
Review Article
J Mol Biol & Mol Imaging. 2015;2(1): 1012.
Molecular Imaging of Angiogenesis in Cardiovascular Diseases
Ziwei Huang1, Wei Du1, Zuo-Xiang He2, Zongjin Li1*
1Department of Pathophysiology, Nankai University School of Medicine, China
2Department of Cardiac Nuclear Imaging, Fuwai Hospital, Peking Union Medical College & Chinese Academy of Medical Sciences, China
*Corresponding author: Zongjin Li, MD, Nankai University School of Medicine, China
Received: November 27, 2014; Accepted: January 02, 2015; Published: January 06, 2015
Abstract
Cardiovascular diseases (CVD) are leading cause of mortality and morbidity worldwide. Recent advances in molecular imaging provide invaluable tools for evaluating angiogenesis in CVD. It has been widely regarded that angiogenic therapy is an attractive approach for treating ischemic heart disease; conversely, a variety of studies suggest that neovascularization contributes to the growth of atherosclerotic lesions and is a key factor in plaque destabilization leading to rupture. Moreover, angiogenesis is not only critical for atherosclerosis and other cardiovascular diseases, but also for assessing the consequences of therapeutic intervention. The developments of potential biological targets for imaging angiogenesis and imaging tools have expanded our eyesight to encompass many important components of the processes of angiogenesis, either in physiological activities or pathological progressions. This review will focus on recent advances on noninvasive approaches for direct evaluation of the molecular events associated with angiogenesis in CVD, as well for prediction responses and tracking therapeutic efficacy of angiogenic therapy.
Keywords: Molecular imaging; Angiogenesis; Cardiovascular disease
Abbreviations
CVD: Cardiovascular Disease; MRI: Magnetic Resonance Imaging; PET: Positron Emission Tomography; SPECT: Single Photon Emission Tomography; RGD: Arg-Gly-Asp peptides; VEGF: Vascular Endothelial Growth Factor
Introduction
The concept and practice of molecular imaging, defined as the in vivo characterization and measurement of biological processes at cellular and molecular level within living organisms, has been present for decades and originated with targeted nuclear imaging [1, 2]. Non-invasive molecular imaging techniques, such as computerized tomography (CT), magnetic resonance imaging (MRI), ultrasound (US), positron emission tomography (PET)/single photon emission computed tomography (SPECT), have already been the backbone for diseases detection and diagnosis [3-5].
Ischemic cardiovascular disease (CVD) accounts for approximately 30% of all deaths in the United States [6]. The attempts to influence the processes of angiogenesis in atherosclerosis and other cardiovascular diseases for therapeutic propose have emerged as a major unresolved issue. Angiogenic therapy has been widely regarded as an attractive approach for treating ischemic heart disease; conversely, a variety of studies suggest that neovascularization contributes to the growth of atherosclerotic lesions and is a key factor in plaque destabilization leading to rupture [7]. Though improvements in understanding the CVD have helped reduce the death rate, a clearer prescription of angiogenesis accounting for the development of atherosclerosis, stroke, myocardial infarction and therapeutic angiogenesis is required. With the abilities of living imaging specific molecular targets and fundamental biological processes in vivo, molecular imaging approaches will be critical for monitoring angiogenesis processes in diseases, as well as evaluating physiological consequences of the therapeutic intervention.
Modalities of Molecular Imaging
Molecular imaging aims at sensing specific molecular targets, fundamental biological processes and certain cell types in living subjects [2]. A number of methods are available to track molecular events in vivo by molecular imaging [4] (Figure 1). The essence of molecular imaging is the interaction between probes and targeted markers. Initially, antigens on cell-specific surface or epitopes with radio-labeled monoclonal antibodies are regarded as the beginning of imaging methods. Often, the probes are associated with specific molecular biological events in physiological or pathological processes [3]. Successful molecular imaging relies on both probes with high affinity, sensitivity, specificity and innovations of imaging technologies. Positron emission tomography (PET) and single photon emission tomography (SPECT) are two traditional main nuclear modalities for imaging of molecular and cellular processes in vivo. Besides the superior sensitivity, these two imaging systems also have another important advantage of wide range of imaging probes accessible for analyzing molecular processes in vivo. Ultrasound and magnetic resonance imaging (MRI) also help to assess cardiovascular diseases and therapeutic interventions. MRI has good spatial resolution and tissue penetration and a high susceptibility to motion artifacts; however, there are not as many probes available as PET and SPECT.
