
Research Article
J Mol Biol & Mol Imaging. 2015;2(1): 1013.
Development of a New Molecular Probe for the Detection of Inflammatory Process
Laurent S1,2*, Stanicki D1, Boutry S2, Roy JC1, Vander Elst L1,2, Muller RN1,2
1Department of General, Organic and Biomedical Chemistry, NMR and Molecular Imaging Laboratory, University of Mons, Belgium
2Center for Microscopy and Molecular Imaging (CMMI), Belgium
*Corresponding author: Sophie Laurent, Department of General, Organic and Biomedical Chemistry, NMR and Molecular Imaging Laboratory, University of Mons, Center for Microscopy and Molecular Imaging (CMMI), Belgium
Received: November 27, 2014; Accepted: January 02, 2015; Published: January 06, 2015
Abstract
With the aim of developing an imaging tool for detecting and localizing inflammation, iron oxide-based contrast agents for magnetic resonance imaging (MRI) were synthesized and targeted to E-selectin; an endothelial protein that is overexpressed at early stages of inflammation. Iron oxide nanoparticles were thus linked to a synthetic E-selectin ligand (sialyl Lewis X) mimetic. The soobtained molecular MRI contrast agent was physico-chemically characterized and tested on cell cultures expressing E-selectin. Those stable and wellcharacterized iron oxide nanoparticles showed significant signs of E-selectin specificity.
Keywords: Inflammation; Sialyl Lewis X; Iron oxide nanoparticles; E-selectin
Introduction
The early detection and localization of inflammatory process constitute one of the greatest challenge of medical imaging[1-3]. E-selectin (CD62E, ELAM-1) is a cell adhesion molecule expressed on endothelial cells during inflammatory processes. It initiates interactions between the endothelial cell and circulating leukocytes, preceding leukocyte diapedesis into inflamed tissue [4]. This protein specifically recognizes and binds the tetrasaccharide sialyl Lewis X located on the surface proteins of leukocytes. This interaction triggers leukocyte « rolling » on inflamed endothelial cells which is the first step of their strong adhesion and transmigration to the surrounding tissues. Although E-selectin plays an important role in the physiological mechanisms regulating inflammation reactions, it is now established that this protein is also heavily involved in several disorders including, among others, inflammatory and cardiovascular disorders or some cancers. Keeping this in mind, we decided therefore to develop a new molecular probe for MRI able to selectively target E-selectin.
Methods
Synthesis of the nanoplatform
A magnetic platform (ultra small super paramagnetic iron oxide nanoparticles) was stabilized after treatment with 3-(triethoxysilyl) propylsuccinic anhydride (TEPSA) as reported elsewhere [5]. Briefly, iron oxide cores were prepared by co-precipitation iron chloride salts in diethyleneglycol (DEG) at 170°C. Carboxylic functions have been introduced by treating the suspension with an organ functional silane (i.e. TEPSA). The chosen vector is a mimetic of sialyl Lewis x (SLex), a natural ligand of selectins. Its synthesis was performed following a protocol previously described [6]. A small amount of mimetic hydrochloride salt (4 μmol; 2.5 mg) was added to an aqueous dispersion of TEPSA-modified nanoparticles (150 mM in iron; 5 ml) in the presence of N-(3-dimethylaminopropyl)- N’-ethylcarbodiimide hydrochloride (50 μmol; 10 mg) as a coupling agent at pH 7.5. After one night under stirring, the ferrofluid was purified by membrane filtration (membrane cut-off = 30,000 kDa) and finally centrifuged (16,500 G; 40 minutes). In order to ensure the colloidal stability of the as-prepared particles, O-(2-aminoethyl)- O’-methylpolyethyleneglycol (120 μmol; 90 mg) was added to the ferrofluid in the presence of EDC (200 μmol; 38 mg). The pH was then adjusted to 7.5 and the mixture stirred at room temperature. After 15 hours of reaction, the suspension was purified by membrane filtration (membrane cut-off = 30 kDa).
Non specific nanoparticles were prepared as explained before by treating TEPSA-modified NPs with O-(2-aminoethyl)-O’- methylpolyethyleneglycol (without the sialyl Lewis X vector; (PEGNPs)).
