented circular mitochondria would be removed and thus elongation would increase while the number of mitochondria would be reduced by MPP+ and 100nM rotenone. Our results show that the number and size of mitochondria in DA neurites were reduced, and mitochondria became less elongated by 5 and 20nM rotenone, strongly indicating mitochondrial fragmentation by PD toxins.
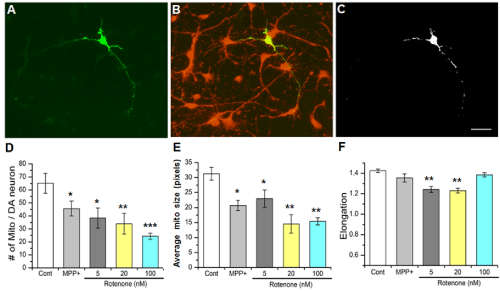
Figure 3: Mitochondria in DA neurites are degenerated by either MPP+ or
rotenone.
(A) A DA neuron stained with anti-TH antibody.
(B) Mitochondria in culture were stained with a mitochondria-specific dye
MitoTracker Orange (MTO, 50nM).
(C) Mitochondrial signals in DA cell body and neurites. The overlapping
fluorescent signals of (B) were used to analyze changes in mitochondrial
structure by PD toxins, MPP+ and rotenone. Staining of cell bodies with MTO
was saturated and eliminated from the analysis. Scale bar = 40µm.
(D-F) Number of mitochondria (D) and the average size (E) of neuritic
mitochondria with or without exposure to MPP+ or rotenone. The number
of mitochondria and the average size (pixels) of the mitochondria were
decreased by these toxins. (F) Mitochondrial elongation was reduced by
rotenone (5 and 20nM). All data from 3 separate experiments. Number (n) of
images analyzed: Control (n=45); MPP+ (n=12); Rotenone 5nM (n=9), 20nM
(n=6), 100nM (n=22). Student t-test, * p≤0.05, ** p≤0.01, ***p≤0.001.
Degeneration of DA synapses by PD toxins, MPP+ and rotenone
A prime cellular locus for DA signaling is the synapse. Therefore, we wanted to examine altered DA synapse morphology after exposure to MPP+ or rotenone. In order to identify synapses in culture, we used a fixable FM1-43fx (50nM) (see Materials and Methods). The observation with FM1-43fx was consistent to the additional experiments identifying DA synapses using another synaptic marker anti-synaptotagmin antibody (Figure 4A). The number of DA synapses (Figure 4C) was drastically decreased by 10µM MPP+ and rotenone (5 and 20nM). Furthermore, no DA synapses could be observed after 100nM rotenone treatment. MPP+ decreased the size of DA synapses whereas rotenone (5 and 10nM) did not show effects on DA synapse size (Figure 4D).
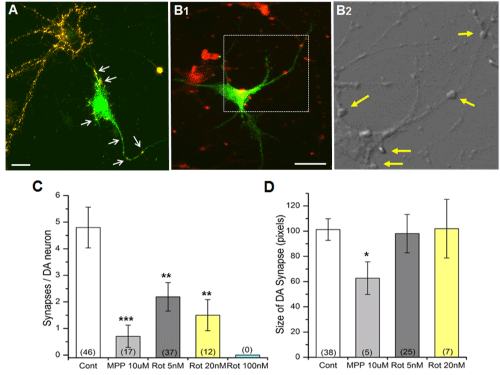
Figure 4: Degeneration of DA synapse by either MPP+ or rotenone. (A) DA synapses identified by overlapping anti-TH (green) and synaptic marker anti-synaptotagmin signals (orange). Examples of DA synapses (antisynaptotagmin positive) indicated by arrows. Scale bar = 30µm. (B1) DA synapses identified by overlapping anti-TH (green) and another synaptic marker FM1-43fx signals (red). Scale bar = 50µm. (B2) Examples of DA synapses (FM1-43fx positive) indicated by arrows. This bright field image is the enlarged image of the rectangle in (B1). (C-D) The number of synapses in DA neurons significantly decreased when the cells were treated with the PD toxins. The number of synapses in DA neurons (C) signficantly. However, the size of DA synapses (D) was not changed by rotenone. The overlapped anti-TH and FM1-43fx images were used to quantify the number of synapses using Image J. No DA synapses were observed when cultures were exposed to 100nM rotenone, and thus DA size was not analyzed for this concentration of rotenone. The numberof neurons analyzed is shown in parentheses. All data from 2 separate experiments. Student’s t-test, * p≤0.05, ** p≤0.01, ***p≤0.001.