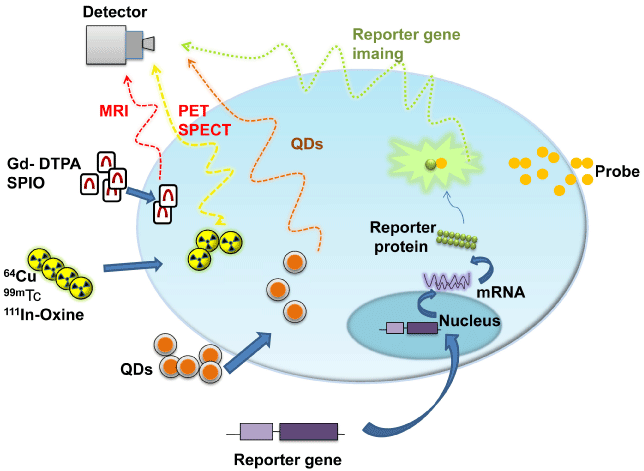
Figure 1: Conceptional introduction of noninvasive molecular imaging. It
prescribes simple principles of imaging techniques including MRI, PET/
SPECT, QDs and gives some example probes and targets for imaging.
Abbreviations: MRI: Magnetic Resonance Imaging; PET: Positron Emission
Tomography; SPECT: Single Photon Emission Tomography; QD: Quantum
Dots; Gd-DTPA: Gadolinium-Diethylenetriamine Penta-acetic Acid; SPIO:
Super Paramagnetic Iron Oxide; 99mTc: 99mTc-hexamethylpropylene amine
oxime; 111In-Oxine, 111In-oxyquinoline. Reproduced with permission from [4].
There’s no imaging modality meeting all clinical needs optimally. The choice of an imaging modality is depended on the reflection that the therapeutic targets cast on the biological processes [3]. Moreover, the success of molecular imaging rests on the development targeted biological markers of molecular and physiological processes, development of new instruments with improved sensitivity and resolution and the establishment of multidisciplinary teams of experimental and clinical investigators with a wide range of expertise [8]. Since the role of angiogenesis plays in the CVD is so controversial that targeted imaging of the process is necessary. To make the goal realizable, the developments of molecular imaging techniques and imaging marker are essential. We expect that targeted imaging of angiogenesis can contribute to improving translation of animal research to the clinical applications in near future.
Angiogenesis in Cardiovascular Diseases
Angiogenesis is generally defined as the sprouting of new capillaries from pre-existing micro vessels, which is a complicated process involving multiple cell types, numerous growth factors and complex regulatory checks and balances orchestrated by both stimulatory and inhibitory factors [9, 10]. During angiogenesis major physiological stimulatory conditions known for certain include tissue ischemia and hypoxia, inflammation and shear stress, et al [11]. To now, a variety of local and circulating factors have been identified to get involved in the angiogenesis process, including vascular endothelial growth factor (VEGF), hypoxia-inducible factor1α (HIF- 1α), transforming growth factor beta (TGF-β) and angiopoietins, in which VEGF is the most critical mediator. Besides, the angiogenic response is also modulated by the composition of the extracellular matrix (ECM) and intercellular adhesions, including integrins [8]. Integrins are a family composed of 24 αβ heterodimeric cell adhesion receptors capable of mediating the attachment of cells to the ECM or the interaction between specialized cells. Particularly, the αvβ3 integrin is expressed in angiogenic vessels. Since allowing cells to interact with the ECM and acting in the migration, proliferation, differentiation and survival of cellular processes, the αvβ3 integrin is considered as another significant novel target for imaging angiogenesis in addition to the VEGF. As a result, the two factors remain as a focus for multiple molecular imaging studies while diagnosing or treating diseases characterized by angiogenesis. In addition, many factors controlling and influencing angiogenesis including soluble growth factors, membrane-bound proteins, cell-matrix and cell-cell interactions and many interacting systems have been used as imaging targets for angiogenesis [11].