Physico-chemical characterization
Size was measured by transmission electron microscopy (TEM, Microscope Leo960E operating at an accelerating voltage of 60kV (Oregon, USA)) and diffusion light scattering (DLS, Zetasizer NanoS from Malvern Instrument (Worcestershire, UK)). The molecular probe was obtained by combining a mimetic of sialyl Lewis X (SLex) to the silanized magnetic platform by amide-bond coupling. The 1H NMRD profiles were recorded using a Stelar Fast Field Cycling relaxometer (Mede, Italy). The system operates over a range of magnetic field extending from 0.25 mT to 0.94 T (0.01-40 MHz) at 37°C. T1 and T2 measurements were performed on Bruker Minispec mq20 and mq60 (Karlsrushe, Germany) working respectively at a Larmor frequency of 20 MHz (0.47T) and 60 MHz (1.41T) at 37°C.
The relaxation rates were measured as a function of the iron molar concentration at 0.47 and 1.41 T in order to calculate the r1 and r1 relaxivities (defined as the enhancement of the relaxation rate of water protons in 1 mmol/L solution of contrast agents). The relaxivities were calculated as the slope of relaxation rate (Ri obs) versus iron concentration according to the equation:
Ri obs = 1/Ti obs = ri [Fe] + 1/Tidia
ribeing the relaxivities and Tidia being the proton relaxation times in aqueous solutions without nanoparticle.
The total iron concentration was determined by measuring the longitudinal relaxation rate R1 according to the method previously described [7]. Briefly, the samples were mineralized by microwave digestion (MLS-1200 Mega, Milestone, Analis, Belgium) and the R1 value of the resulting solutions was recorded at 0.47 T and 37 °C, which allowed the determination of iron concentration using the equation:
[Fe] = (R1sample – R1dia) × 0.0915
Where R1diam (s-1) is the diamagnetic relaxation rate of acidified water (0.36 s-1) and 0.0915 (s.mM) is the slope of the calibration curve.
In vitro characterization
In vitro studies were performed by using a Chinese hamster ovary (CHO) cell line stably transfected to express human E- selectin. These cells are a generous gift from Dr. Robert Sackstein (Harvard Medical School, Boston, MA). CHO cells (Sigma-Aldrich, Belgium) were used as negative control.
SLex-PEG-NPs or PEG-NPs were added to cultures at 2 mM Fe and were gently stirred for 10 minutes, then washed. After centrifugation, the cell pellet was washed with annexin buffer (composition of the buffer: 2.5 mM CaCl2, 150 mM NaCl, 10 mM Hepes, the pH of the mixture is adjusted to 7.4), then re-suspended in 1 ml of gelatin/annexin buffer mixture (2%). Each measurement was realized 4 times.
The interaction has been estimated by measuring the transverse relaxation time at 60MHz and 15°C. The results are expressed as the relative increase of relaxation (RIR) rate R2 calculated following the equation:
RIR = 100 x (R2-R2C)/R2C
Where R2 = 1/T2 is the transverse relaxation rate of the cells incubated with the nanoparticles (s-1) and R2C is the relaxation rate of the cells incubated with buffer (i.e. without nanoparticles)
In vivo MRI in an inflammation model: preliminary approach
The oxazolone contact hypersensitivity ear model was used to induce inflammation [8, 9]. Briefly, 8 weeks old BALB/c mice were purchased from Charles River Laboratories (L’Abresle, France). They were sensitized by application of 150 μl of 5% oxazolone (Sigma, Belgium) in ethanol and acetone 4/1 vol/vol to the shaved abdomen. Challenge was performed 4 days later by local application of oxazolone (1% in 15 μl) on the left ear (LA1500020, protocol MU-16-01). MRI was performed with a 7T scanner (Bruker Pharmascan, Ettlingen, Germany) before and after injection of NPs (200 μmol/kg) using a T2-weighted RARE sequence (TR/TE : 5000/40,1 ms, RARE factor : 4, Matrix : 256x192, FOV : 2x2 cm). Mice were kept anesthetized under 2% isoflurane vaporized in O2 (0, 7 L/min).
