
Perspective
J Mol Biol & Mol Imaging. 2015;2(1): 1014.
DUBs: Regulation by Reversible Ubiquitination
Manisha Sharma and Girdhar K. Pandey*
Department of Plant Molecular Biology, University of Delhi South Campus, India
*Corresponding author: Girdhar K Pandey, Department of Plant Molecular Biology, University of Delhi South Campus, India
Received: February 14, 2015; Accepted: March 05, 2015; Published: March 06, 2015
Abstract
Deubiquitinases (DUBs) are the class of enzymes that serve to reverse the ubiquitination by cleaving and releasing ubiquitin moity from the modified proteins. Corresponding to E3 ligases, eukaryotic genomes also encode several DUB proteins that are further divided into subfamilies. The activity and functions of DUB proteins are enigmatic in the sense that upon binding ubiquitin or a protein scaffold they frequently undergo a confirmational change. The association of DUBs with different substrate protein complexes regulate their specificity towards target proteins. Recent studies in human and yeast have led to new insights into the role of DUBs in various cellular processes.
Keywords: Ubiquitin; DUBs; Enzymes; Protein
DUB Protein Complement in Plants
Plants have evolved a sophisticated armoury of proteins for the regulation of critical cellular signalling events. Post-translational modifications of proteins typically by reversible ubiquitination is associated with proteolysis of target protein by the 26S proteasome [1-2]. Moreover, the covalent attachment of ubiquitin to proteins not only target them for degradation but also influence their localization, activation and hence their stability. Ubiquitylation is sequentially regulated by a set of activating (E1), conjugating (E2) and ligase (E3) enzymes to facilitate the covalent attachment of ubiquitin on the substrate protein [1-3]. The substrate protein can be modified by the addition of single or multiple ubiquitin residues. The fate of ubiquitin bound substrate largely depends on the length and topology of ubiquitin oligomer. The subsequent addition of more than four ubiquitin residues facilitates efficient binding and degradation of modified protein by the 26S proteasome [3] (Figure 1).
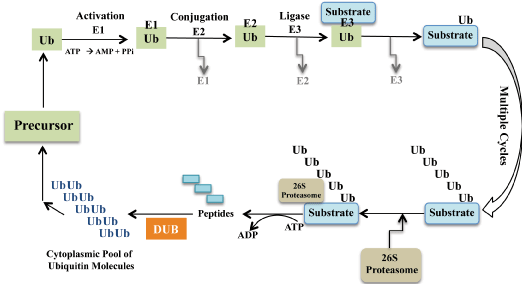
Figure 1: The ubiquitination of substrate protein involves sequential cascade
of enzymes E1, E2 and E3 ligase. E1 is an activating enzyme as it forms a
thioester bond with the ubiquitin molecule in an ATP dependent manner. In
the next step, the activated ubiquitin is carried by the conjugating enzyme E2
to the E3 ubiquitin ligase. E3 ligase facilitates attachment of activated ubiquitin
to the targeted substrate protein. Subsequent conjugation steps results in the
formation of polyubiquitin chain on substrate. The polyubiquitinated protein
is recognized and degraded by the 26S proteasomal machinery. The DUBs
(deubiquitylating enzymes) are responsible for recycling free ubiquitin pool
back into the cell for subsequent reactions.
Another set of relatively less examined ubiquitylation regulatory proteins are known as deubiquitinating enzymes (DUBs) [4-5]. These deubiquitinases are counter proteins since they remove the ubiquitin molecules from the substrates by the hydrolysis of peptide bond between them. The ubiquitin binding domain of DUB proteins efficiently recognize substrate anchored with polyubiquitin chain assemblies and selectively cleave them [6]. The antagonistic role of DUBs in the ubiquitin pathway is indispensable in several ways. They are required for the co-translational activation of the ubiquitin proprotein, recycle and regeneration of monoubiquitin released from the degraded protein inside the cell [4-5]. Human genome encodes nearly 400 DUBs divided into five families (Table 1). So far, concerted functional studies have been successful to identify only a few substrates of these proteins and physiological roles of majority of these are not defined.