Angiogenesis in atherosclerosis
The growing worldwide health challenge of cardiovascular diseases (CVD) has caused a remarkable need for early diagnosis and sequential treatment. The majority deaths nowadays can be accounted for myocardial infarction and stroke resulting from atherosclerosis. During the process, one of the major pathologic features of atherosclerosis is atherosclerotic lesion. In fact, atherosclerosis is a result of a series of highly specific molecular and cellular inflammatory responses of the vessel wall. At first, the lesion appears as a reversible fatty-streak state. Then it develops to a plaque containing foam cells, mast cells, macrophages and other cells, forming a necrotic core. Eventually, luminal stenosis happens, leading to myocardial ischemia [12]. What’s more dangerous is that the atherosclerotic lesion can sometimes rupture and form thrombus in the vessels.
With the burgeoning in the understanding of the molecular pathways involved in atherogenesis and lesion progression and the mechanisms underlying the complications of human atherosclerotic plaques [13], new techniques and therapy methods are on the way to help. Imaging methodologies are introduced to identify the lesions in relevant vascular beds, which may then alter or guide current therapies in patients with cardiovascular diseases. A large amount of imaging targets for CVD have been reported [14], including macrophages, annexin V, protease activity, angiogenesis, thrombosis, fibrin and platelets, targeting the inflammation progression and rupture (Figure 2).
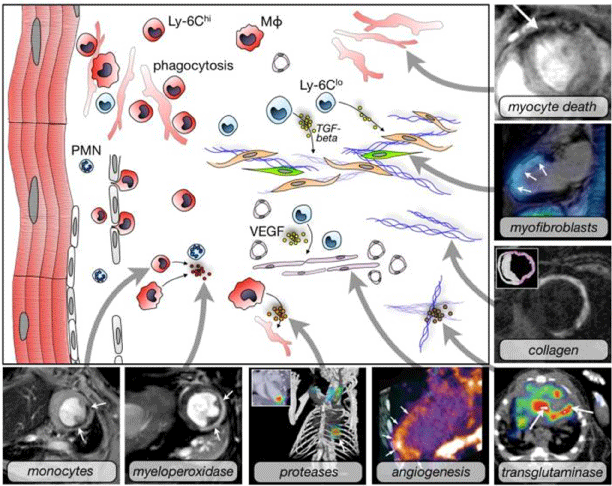
Figure 2: Molecular imaging targets for cardiovascular disease. It introduces
some imaging targets using different imaging techniques or multimodality
imaging platforms. The set includings are monocytes (iron oxide
nanoparticles, T2-weighted MRI), myeloperoxidase (MPO-Gd, T1-weighted
MRI), proteases (FMT-CT), angiogenesis (integrin, PET-CT), myocyte death
(annexin V-decorated iron oxide nanoparticles), myofibroblasts (integrin,
fused SPECT/MR), collagen (MRI), transglutaminase (factor XIII, SPECTCT).
Among them, angiogenesis is one of the most characteristic targets
used in molecular imaging in monitoring and diagnosis. Reproduced with
permission from [14].
Angiogenesis is one of the most leading candidates which promise to affect the clinical applications. Different from the tumor growth, the role of angiogenesis in atherosclerosis is remained unsolved and consensus cannot be reached on the problem. Debates surrounding the pathogenic role of angiogenesis in cardiovascular diseases such as atherosclerosis has been particularly energetic [15]. The controversy to the problem shows that the pathological process of cardiovascular diseases, particularly atherosclerosis is extensively complicated. Koester noted the association between neovascularization and atherosclerosis in 1876 [16], which was observed by others later. More specific mechanisms are discovered in the following century and the concept that angiogenesis is not just a bystander but a key player in atherosclerosis is evidenced by mouse models of atherosclerosis [12]. Recent researches reveal that pathogenic angiogenesis often emerges in the plaque of atherosclerosis, which promotes the development of atherosclerosis or even triggers in traplaque hemorrhage and potential complications [14]. However, the promotion of angiogenesis is an attracting therapy on myocardial ischemia. Although more studies are still on the way about the mechanisms, there is no doubt that therapeutic angiogenesis is an exciting field of cardiovascular medicine, filling some limitations that surgeries cannot break through. As a result, it’s a highly contentious tissue whether angiogenesis is a key causative factor in the pathogenesis of atherosclerotic plaque formation or is a way to treat coronary heart disease [17].