Results
Iron oxide nanoparticles have been synthesized following a welldescribed method of co-precipitation of iron salts in DEG, at high temperature in the presence of sodium hydroxide as the alkaline source. In addition, to allow the easy transfer of the particle in aqueous media, the use of DEG leads to a significant reduction of the particle’s size distribution in comparison to the classical co-precipitation method. The mean core diameter determined by TEM has been evaluated to be 8 ± 2.2 nm. TEPSA was chosen as a stabilizing matrix for the NPs. This molecule exhibits, after hydrolysis, the carboxylic functions necessary for stabilization and further conjugation. DLS measurements revealed a slight increase of the hydrodynamic diameter (16.4 ± 2.1 nm for bare NPs and 18.8 ± 1.9 nm for TEPSAcoated NPs) suggesting the formation of a thin layer surrounding the NP as previously described (Figure 1). After synthesis, the sialyl Lewis X mimetic was covalently grafted to the NP surface by using EDC as a coupling agent. In order to ensure the NPs stealthiness and to increase their colloidal stability, short polyethyleneglycol chains were introduced to the NPs surface to cap remaining carboxylate functions. Unspecific NPs have been similarly obtained by treating TEPSA-NPs only with PEG.
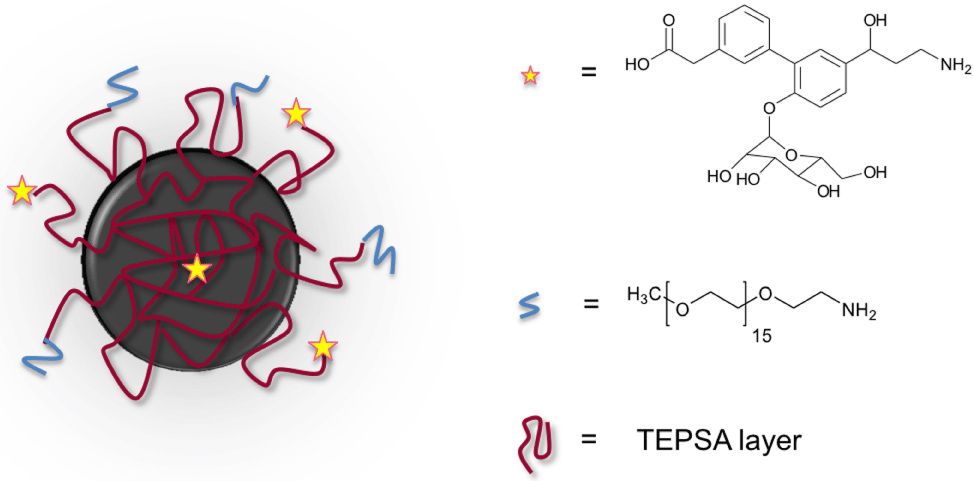
Figure 1: Schematic representation of the synthesized specific NPs (SLex-
NP).
The physico-chemical characteristics of both NPs batches are summarized below (Figure 2, Table 1). As one can observe, a difference of the size distribution between NP-PEG and NP-SLex-PEG is noticed. Such increase is likely due to the presence of some agglomerates which could have formed during the vectorization process. This hypothesis was attested by the relaxometric measurements. As depicted in table 1, we can observe larger r2/r1 ratio for NP-SLex-PEG when comparing with NP-PEG.
DPCS (nm)
Relaxivities (s-1.mM-1) (37°C)
20 MHz
60 MHz
r1
r2
r1
r2
NP-PEG
24 ± 2
27.8 ± 1.0
63.8 ± 1.7
10.5 ± 0.9
62.0 ± 1.4
NP-SLex-PEG
40 ± 2
30.4 ±1.8
85.8 ± 2.7
12.3 ± 0.7
80.6 ± 1.9
Table 1: Hydrodynamic sizes (DPCS) and relaxivity values at 20 and 60 MHz of NP-PEG and NP-SLex-PEG.
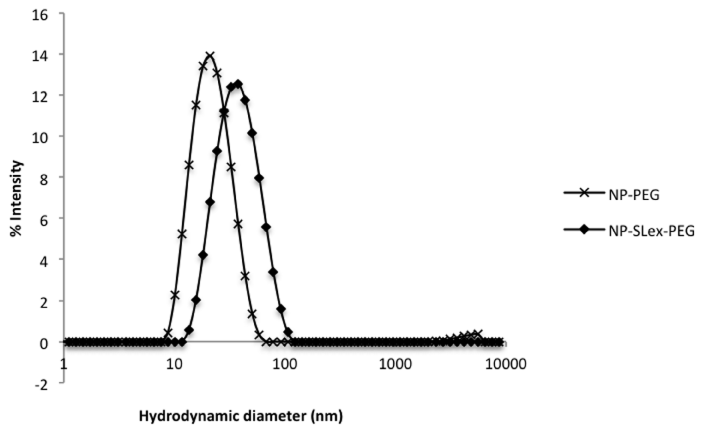
Figure 2: Hydrodynamic diameters (obtained by DLS) of NP-PEG and NPSLex-
PEG.