Species
USP/UBP
UCH
OTP
MJD
JAMM
Arabidopsis thaliana
27
3
12
3
8
Oryza sativa
46
5
23
2
19
Physcomitrella patens
27
2
16
2
18
Chlamydomonas reinhardtii
18
2
12
2
10
Homo sapiens
236
25
41
45
45
Caenorhabditis elegans
44
5
9
4
16
Table 1: Number of DUB protein subfamily members in different organisms.
DUB Subfamilies in Plants
In comparison to animals, plants encode a large number of E3 ligases facilitating interaction and targeted degradation of diverse proteins [7]. However, animal’s genome encodes a large number of DUB proteins that are not as abundant in plants (Table 1). The relatively small number of DUBs in comparison to ubiquitylating enzymes suggests their enormous range of substrates in plants. Another view supports that DUBs identify and interact with the polyubiquitin chain attached to the target protein instead of the protein itself. Reversal of ubiquitination by DUBs has been implicated in several cellular events including cell cycle regulation, proteasomal degradation, gene expression and enzyme activation in mammals [8-14]. However, the cellular functions of DUBs in plants are poorly defined and require detail in planta study.
The DUB proteins in both plants and animals are divided into five families based on their different catalytic domains namely USP/ UBPs (ubiquitin-specific protease), UCHs (ubiquitin C-terminal hydrolase), OTUs (the ovarian tumor domain), MJD (the Josephin domain) encoding cysteine proteases and JAMM (JAB1/MPN/ Mov34) encoding zinc-dependent metalloproteases [5].
Recent analysis in Arabidopsis thaliana resulted in the identification of all together 65 DUB proteins in its genome [15]. Further, database searches have predicted nearly 95 DUB proteases in the genome of Oryza sativa. Similar search in Physcomitrella patens and Chlamydomonas reinhardtii have identified 65 and 44 DUBs in their genome, respectively (Table 1) (https://sysimg.ifrec. osaka-u.ac.jp/udb/index.html). These figures suggest the evolution of DUB multigene family in plants corresponds with the complexity of their genomes. Astonishingly, representatives of all five subfamily members steadily exists in dicots, monocots, including bryophyte and algal genome indicating unique role of each subfamily in all of them.
Functions of DUBs in Plants
Identification of DUBs in plants reveals that among all five subfamilies, UBP/USP cysteine protease is the prevalent group with maximum individual members. The domain analysis of the UBP/USP members suggest that despite low conservation at the sequence level, they all share conserved tertiary structure.
Apart from an active-site core domain, many DUB proteins consist of insertions and extensions at their N- and C- terminal. These additional extensions contribute in direct substrate identification and in the regulation of protein- protein interaction, subcellular localization and catalytic activity of DUB proteases [9-16].
Several reports in Arabidopsis suggest essential function of USP class of proteins in plant development [17]. The closest ortholog of yeast Ubp14p in Arabidopsis is UBP14, which has been proposed to function in embryo development and in nutrient deficient conditions [18]. Redundant role of UBP15 and UBP16 were also recognized in the cell cycle progression and flowering induction in Arabidopsis [18]. Similarly, the positive role of UBP1 and 2 has been described in unusual protein turnover in plants by conferring resistance towards canavanine [19].
A different class of DUBs i.e. UCH proteases are functionally distinct from other classes in their efficiency to cleave ubiquitin moiety based on their size. Genetic analysis of UCH1 and 2 in Arabidopsis have established their significant role in plant development by regulating auxin signalling pathway [20]. Further, akin to animals, the metalloprotease class of DUBs form a multiprotein complex with BRCC36 and BRCA1 proteins to exert their physiological functions such as DNA damage repair and chromosomal homologous recombination in plants [21]. In animals, DUBs have a well documented role in mediating TGFβ signalling leading to metastasis in breast cancer. The inactivation of DUB protein known as BAP1 (BRCA1-associated protein 1) results into proliferation of cancerous cells thus serving as a major tumor suppressor [22-23]. However, the homologs of BRCA1 in plants have evolved with plant specific functional domains contributing different biological roles for them [24].