Angiogenesis in myocardial infarction
Angiogenesis plays an important role in infarct healing and left ventricular remodeling following myocardial infarction and infarct remodeling has important implications for the prognosis following myocardial infarction [18]. Myocardial angiogenesis will help treat cardiac disorders concerning ischemia. During the process of angiogenesis, a number of cytokines and related cells can be detected to help evaluate. Recently, a preliminary SPECT study of 10 acute myocardial infarction patients imaging integrin αVβ3-targeted agent to predict left ventricular remodeling (99mTc-Nc 100692, GE Healthcare, Oslo, Norway) [19]. Further analysis will also help allow assessment in post-myocardial infarction patients.
Therapeutic angiogenesis
The consequences of cardiovascular disease are likely to be lethal (eg, myocardial infarction, heart failure), even if multiple surgical therapies are commonly used to help patients. In fact, ischemic cardiovascular diseases are the number one cause of mortality in the United States and a major cause of morbidity and health-care use [20], which appeals for more effort on the better and more useful treatments. As a result, stem cell therapy has appeared as a powerful option and generated significant interest [21-23].
As mentioned before, angiogenesis can occur following many physiologic and pathologic processes, for example, it is associated with recovery from accidental or surgical wounds. With more bioassays and pre-clinical experiments performed, progress in understanding the relationship between cardiovascular diseases and angiogenesis has created a new perspective on therapeutic angiogenesis. Previous preclinical trials have already erected the concept that new vessels can help the ischemia. However, the translation to the clinical application is frustrating. Angiogenesis here is a kind of capillary proliferation based on ischemia beds and hypoxia is the major driver. It’s not already clear that whether it is sufficient to compensate for ischemia resulting from a proximal occlusion of a major arterial trunk, as is the case with many patients with coronary or peripheral vascular disease [24]. However, the host of stimulatory and inhibitory factors offers a huge amount of targets for therapeutic interventions and molecular imaging, which will promote a further understanding over the real role of angiogenesis in cardiovascular diseases.
Molecular Imaging of Angiogenesis in Cardiovascular Diseases
To help noninvasive observe the cardiovascular system, molecular imaging tools and targets are two important fields to explore for the availability to be used in clinical assessment [14]. Usually, techniques are needed to image the vulnerable lesions in blood vessels and myocardial ischemia condition. When diagnosing atherosclerosis, the imaging of macrophages, annexin V, protease activity and angiogenesis can help to define the disease stage. When diagnosing thrombosis, imaging markers like fibrin and platelets are useful. When diagnosing myocardial infarction, angiogenesis and stem cells for regeneration are developed into the clinical application. As a result, imaging angiogenesis can reflect the cardiovascular condition, besides imaging specific targets in the activity indirectly means cardiovascular imaging. As mentioned above, imaging techniques like MRI, PET/SPECT are indispensable tools to help observe and figure out the exact condition of cardiovascular system.
Due to the important role the angiogenesis plays in many kinds of diseases as mentioned above, angiogenesis is regarded as an exciting frontier of therapy. Since the role of angiogenesis in atherosclerosis and other CVD has emerged as a major unresolved issue [12], the molecular mechanisms involved in angiogenesis need analyzing and the observation through molecular targeted imaging is necessary. Molecular imaging of reliable biomarkers to monitor the activity of the angiogenic factors will flourish the development in this frontier.
