
Mini Review
J Mol Biol & Mol Imaging. 2015;2(2): 1016.
Considerations on the Advantages of Small Tracers for Optical Molecular Imaging
Sabrina Oliveira*
Molecular Oncology, Division of Cell Biology, Department of Biology, Faculty of Science, Utrecht University, Netherlands
*Corresponding author: Sabrina Oliveira, Molecular Oncology, Division of Cell Biology, Department of Biology Faculty of Science, Utrecht University, Netherlands
Received: February 23, 2015; Accepted: March 25, 2015; Published: March 26, 2015
Abstract
Molecular imaging is nowadays gaining a complementary role in cancer therapy, specifically in diagnosis, selection of therapy, monitoring of therapeutic efficacy, and also in the surgical setting. Many tumor-targeted tracers have been developed for these applications. Optical molecular imaging has been gaining more importance, particularly due to the absence of ionizing radiation, rendering its use friendlier and safer for patients and medical personnel. Recently, clinical trials have been initiated for tumor optical imaging with monoclonal antibodies conjugated to the near-infrared fluorophore IRDye 800CW. Yet, preclinical studies clearly indicate several aspects that favor smaller tracer formats over antibodies, namely, their rapid distribution, efficient penetration, and the rapid clearance of unbound tracers. Altogether, these small tracers lead to adequate contrast within minutes or hours after administration, instead of days. Here, the discussion will be centered on the properties of the different formats of tracers, in particular the small formats. Although, the conventional antibody format has already reached clinical trials, optical imaging trials with smaller formats are eagerly awaited to clarify whether their advantages over antibodies are also evident in patients.
Keywords: Optical imaging; Tumor targeting; Antibody; Nanobody; Affibody
Introduction
In the current era of increased awareness of the complementary role of molecular imaging in cancer therapy, tremendous efforts are being made in the development and evaluation of new tumortargeted tracers for molecular imaging. Not only can these tracers be relevant for diagnosis, they can also be determinant for selection of therapy, for monitoring of therapeutic efficacy, as well as for drug development [1, 2]. In addition, some of these can be employed in the surgical setting, to guide tumor resections. In this particular context, optical molecular imaging has been recently gaining more importance, due to advances in optical imaging technologies, and due to the absence of ionizing radiation, rendering its use friendlier and safer for patients and medical personnel [3, 4]. Recently, clinical trials have been initiated with monoclonal antibodies (mAbs) targeting the tumor markers VEGF or EGFR, that are conjugated to the nearinfrared fluorophore IRDye 800CW. On the other hand, many preclinical studies suggest that smaller tracers have advantages in molecular imaging, when compared to conventional mAbs. Here, the discussion will be centered on the properties of the different formats of tracers, in particular highlighting studies in which small tracers have been conjugated to the fluorophore IRDye 800CW.
Optical Imaging Principles
In general, optical imaging relies on the detection of fluorescence emitted by fluorophores, upon light excitation. For more effective imaging of structures below a couple of centimeters from the surface of the skin, fluorophores with maximum excitation and emission within the near- infrared wavelength should be employed (i.e. 700- 1000 nm). In this range of wavelength, auto fluorescence of tissues is at the lowest possible level and the lowest amount of fluorescence is lost through absorption by tissue components [5]. Currently, methylene blue (700 nm) and indocyanine green (800 nm) are the only nearinfrared fluorescent agents approved for human use, where the latter, besides being employed for tumor imaging, has particularly been explored for sentinel lymph node mapping. This procedure refers to the identification of the closest lymph node to the tumor, which is then tested for the presence of tumor cells before other lymph nodes are resected, as would be done in common practice. Importantly, two more near-infrared fluorophores have been subjected to numerous pre-clinical studies and are in the process of clinical translation: IRDye 800CW (LI-COR Biosciences, Lincoln, NE) and ZW800-1 (The FLARE Foundation, Wayland, MA) [3], being the former in a relatively more advanced stage.
Tumor targeting and contrast
For tumor imaging in particular, the near-infrared fluorophores should accumulate specifically and only in tumors, so that high tumor-to-background ratios are obtained (at least 2, but as high as possible) and images with good contrast are acquired. Nevertheless, the intravenous or local administration of these fluorophores leads to an inevitable distribution in normal tissues. Thus, higher contrast can be obtained by 1) targeting moieties, such as antibodies (e.g. [6]), to which these fluorophores are conjugated (active targeting), or 2) by systems used for drug delivery, i.e. nanoparticles, such as liposomes (e.g. [7]), that are employed to protect normal tissues and favor the accumulation of optical tracers in the tumor area (passive targeting). In both cases, after the intravenous administration of the tracers, these have to extravasate at the tumor and be retained specifically on or in tumor cells.
Normal vasculature possesses gaps between endothelial cells of 6-7 nm [8]. Notably, tumor vasculature is in general leakier due to the rapid and unorganized growth of tumor blood vessels. With pores between endothelial cells that may reach approximately 800 nm, extravasations becomes possible for molecules or nanoparticles in the range of 10-200 nm [9]. Moreover, this property is in the ‘enhanced permeability and retention’ effect (EPR) [10] combined with the lack of proper lymphatic drainage. Together, these lead to the accumulation of molecules or nanoparticles at the tumor site, by passive targeting, provided that they circulate long enough in the bloodstream [11]. Alternatively and simplistically, when tracers are smaller and have a short half-life in the bloodstream (e.g. hours or minutes), their binding affinity becomes the most important parameter, as these molecules may encounter the target only once, and thus may have only one chance for specific binding. In this case of active targeting, binding affinities in the range of 0.1 – 10 nM have been described to be essential for sufficient tumor accumulation [12].