The members of ovarian tumor proteases have not been well characterized in plants except OTLD1 that function in histone deubiquitylation [25]. MJD domain containing cysteine proteases associates with 26S proteasome to perform protein quality check in animals [26]. However, so far none of the MJD domain containing proteases has been functionally characterized in plants.
Modulation of Signaling Pathways
Remarkably, the course of ubiquitinating (E3 ligases) and deubiquitinating (DUBs) activity is identical to the kinase/ phosphatase mediated regulatory switches in signalling pathway. Similar to the substrate phosphorylation by kinases, E3 ligases attach ubiquitin monomer to the target protein whereas; analogous to phosphatases DUBs remove the attached ubiquitin from the modified protein [27-28]. Ubiquitination mark the target protein for the binding of signalling molecules such as adapters and kinases while deubiquitination regulate the signalling cascade by supervising the half-life of protein complexes by their hydrolysis [28]. Deubiquitination thus prevents the prolonged signalling response by regulating the fate of protein complexes inside the cell. The chromatin remodeling by histone modifications has been linked with gene expression and is mediated by several DUB proteins. Many studies demonstrated that both histone ubiquitination and deubiquitination act in combination to regulate gene transcription. However, presence of several histone DUBs indicate that they may have redundant functions in various processes [29-31].
Subsequently, some of the recent studies have identified DUB proteins to be instrumental in ubiquitination reversal of H2A and H2B proteins [32-33].The inactivation of H2B deubiquitinase, UBP26 activity in Arabidopsis significantly disrupts flowering transition by FLOWERING LOCUS C (FLC) (34). Ubiquitination of histones directly influence their methylation level and consequently controls gene activation in the chromatin [34]. Accumulating evidences in plants suggest DUBs to be involved in nearly all aspects of development and stress responses [35].
Methods to Define Substrate Specificity of DUBs
Emerging evidence propose the importance of DUB proteins in the substrate fate regulation equivalent to ubiquitylating enzymes. However, the elucidation of the molecular function of DUBs in plants is in its early stages. Studies using yeast and mammalian system are employing various qualitative and quantitative means to determine substrate specificity of DUB proteins [36]. The protein interactions mediated by DUBs is usually validated using pull-down assays by overexpressing both the interacting proteins in cells. This method is regularly employed in plant system as well. Nevertheless, this method suffers several limitations such as lack of physiological interaction environment and need of low dissociation rate of the interaction complex. Additionally, various fluorescence substrate derivatives produced by ubiquitin-intein technology are used for easy quantitation of DUBs activity [38-40]. One such example of fluorogenic derivative is Ubiquitin-rhodamine110-glycine (Ub-Rho110-G). The rhodamine moiety in itself is non-fluorogenic however, splitting of disubstituted moiety into monosubstituted residue results in intense fluorescence emission at the wavelength of 485nm [40]. The attributes like high
dynamic range, optical properties and sensitivity of this fluorescence intensity assay substantially contribute towards efficient monitoring of deubiquitinating proteases activity [40]. The other class of reagents used for determining DUBs specificity are the UBL-electrophile fused protein that exclusively binds on the active site of DUBs [37]. One of the first synthesized electrophile used for labeling specific DUB proteases was ubiquitin vinylsulfone [41]. It mediates covalent labeling of specific DUB proteases by linking catalytic thiol group present on their active sites to electrophile. Near to none crossreactivity with other proteins and short incubation time for DUBs labeling are some of the advantages of this method [42]. The progress in quantitative assay development together with the use of genetic and proteomic tools undertake a better understanding of DUB targets in plants.
The groundwork efforts in animal system are encouraging and suggest possible implications in plants also to decipher the cryptic functions of individual DUB protein. Future studies aimed towards resolving the fate of DUB target proteins may prove promising for defining the significance of complementary mechanisms of ubiquitination and deubiquitination in plant growth and development.