Imaging ofαVβ3 integrin
Vascular biology of atherosclerosis suggests several molecular processes that can serve as imaging targets [13], includingαVβ3 integrin and VEGF as angiogenesis markers, adhesion molecules in endothelial activation, etc. They can be markers both for tracking the process of atherosclerosis and for emerging therapeutic interventions. TheαVβ3 integrin is found abundant on the surface of proliferating endothelial cells and known to modulate angiogenesis, thus representing a main target for imaging. Advanced molecular probes relevant toαVβ3 integrin has been developed in PET and SPECT imaging for assessing angiogenesis, thus multiple studies are undertaken and confirm that increasedαVβ3 integrin activity is present within the myocardium at both early and late time points post-infarction [19, 25, 26] (Figure 3). The earliest research focused on in vivo using magnetic resonance imaging targetedαVβ3 integrin can be dated back to the noninvasive detection via a paramagneticlabeled monoclonal antibody examination. However, further studies have been limited by the poor clearance of the antibody from the blood. Then, Haubner et al focused on the Arg-Gly-Asp (RGD) peptides, which are known to have high affinities for theαVβ3 integrin [27]. Given these preliminary work, it is supported that radio labeled targets of theαVβ3 integrin for imaging of angiogenesis provide a useful technique that can be applied to clinical researches.
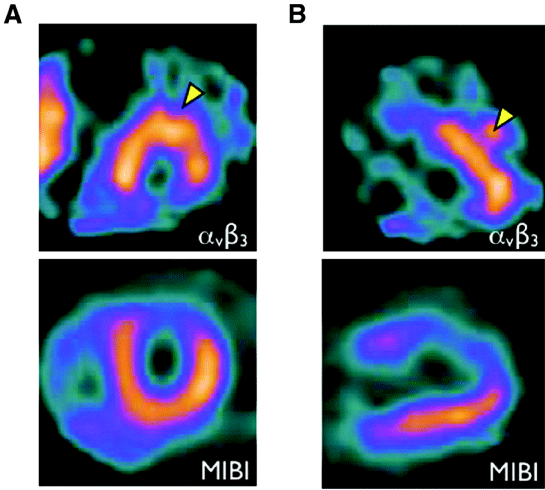
Figure 3: Imaging of αVβ3 integrin to monitor angiogenesis. Three weeks
after myocardial infarction, the patient was injected integrin αVβ3-targeted
agent (99mTc-NC100692). As arrow heads, in the infarct zone, focal signal
enhancement shows the expression of integrin αVβ3, which reflects the
condition of angiogenesis. A: the short-axis; B: long-axis. Reproduced with
permission from [19].
Specifically, 111In-RP748 and99mTc-labeled peptide (NC100692) are further reported for in vivo imaging of myocardial angiogenesis. It appears that 111In-RP748 has association with the activation of theαVβ3 integrin in infarct region, additionally; trials of rats also supported that regional myocardial retention of 111In-RP748 related to the focal uptake of a radio labeled nitroimidazole (BRU-5921) in the reperfused infarcted region [28]. Therefore, the argument that 111In-RP748 is a targeted marker of angiogenesis is supported, which is also a stimulator in myocardial hypoxia regions.
99mTc-labeled peptide is another technetirum-labeled cyclic RGD peptide that has been used in a variety of studies to noninvasively assess angiogenesis [29]. It was demonstrated by a group at Yale University in rodent models of hind limb ischemia [5]. The result was further confirmed by the close correlation of ex vivo tissue analysis (gamma counting) and immune fluorescence staining [5]. In addition to these markers, promising targeted imaging of other integrin like αVβ5 can be also taken into account. And the goals for the years to come may covert the translation from experiment work to visualization of these appealing biologic targets in humans. It seems considerable challenges to bridge the gap between laboratory experiments to the clinic.
Imaging of VEGF or VEGF receptors
Another indispensable target in angiogenesis is VEGF. The close association of angiogenesis with the expression of VEGF and VEGF receptors is well recognized, while the impact of VEGF on atherosclerosis plaque is in vigorous debate. There are four known isoforms of VEGF ligands, modulating the angiogenic effects by binding to specific receptors. Thus the VEGF receptors (VEGFR-1, VEGFR-2,VEGFR-3) are rational targets for imaging ischemiainduced angiogenesis. It appears receptor dimerization and subsequent intracellular signal transduction via tyrosine kinases [30]. Varieties of researches have targeted imaging of angiogenesis by radio labeling (124I and 123I) angiogenic receptors in animal models to identify ischemia tissue. A recombinant human form of VEGF121 associated with antibodies is examined in initial studies of VEGF. However, the preclinical applications of these approaches are partly limited by the total VEGF121 receptor density and the slower than usual clearance rates of these antibodies. Then evolution in new probes comes into existence. Marina V Backer et al described single-chain VEGF-based probes [31], which makes investigations more efficient and helps monitor the up regulation of VEGF during angiogenesis. There is also 64Cu-6DOTA-VEGF121 imaged as the tracer in a rat model of myocardial infarction for angiogenesis [32].