Degeneration of DA synaptic mitochondria by MPP+ or rotenone
Finally, we examined effects of PD toxins on mitochondria closely associated with DA synapses (DA synaptic mitochondria). Identification of DA synaptic mitochondria required triple staining of neuronal cultures with anti-TH, MitoTracker Orange (MTO) and FM1-43fx dyes. Figure 5A shows three examples of mitochondria identified with MTO that are in close association with functional synapses in a DA neuron. Mitochondria such as these were analyzed using ImageJ. MPP+ and rotenone drastically decreased the number of DA synaptic mitochondria (Figure 5B). MPP+ also decreased the size of DA synaptic mitochondria. However, there was no difference in synaptic mitochondrial size and elongation after rotenone exposure (Figure 5C & D). This result is different from Figure 3F. At this time, we are not certain whether this observation is related to the potential limitation of triple staining. This might be addressed in future experiments using GFP-tagged mitochondria specifically expressed in DA neurites and a synaptic dye. We observed that some DA synapses possess more than one mitochondrion, thus DA synaptic mitochondria (Figure 5B) outnumbered DA synapses (Figure 4C). Nonetheless, our triple staining protocol enabled us to identify and quantify DA synaptic mitochondria that were altered by two PD toxins, MPP+ and rotenone.
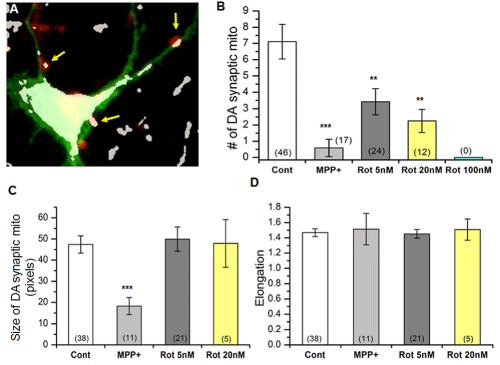
Figure 5: Degeneration of DA synaptic mitochondria by either MPP+ or
rotenone.
(A) Identification of DA synaptic mitochondria using triple staining method
(see Materials and Methods). Using SPOT Advanced software, the anti-
TH (green), MitoTracker Orange (white – pseudocolor) and FM1-43fx (red)
images were overlapped. The resulting overlapped image was used with
ImageJ to quantify synaptic mitochondria. The colocalization function of
ImageJ identified areas where all three images overlapped (indicated by
arrows); considered to be DA synaptic mitochondria.
(B) Reduction of DA synaptic mitochondria after exposure to PD toxins. No
DA synapses were observed when exposed to 100nM rotenone.
(C-D) Changes in size (pixels) (C) of DA synaptic mitochondria and elongation
(D) in the absence or presence of either MPP+ or rotenone. Number of images
analyzed in parentheses. All data from 2 separate experiments from two
different rats. Student’s t-test, * p≤0.05, ***p≤0.001.
Discussion
In this study, we show that DA neurons in rat primary culture are selectively degenerated by PD toxins. Also our data demonstrate that sub-cellular structures (e.g. neurites, synaptic mitochondria) present in DA neurons are degenerated by either MPP+ or rotenone. Morphological properties of mitochondria in DA neurites and synapses were quantified using epifluorescence microscopy and image analysis software (e.g. ImageJ, Neurolucida). Additionally, our data indicates that sub-cellular structure (e.g. mitochondria, synapse) of DA neurons is more sensitive to PD toxins because 5nM rotenone fragmented mitochondria in DA neurites and decreased the number of DA synapses whereas the same concentration did not alter the number of DA neurons (Figure 1). These data are consistent with the hypothesis that DA synapses should be compromised prior to the loss of DA neurons, leading to failure of DA synaptic transmission and ultimately to the development of PD motor symptoms. Better understanding of these early alterations may provide new information related to the onset of PD symptoms and new strategies to better treat patients at this stage of the disease.
We believe this primary DA neuronal culture provides certain advantages over animal or tissue-based models. The first advantage is the ability to observe sub-cellular structures with enhanced resolution. Compared to the traditional rat PD model using the intact midbrain which is three dimensional, the cellular model shows a two dimensional arrangement of neuronal processes and thus allows easy quantification of changes in sub-cellular structures. In contrast, the intact midbrain contains far too many synapses and mitochondria in close proximity to allow such meaningful quantification. It is essential to characterize individual DA synapses and mitochondria to study presymptomatic changes by PD toxins. Second, the DA neuronal culture is easily amenable to pharmacological and biochemical manipulations in order to further dissect pathophysiological mechanisms of PD onset at the sub-cellular level as drugs or chemicals can be directly added to the culture medium. In contrast, for a whole animal model, these chemicals should be introduced systematically, orally or via injection. Given that some mouse models expressing mutant PD genes do not show typical cellular and behavioral symptoms of PD [21, 22], rat DA neuronal culture appears to be a promising model to study early alterations in DA neuronal system.