Acknowledgement
GKP is thankful to Department of Biotechnology (DBT), Department of Science and Technology (DST) and University Grant Commission (UGC), India for research funding.
References
- Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004; 1695: 55-72.
- Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004; 8: 610-616.
- Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001; 70: 503-533.
- Wilkinson KD. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J. 1997; 11: 1245-1256.
- Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, et al. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005; 123: 773-786.
- Reyes-Turcu FE, Wilkinson KD. Polyubiquitin binding and disassembly by deubiquitinating enzymes. Chem Rev. 2009; 109: 1495-1508.
- Vierstra RD. The expanding universe of ubiquitin and ubiquitin-like modifiers. Plant Physiol. 2012; 160: 2-14.
- Song L, Rape M. Reverse the curse--the role of deubiquitination in cell cycle control. Curr Opin Cell Biol. 2008; 20: 156-163.
- Guterman A, Glickman MH. Deubiquitinating enzymes are IN/(trinsic to proteasome function). Curr Protein Pept Sci. 2004; 5: 201-211.
- Komada M. Controlling receptor downregulation by ubiquitination and deubiquitination. Curr Drug Discov Technol. 2008; 5: 78-84.
- Schmidt M, Hanna J, Elsasser S, Finley D. Proteasome-associated proteins: regulation of a proteolytic machine. Biol Chem. 2005; 386: 725-737.
- Daniel JA, Grant PA. Multi-tasking on chromatin with the SAGA coactivator complexes. Mutat Res. 2007; 618: 135-148.
- Kennedy RD, D'Andrea AD. The Fanconi Anemia/BRCA pathway: new faces in the crowd. Genes Dev. 2005; 19: 2925-2940.
- Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007; 26: 3214-3226.
- Yang P, Smalle J, Lee S, Yan N, Emborg TJ, Vierstra RD. Ubiquitin C-terminal hydrolases 1 and 2 affect shoot architecture in Arabidopsis. Plant J. 2007; 51: 441-457.
- Koulich E, Li X, DeMartino GN. Relative structural and functional roles of multiple deubiquitylating proteins associated with mammalian 26S proteasome. Mol Biol Cell. 2008; 19: 1072-1082.
- Liu Y, Wang F, Zhang H, He H, Ma L, Deng XW. Functional characterization of the Arabidopsis ubiquitin-specific protease gene family reveals specific role and redundancy of individual members in development. Plant J. 2008; 55: 844–856.
- Li WF, Perry PJ, Prafulla NN, Schmidt W. Ubiquitin-specific protease 14 (UBP14) is involved in root responses to phosphate deficiency in Arabidopsis. Mol Plant. 2010; 3: 212-223.
- Yan N, Doelling JH, Falbel TG, Durski AM, Vierstra RD. The ubiquitin-specific protease family from Arabidopsis. AtUBP1 and 2 are required for the resistance to the amino acid analog canavanine. Plant Physiol. 2000; 124: 1828-1843.
- Yang P, Smalle J, Lee S, Yan N, Emborg TJ, Vierstra RD. Ubiquitin C-terminal hydrolases 1 and 2 affect shoot architecture in Arabidopsis. Plant J. 2007; 51: 441-457.
- Block-Schmidt AS, Dukowic-Schulze S, Wanieck K, Reidt W, Puchta H. BRCC36A is epistatic to BRCA1 in DNA crosslink repair and homologous recombination in Arabidopsis thaliana. Nucleic Acids Res. 2011; 39: 146-154.
- Yu H, Pak H, Hammond-Martel I, Ghram M, Rodrigue A, Daou S, et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc Natl Acad Sci U S A. 2014; 111: 285-290.
- Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G. BAP1 and cancer. Nat Rev Cancer. 2013; 13: 153-159.
- Trapp O, Seeliger K, Puchta H. Homologs of breast cancer genes in plants. Front Plant Sci. 2011; 2: 19.