The development of VEGF receptor imaging raises the hope for applications in clinical intervention and the evaluation of therapeutic angiogenic strategies. In cardiovascular disease and other peripheral vascular diseases, the identification of VEGF receptors contributes to the option of sites for local injection of angiogenic treatments. Moreover, various angiogenesis-stimulating factors like platelet-derived growth factor (PDGF), fibroblast growth factors (aFDF and bFGF), TGF-β, Eph-B4/ephrin-B2, VE-cadherin and angiopoietin can be used for angiogenesis imaging (Figure 4). Furthermore, several molecular processes involved in the vascular biology of atherosclerosis, such as the leukocyte adhesion molecules in endothelial dysfunction, activated macrophage and ongoing leukocyte recruitment in inflammatory activation, can shed light on the imaging targets of angiogenesis.
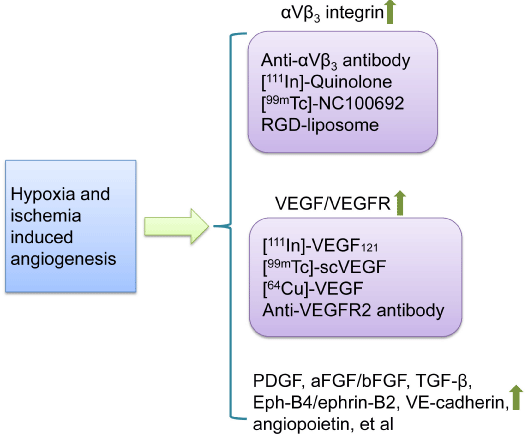
Figure 4: Targets for molecular imaging angiogenesis. Resulting from
ischemia and hypoxia in the organs and tissues like heart, the expression
of an amount of molecular mediating angiogenesis are up regulated to
response. Imaging probe scan be developed for targeting those molecules.
Abbreviations: RGD peptide: Peptide Composed of Arg-Gly-Asp Sequence;
VEGF: Vascular Endothelial Growth Factor; VEGFR: Vascular Endothelial
Growth Factor Receptor; scVEGF: single-chain VEGF; PDGF: Platelet-
Derived Growth Factor; aFGF and bFGF: acidic and basic Fibroblast Growth
Factors; TGF-β: Transforming Growth Factor-β.
Multimodality imaging
As mentioned, imaging techniques are expected to supply anatomic, structural, functional, molecular and genomic information for the diagnosis and therapy while none of them can meet all the clinical needs currently. As a result, multimodality imaging has gained appeal for having the advantage of harnessing the strengths of different imaging methods [32] (Figure 5). Often, CT and MRI possess high anatomic resolution while SPECT/PET can provide high detection of sensitivity. Thus optical modalities and radionuclide methods can be combined to furnish synergistic and complementary information clinically. With the appearance of the first commercial PET/CT system, the approach was rapidly embraced by increasingly medical professions. There are also some outstanding issues though including partial volume effect, lesion segmentation, reconstruction timing and protocol workflows, which restrict the acceptance to increase in clinical assessment. In addition to further developments of PET/CT system, PET/MRI system is on the way. However, more challenges exist and many troubles need solving. Multimodal probes are another important field for multimodality imaging, which can be divided into two categories: (1) probes that enable multiple in vivo imaging molecular readouts and (2) probes that enable in vivo imaging and concomitant targeted therapy [33]. Nanoparticles platform is promising for multimodality imaging in vivo [3].