To check whether the apparition of such agglomerates was the result of the synthesis process or was the consequence of a poor stability of the sample, we measured the evolution of the longitudinal (r1) and transverse (r2) relaxivities at 1.41T for a diluted sample (1mM in iron). The clustering of several magnetic cores usually leads to an increase of the r2/r1 ratio that can reach values up to several hundred depending on the magnetic field (Larmor frequency). The data indicated no evolution of that ratio suggesting thus a good colloidal stability of the as-prepared nano-systems (Figure 3).
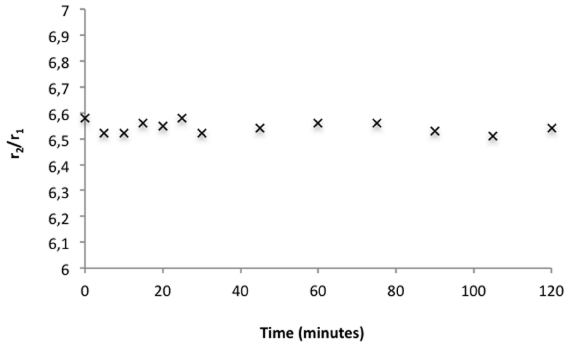
Figure 3: Evolution of the r2/r1 ratio versus time at 60 MHz and 37°C.
The specificity of the as-obtained nanoparticles (SLex-NP) was evaluated in vitro by incubating them either with Chinese hamster ovary (CHO) cells or with CHO cells expressing E-selectin (CHO-E). Similar manipulations were performed with non-vectorized NPs. Interaction between cells and NPs was then evaluated by measuring the transverse (R2) relaxation rates at 60 MHz.
Relaxation measurements suggested that SLex-NP binds to CHO-E cells in vitro unlike non-vectorized NPs (Figure 4). After incubation of CHO cells, no significant difference of R2 relative increase was observed between SLex-NPs-incubated cells and nonvectorized NPs-incubated cells. The specific retention of the probe by E-selectin-expressing CHO cells was suggested by a significantly greater R2 relative increase of the cells incubated with SLex-NPs, as compared to those incubated with non-specific particles (p<0.0001). No significant difference has been observed between the two kinds of particles when incubating control (E-selectin negative) cells.
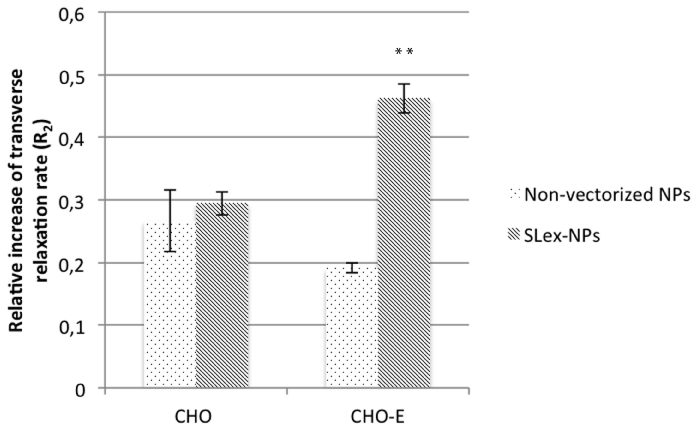
Figure 4: Relative increase of R2 for incubation of specific and non specific
NPs with CHO (n=4) and CHO-E (n=4) cells. **: p<0, 0001 (Student’s t-test;
SLex-NPs vs NPs on CHO-E cells).
From 20 hours post-challenge, the ear became more easily visible in MRI due to inflammation-related phenomena that are thickening it (Figure 5A). Immediately after injection of NPs, « line-shaped » hypo signals could be seen in the ear, probably corresponding to circulating NPs in vessels (Figure 5B). Those preliminary in vivo results suggest that NPs are detectable in vivo. Nevertheless, post-challenge imaging time has to be adequately chosen, so that vessels are not already visible before injection of NPs. Indeed, blood vessels could become observable also as a line-shaped hypo signal at the inflammatory peak (20-24h post-challenge), probably related to vessel dilation. For future E-selectin-specificity tests in vivo, experimental conditions will have to be optimized for higher sensitivity MRI, maybe using another model of inflammation.