- Krichevsky A, Zaltsman A, Lacroix B, Citovsky V. Involvement of KDM1C histone demethylase-OTLD1 otubain-like histone deubiquitinase complexes in plant gene repression. Proc Natl Acad Sci U S A. 2011; 108: 11157-11162.
- Costa Mdo C, Paulson HL. Toward understanding Machado-Joseph disease. Prog Neurobiol. 2012; 97: 239-257.
- Wilkinson KD, Tashayev VL, O'Connor LB, Larsen CN, Kasperek E, Pickart CM. Metabolism of the polyubiquitin degradation signal: structure, mechanism, and role of isopeptidase T. Biochemistry. 1995; 34: 14535-14546.
- Piotrowski J, Beal R, Hoffman L, Wilkinson KD, Cohen RE, Pickart CM. Inhibition of the 26 S proteasome by polyubiquitin chains synthesized to have defined lengths. J Biol Chem. 1997; 272: 23712-23721.
- Ventii KH, Wilkinson KD. Protein partners of deubiquitinating enzymes. Biochem J. 2008; 414: 161-175.
- Zhao Y, Lang G, Ito S, Bonnet J, Metzger E, Sawatsubashi S, et al. A TFTC/STAGA module mediates histone H2A and H2B deubiquitination, coactivates nuclear receptors, and counteracts heterochromatin silencing. Mol Cell. 2008; 29: 92-101.
- Zhang XY, Varthi M, Sykes SM, Phillips C, Warzecha C, Zhu W, et al. The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol Cell. 2008; 29: 102-111.
- Zhang XY, Pfeiffer HK, Thorne AW, McMahon SB. USP22, an hSAGA subunit and potential cancer stem cell marker, reverses the polycomb-catalyzed ubiquitylation of histone H2A. Cell Cycle. 2008; 7: 1522-1524.
- Nicassio F, Corrado N, Vissers JH, Areces LB, Bergink S, Marteijn JA, et al. Human USP3 is a chromatin modifier required for S phase progression and genome stability. Curr Biol. 2007; 17: 1972-1977.
- Schmitz RJ, Tamada Y, Doyle MR, Zhang X, Amasino RM. Histone H2B deubiquitination is required for transcriptional activation of FLOWERING LOCUS C and for proper control of flowering in Arabidopsis. Plant Physiol. 2009; 149: 1196-1204.
- Inui M, Manfrin A, Mamidi A, Martello G, Morsut L, Soligo S, et al. USP15 is a deubiquitylating enzyme for receptor-activated SMADs. Nat Cell Biol. 2011; 13: 1368-1375.
- Isono E, Nagel MK1. Deubiquitylating enzymes and their emerging role in plant biology. Front Plant Sci. 2014; 5: 56.
- Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009; 78: 363-397.
- Wilkinson KD, Gan-Erdene T, Kolli N. Derivitization of the C-terminus of ubiquitin and ubiquitin-like proteins using intein chemistry: methods and uses. Methods Enzymol. 2005; 399: 37-51.
- Dang LC, Melandri FD, Stein RL. Kinetic and mechanistic studies on the hydrolysis of ubiquitin C-terminal 7-amido-4-methylcoumarin by deubiquitinating enzymes. Biochemistry. 1998; 37: 1868-1879.
- Hassiepen U, Eidhoff U, Meder G, Bulber JF, Hein A, Bodendorf U, et al. A sensitive fluorescence intensity assay for deubiquitinating proteases using ubiquitin-rhodamine110-glycine as substrate. Anal Biochem. 2007; 371: 201-207.
- Love KR, Catic A, Schlieker C, Ploegh HL. Mechanisms, biology and inhibitors of deubiquitinating enzymes. Nat Chem Biol. 2007; 3: 697-705.
- Borodovsky A, Kessler BM, Casagrande R, Overkleeft HS, Wilkinson KD, Ploegh HL. A novel active site-directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO J. 2001; 20: 5187-5196.