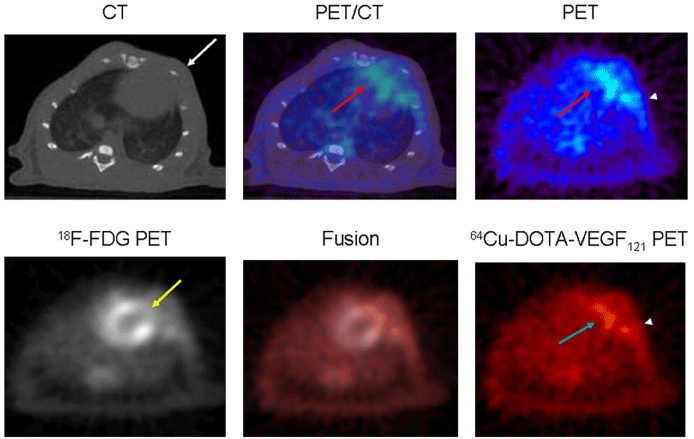
Figure 5: Multimodality imaging of angiogenesis in a rat model of MI. Micro CT (up left) image provides the anatomic landscape for localizing and PET (up/down
right) generates the functional and molecular data. The fusion in the middle is an example explored in multimodality molecular imaging. There is increased uptake
in surgical wound area (arrowheads) in both PET images and the fusion images. Reproduced with permission from [32].
Translational Prospects
Treatment of patients with cardiovascular diseases increasingly incorporates molecular and cellular markers of disease into management algorithms [2]. Various imaging approaches that can visualize molecular targets have limitations from many aspects. Before using in human, one clinical-grade material, especially probes and agents, require extensive toxicological testing in trials. Another important issue is the practicality, for example, the use of MRI in the coronary circulation is impeded by the cardiac and respiratory motion where plaque rupture is likely to have considerable clinical impact on the organ [34]. As for the signal, imaging techniques should satisfy the requirements for spatial and temporal resolution, which means high sensitivity and penetration to guarantee the signal strength. Though molecular imaging is still in the infancy, it shows promise for providing biological details of atherosclerosis, myocardial infarction and others.
Acknowledgments
This work was partially supported by grants from the National Natural Science Foundation of China (81320108014, 81371620), Tianjin Natural Science Foundation (12JCZDJC24900) and Program for Changjiang Scholars and Innovative Research Team in University (IRT13023).
References
- Massoud TF, Gambhir SS . Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003; 17: 545-580.
- Lindner JR . Molecular imaging of thrombus: technology in evolution. Circulation. 2012; 125: 3057-3059.
- Wang L, Wang Y, Li Z. Nanoparticle-based tumor theranostics with molecular imaging. Curr Pharm Biotechnol. 2013; 14: 683-692.
- Tong L, Zhao H, He Z, Li Z. Current Perspectives on Molecular Imaging for Tracking Stem Cell Therapy. OF E, editor: InTech; 2013 2013-02-20.
- Mitchel R. Stacy P, Mark W. Maxfield, MD, and Albert J. Sinusas, MD. Targeted molecular imaging of angiogenesis in PET and SPECT: A review. Yale J Biol Med. 2012; 85: 75-86.
- Li Z, Han Z, Wu JC. Transplantation of human embryonic stem cell-derived endothelial cells for vascular diseases. J Cell Biochem. 2009; 106:194-199.
- Khurana R, Simons M, Martin JF, Zachary IC. Role of Angiogenesis in Cardiovascular Disease: A Critical Appraisal. Circulation. 2005; 112: 1813-1824.
- Dobrucki LW, Sinusas AJ . Cardiovascular molecular imaging. Semin Nucl Med. 2005; 35: 73-81.
- Mitsos S, Katsanos K, Koletsis E, Kagadis GC, Anastasiou N, Diamantopoulos A, et al. Therapeutic angiogenesis for myocardial ischemia revisited: basic biological concepts and focus on latest clinical trials. Angiogenesis. 2012; 15:1-22.
- Neeman M, Gilad AA, Dafni H, Cohen B. Molecular imaging of angiogenesis. J Magn Reson Imaging. 2007; 25:1-12.
- Papetti M, Herman IM . Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002; 282: C947-970.
- Pant S, Deshmukh A, Mehta JL. Angiogenesis in atherosclerosis: An overview. In: Mehta J, Dhalla, Naranjan S. (Eds.), editor. Biochemical Basis and Therapeutic Implications of Angiogenesis. 6: Springer; 2013. p. 209.