During the last several decades, many PD models have been developed either using genetic manipulation or challenge by PD toxins [23, 24, 6]. So far, the vast majority of studies using those models have been focusing on mechanisms underlying selective loss of DA neurons and behavioral PD symptoms. However, few PD models have been developed to study early alterations in DA neurons by PD causing factors, either genetic or environmental toxins. To the best of our knowledge, the present study is the first to characterize mitochondria at DA synapses. Considering motor dysfunction in PD is a DA synaptic signaling failure, our ability to examine properties of DA synaptic mitochondria altered by PD causing factors (mutant genes or toxins) will provide a unique opportunity to understand important early alterations in DA synaptic transmission and resulting onset of PD pathology, allowing possible identification of molecular markers for the presymptomatic stage of PD.
MPP+ and rotenone are known to have the same target: complex I in the mitochondria [25]. In general, our data is consistent with such findings because in our study MPP+ and rotenone caused mitochondrial degeneration in DA neurites as evidenced by decreased number and size of mitochondria. However, MPP+ did not alter mitochondrial elongation whereas elongation did decrease in the presence of rotenone. In addition, the effects of these two toxins on DA synaptic morphology were different (i.e. see DA synapse size in Figure 4D). Interestingly, it has been reported that rotenone also disrupts microtubule structure [26] and microglia activation [27], which contributes to rotenone toxicity. Controversy remains as to whether complex I inhibition is required for rotenone toxicity in DA neurons [28]. In our study, mitochondrial morphology was altered because MPP+ and rotenone likely interfered with mitochondrial fusion and/or fission. For example, rotenone causes fragmentation of DA mitochondria (i.e. reduced number, size and elongation of mitochondria; Figure 3), possibly by inhibiting fusion or enhancing fission. This can be validated by blocking drp1 or opa 1 using RNAi gene transfection. Drp1 suppression will result in impairment of fission while Opa1 suppression inhibits fusion [25, 20]. Alternatively, photo-convertible MitoDendra2 can be used to determine whether fusion or fission is inhibited by toxins as this approach allows the live tracking of mitochondrial dynamics such as morphological changes (fission/fusion) [29, 30]. This future study will elucidate new information on mechanisms underlying fragmentation of mitochondria by either MPP+ or rotenone, and possibly distinguish different actions of the two toxins.
Our study shows that sub-cellular structures (e.g. synaptic mitochondria) of DA neurons are degenerated by either MPP+ or rotenone, but we expect the functional properties of DA neuronal signaling to change prior to the alterations of sub-cellular structures. Since alterations of electrophysiological properties by PD causing factors have been understudied, it will be very interesting to examine the functional properties of DA neuronal signaling such as excitability of DA neurons and synaptic DA release [31, 32]. This will be a logical outgrowth of the current study, providing a complete analysis of early alterations induced by PD toxins at the level of DA neuronal function and sub-cellular structure. One more interesting future study will be to examine chronic effects of PD toxins on early changes in DA neuronal signaling as the current study showed only their acute effects (24 hours). Although many PD studies addressed acute effects of PD toxins on DA neurons [33, 30], a chronic PD toxin model with rat DA neuronal culture (e.g. treatments lasting 7 days to 5 weeks) will provide time-dependent changes of presymptomatic structural and functional properties of DA signaling.
Conclusion
- Both rotenone and MPP+ selectively degenerated DA neurons.
- Neurite length of DA neurons was decreased after exposure to either PD toxin.
- The number and size of mitochondria in DA neurites were reduced as a result of either rotenone or MPP+ exposure.
- The number of DA synapses and DA synaptic mitochondria decreased when neurons were exposed to either PD toxin.
- A cell model was successfully developed for primary DA neuronal cultures that can examine sub-cellular changes in DA neurite length and DA synaptic mitochondria.
Acknowledgement
This work was partially supported by the Ohio Musculoskeletal and Neurological Institute (OMNI) and Ohio University Research Committee (OURC) awards.
References
- Beaulieu JM, Gainetdinov RR . The physiology, signaling, and pharmacology of dopamine receptors. below Pharmacol Rev. 2011; 63: 182-217.
- de Silva HR, Khan NL, Wood NW . The genetics of Parkinson's disease. below Curr Opin Genet Dev. 2000; 10: 292-298.
- Gupta A, Dawson VL, Dawson TM . What causes cell death in Parkinson's disease? below Ann Neurol. 2008; 64 Suppl 2: S3-15.
- Dawson TM, Ko HS, Dawson VL . Genetic animal models of Parkinson's disease. below Neuron. 2010; 66: 646-661.
- Fahn S . Description of Parkinson's disease as a clinical syndrome. below Ann N Y Acad Sci. 2003; 991: 1-14.
- Lim KL, Ng CH . Genetic models of Parkinson disease. below Biochim Biophys Acta. 2009; 1792: 604-615.
- Cookson MR . The biochemistry of Parkinson's disease. below Annu Rev Biochem. 2005; 74: 29-52.