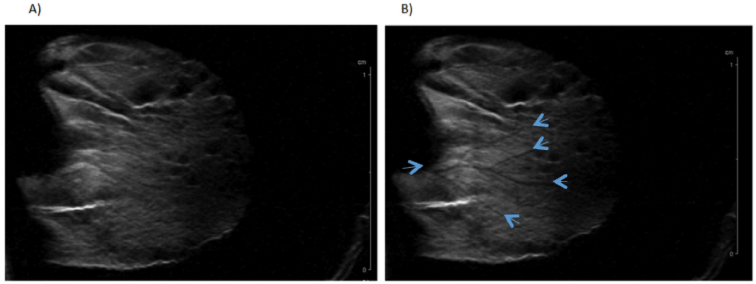
Figure 5: T2-weighted MRI of an inflamed mouse ear before (A) and
immediately after (B) injection of non-targeted USPIO (200μmol/kg).
Arrows are indicating hypo signals probably corresponding to blood vessels
containing circulating USPIO.
Conclusions
In this work, we successfully developed a new super paramagnetic platform surrounded by a thin polysiloxan shell exhibiting carboxylate functions. The as-developed nanosystems have been successfully used for the development of molecular probe able to recognize E-selectins. The nano-systems showed a great selectivity in vitro. This study indicates that SLex-NP systems can be able to target specifically cells expressing E-selectin. This could be valuable for diagnosing and monitoring early inflammation and could be useful for investigating some solid tumors and metastases by targeting tumor-associated neo vasculature or tumor cells that express E-selectin. The combination with other imaging modalities is currently under development in our laboratory.
Acknowledgements
The authors would like to thank the Center for Microscopy and Molecular Imaging (CMMI, supported by the European Regional Development Fund and Wallonia), FNRS, FEDER, ARC, COST, IUAP and ENCITE programs.
References
- Boutry S, Laurent S, Elst LV, Muller RN. Specific E-selectin targeting with a superparamagnetic MRI contrast agent. Contrast Media Mol Imaging. 2006; 1: 15-22.
- Radermacher KA, Beghein N, Boutry S, Laurent S, Vander Elst L, Muller RN, et al. In vivo detection of inflammation using pegylated iron oxide particles targeted at E-selectin: a multimodal approach using MR imaging and EPR spectroscopy.Invest Radiol. 2009; 44: 398-404.
- Boutry S, Burtea C, Laurent S, Toubeau G, Vander Elst L, Muller RN. Magnetic resonance imaging of inflammation with a specific selectin-targeted contrast agent.Magn Reson Med. 2005; 53: 800-807.
- Yang S, Lei X, Wu L, Liu L. The Role of Endothelial Cell Adhesion Molecules P-selectin, E-selectin and Intercellular Adhesion Molecule-1 in Leucocyte Recruitment Induced by Exogenous Methylglyoxal. Immunology. 2012; 137: 65-79.
- Bridot JL1, Stanicki D, Laurent S, Boutry S, Gossuin Y, Leclère P, Lazzaroni R . New carboxysilane-coated iron oxide nanoparticles for nonspecific cell labelling.Contrast Media Mol Imaging. 2013; 8: 466-474.
- Fu Y, Laurent S, Muller RN. Synthesis of a Sialyl Lewis X Mimetic Conjugated with DTPA, Potential Ligand of New Contrast Agents for medical imaging. Eur. J. Org. Chem. 2002; 3966-3973.
- Boutry S, Forge D, Burtea C, Mahieu I, Murariu O, Laurent S, et al. How to quantify iron in an aqueous or biological matrix: a technical note.Contrast Media Mol Imaging. 2009; 4: 299-304.
- Reynolds PR, Larkman DJ, Haskard DO, Hajnal JV, Kennea NL, George AJ, et al. Detection of vascular expression of E-selectin in vivo with MR imaging.Radiology. 2006; 241: 469-476.
- Harari OA, McHale JF, Marshall D, Ahmed S, Brown D, Askenase PW, et al. Endothelial cell E- and P-selectin up-regulation in murine contact sensitivity is prolonged by distinct mechanisms occurring in sequence.J Immunol. 1999; 163: 6860-6866.