- Libby P, DiCarli M, Weissleder R. The vascular biology of atherosclerosis and imaging targets. J Nucl Med. 2010;51 Suppl 1(Supplement 1): 33S-37S.
- Leuschner F, Nahrendorf M. Molecular imaging of coronary atherosclerosis and myocardial infarction: considerations for the bench and perspectives for the clinic. Circ Res. 2011;108: 593-606.
- Tahara N, Imaizumi T, Virmani R, Narula J. Clinical feasibility of molecular imaging of plaque inflammation in atherosclerosis. J Nucl Med. 2009; 50: 331-334.
- Koester W. Endarteritis and arteritis. Berl Klin Wochenschr. 1876; 13: 454-455.
- Khurana R, Simons M, Martin JF, Zachary IC . Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005; 112: 1813-1824.
- Gao H, Lang L, Guo N, Cao F, Quan Q, Hu S, et al. PET imaging of angiogenesis after myocardial infarction/reperfusion using a one-step labeled integrin-targeted tracer 18F-AlF-NOTA-PRGD2. Eur J Nucl Med Mol Imaging. 2012; 39: 683-692.
- Jaffer FA, Libby P, Weissleder R. Molecular Imaging of Cardiovascular Disease. Circulation. 2007; 116: 1052-1061.
- Laufer EM, Winkens MHM, Narula J, Hofstra L. Molecular Imaging of Macrophage Cell Death for the Assessment of Plaque Vulnerability. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009; 29:1031-1038.
- Li Z, Wilson KD, Smith B, Kraft DL, Jia F, Huang M, et al. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS One. 2009; 4: e8443.
- Li Z, Lee A, Huang M, Chun H, Chung J, Chu P, et al. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009; 53: 1229-1240.
- Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, et al. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007; 116: I46-54.
- Ruggiero A, Thorek DL, Guenoun J, Krestin GP, Bernsen MR. Cell tracking in cardiac repair: what to image and how to image. Eur Radiol. 2012; 22: 189-204.
- Kalinowski L, Dobrucki LW, Meoli DF, Dione DP, Sadeghi MM, Madri JA, et al. Targeted imaging of hypoxia-induced integrin activation in myocardium early after infarction. J Appl Physiol 2008; 104: 1504-1512.
- McAteer MA, Akhtar AM, von Zur Muhlen C, Choudhury RP. An approach to molecular imaging of atherosclerosis, thrombosis, and vascular inflammation using micro particles of iron oxide. Atherosclerosis. 2010; 209:18-27.
- Haubner R, Weber WA, Beer AJ, Vabuliene E, Reim D, Sarbia M, et al. Noninvasive visualization of the activated alphavbeta3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med. 2005; 2: e70.
- Choudhury RP, Fisher EA. Molecular imaging in atherosclerosis, thrombosis, and vascular inflammation. Arterioscler Thromb Vasc Biol. 2009; 29: 983-991.
- Cormode DP, Skajaa T, Fayad ZA, Mulder WJ. Nanotechnology in medical imaging: probe design and applications. Arterioscler Thromb Vasc Biol. 2009; 29: 992-1000.
- Jaffer FA, Libby P, Weissleder R. Optical and multimodality molecular imaging: insights into atherosclerosis. Arterioscler Thromb Vasc Biol. 2009; 29: 1017-1024.
- Kraitchman DL, Bulte JW. In vivo imaging of stem cells and Beta cells using direct cell labeling and reporter gene methods. Arterioscler Thromb Vasc Biol. 2009; 29: 1025-1030.
- Rodriguez-Porcel M, Cai W, Gheysens O, Willmann JK, Chen K, Wang H, et al. Imaging of VEGF receptor in a rat myocardial infarction model using PET. J Nucl Med. 2008; 49: 667-673.
- Jaffer FA, Libby P, Weissleder R. Optical and Multimodality Molecular Imaging: Insights Into Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009; 29: 1017-1024.
- Chiribiri A, Ishida M, Nagel E, Botnar RM. Coronary imaging with cardiovascular magnetic resonance: current state of the art. Prog Cardiovasc Dis. 2011; 54: 240-252.