- Moore DJ, West AB, Dawson VL, Dawson TM . Molecular pathophysiology of Parkinson's disease. below Annu Rev Neurosci. 2005; 28: 57-87.
- Dauer W, Przedborski S . Parkinson's disease: mechanisms and models. below Neuron. 2003; 39: 889-909.
- Qin Y, Thomas D, Fontaine CP, Colvin RA . Mechanisms of Zn2+ efflux in cultured cortical neurons. below J Neurochem. 2008; 107: 1304-1313.
- Fath T, Ke YD, Gunning P, Götz J, Ittner LM . Primary support cultures of hippocampal and substantia nigra neurons. below Nat Protoc. 2009; 4: 78-85.
- Przedborski S1, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, et al . The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. below J Neurochem. 2001; 76: 1265-1274.
- Dagda RK, Cherra SJ 3rd, Kulich SM, Tandon A, Park D, Chu CT . Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. below J Biol Chem. 2009; 284: 13843-13855.
- Wong MY, Sulzer D, Bamford NS . Imaging presynaptic exocytosis in corticostriatal slices. below Methods Mol Biol. 2011; 793: 363-376.
- Park SS, Lee D . Selective loss of dopaminergic neurons and formation of Lewy body-like aggregations in alpha-synuclein transgenic fly neuronal cultures. below Eur J Neurosci. 2006; 23: 2908-2914.
- Wiemerslage L, Schultz BJ, Ganguly A, Lee D . Selective degeneration of dopaminergic neurons by MPP(+) and its rescue by D2 autoreceptors in Drosophila primary culture. below J Neurochem. 2013; 126: 529-540.
- Bové J, Prou D, Perier C, Przedborski S . Toxin-induced models of Parkinson's disease. below NeuroRx. 2005; 2: 484-494.
- Cannon JR, Greenamyre JT . Neurotoxic in vivo models of Parkinson's disease recent advances. below Prog Brain Res. 2010; 184: 17-33.
- Imai Y, Lu B (2011). Mitochondrial dynamics and mitophagy in Parkinson’s disease: disordered cellular power plant becomes a big deal in a major movement disorder. Current Opinion in Neurobiology 21: 935-941.
- Itoh K, Nakamura K, Iijima M, Sesaki H . Mitochondrial dynamics in neurodegeneration. below Trends Cell Biol. 2013; 23: 64-71.
- Abeliovich A, Schmitz Y, Fari&nTilde;as I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N . Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. below Neuron. 2000; 25: 239-252.
- Beal MF (2010) Parkinson’s disease: a model dilemma. Nature Outlook – Parkinson’s disease S8.
- Shimohama S, Sawada H, Kitamura Y, Taniguchi T . Disease model: Parkinson's disease. below Trends Mol Med. 2003; 9: 360-365.
- Botella JA, Bayersdorfer F, Gmeiner F, Schneuwly S . Modelling Parkinson's disease in Drosophila. below Neuromolecular Med. 2009; 11: 268-280.
- Perier C, Vila M . Mitochondrial biology and Parkinson's disease. below Cold Spring Harb Perspect Med. 2012; 2: a009332.
- Choi WS, Palmiter RD, Xia Z . Loss of mitochondrial complex I activity potentiates dopamine neuron death induced by microtubule dysfunction in a Parkinson's disease model. below J Cell Biol. 2011; 192: 873-882.
- Gao HM, Hong JS, Zhang W, Liu B . Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. below J Neurosci. 2002; 22: 782-790.
- Marella M, Seo BB, Nakamaru-Ogiso E, Greenamyre JT, Matsuno-Yagi A, Yagi T . Protection by the NDI1 gene against neurodegeneration in a rotenone rat model of Parkinson's disease. below PLoS One. 2008; 3: e1433.
- Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, et al . Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. below Proc Natl Acad Sci U S A. 2008; 105: 19318-19323.
- Hwang RD, Wiemerslage L, LaBreck CJ, Khan M, Kannan K, Wang X, Zhu et al (2014) The neuroprotective effect of human uncoupling protein 2 (hUCP2) requires cAMP-dependent protein kinase in a toxin model of Parkinson's disease. Neurobiol Dis. 69:180-191.
- Koh DS, Hille B . Rapid fabrication of plastic-insulated carbon-fiber electrodes for micro-amperometry. below J Neurosci Methods. 1999; 88: 83-91.
- Kim KT, Koh DS, Hille B . Loading of oxidizable transmitters into secretory vesicles permits carbon-fiber amperometry. below J Neurosci. 2000; 20: RC101.
- Stephans SE, Miller GW, Levey AI, Greenamyre JT . Acute mitochondrial and chronic toxicological effects of 1-methyl-4-phenylpyridinium in human neuroblastoma cells. below Neurotoxicology. 2002; 23: 569-580.