Background and contrast
Next to the specific accumulation at the tumor, the elimination of the remaining tracer from the bloodstream and normal tissues is essential for low background fluorescence, so that adequate contrast is obtained. Two main routes are responsible for clearance of unbound tracers: hepatic or renal. The kidneys are mainly responsible for clearing small sized particles from the bloodstream, through the urine. The glomerular filtration is dependent on the size of the molecule, where molecules with an in vivo hydrodynamic diameter (HD) under 6 - 8 nm are filtered, which in general is related to an average molecular weight cutoff of approximately 60 kDa [13, 14]. This process is also dependent on the charge or the molecules, where positively charged molecules are more likely to be cleared. Alternatively, the charge of the molecule may provide interactions with plasma proteins, increasing the HD and preventing renal clearance. In that case, and for other large particles (HD >10 - 20 nm) which are not cleared by the kidneys, e.g. antibodies and nanoparticles, the liver is the main responsible for clearance. Such particles are endocytosed by Kupffer cells and hepatocytes, which are then excreted into the bile. In addition, other phagocytic cells, such as monocytes and macrophages located in lymph nodes and in the spleen, are responsible for clearance of large particles [14, 15].
In principle, the quicker the unbound tracer is removed from the normal tissues and blood, the faster the contrast is created and the image can be obtained, provided that the tracer is retained at the tumor. For diagnosis and selection of therapy, the sooner the image is obtained (hours instead of 3 to 7 days), the sooner the patient can initiate therapy, the shorter the time period spent in the hospital, and likely the lower the costs involved. On the other hand, rapid clearance decreases the chance of accumulation at the tumor, and overall tumor uptake may be lower, unless such tracers compensate their short halflife by high binding affinities, as discussed earlier [12].
Formats of Targeting Moieties
Although nanoparticles have very interesting applications in imaging, multi-modal imaging, therapy and even combinations of these, this section focuses on active targeting by antibodies, their fragments and other small targeting moieties (Figure 1). mAbs have been used in molecular imaging for their binding specificity, to target particular tumor antigens (or tumor markers) that are solely expressed or over expressed in tumors, compared to normal tissues. mAbs have been conjugated to radio ligands for PET/SPECT or to fluorophores for optical imaging [16-18], being the latter addressed here in more detail. mAbs targeting EGFR (cetuximab, panitumumab), HER2 (trastuzumab), and VEGF (bevacizumab) have been conjugated to IRDye 800CW and employed pre-clinically to image subcutaneous xenografts of breast [6, 19], vulva [20], and ovarian [21] tumors, of gliomas [22], and of peritoneally disseminated ovarian or gastric tumors [21]. In general, encouraging results have been obtained, which combined with the fact that these mAbs are already approved for therapy in the clinic, stimulated and accelerated the process for clinical translation of antibody-targeted optical imaging. In fact, clinical trials have been initiated with Bevacizumab-IRDye 800CW (ClinicalTrials.gov: NCT01508572, NCT01972373, NCT02129933) and Cetuximab-IRDye 800CW (ClinicalTrials.gov: NCT01987375).
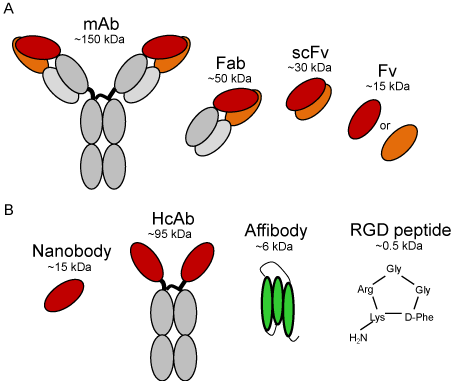
Figure 1: Schematic representation of antibodies and smaller targeting
moieties that have been investigated conjugated to IRDye 800CW: A.
monoclonal antibody (mAb) and its fragments, i.e. antigen-binding fragment
(Fab), variable fragment (Fv), and single-chain variable fragment (scFv); B.
nanobody derived from a heavy chain antibody (HcAb), affibody, and cyclic
RGD peptide. The approximate molecular weight of each type of molecule is
depicted for relative comparison.
However, the molecular weight of mAbs (~150 kDa) and dimensions of 14.2 nm × 8.5 nm × 3.8 nm [23], together with the ‘binding site barrier’ effect, have been shown to hamper their distribution and penetration through tumors [24]. Furthermore, and more importantly for imaging, their relative long half-life in the bloodstream leads to high background levels, which reduce contrast for imaging and thus delays image acquisition for 3 to 7 days after tracer administration. To improve tissue penetration and to accelerate the imaging procedure, efforts have been made to investigate the potential of smaller antibody fragments (Figure 1A). Naturally derived or synthetic antigen-binding fragments (Fabs; ~50 kDa), variable fragments (Fvs; ~15 kDa) or single-chain variable fragments (scFvs; ~30 kDa) have been evaluated for their capacity to overcome the drawbacks of mAbs. In general, these studies show improvements in tumor penetration with size reduction, concomitantly with earlier contrast and more rapid clearance. Unfortunately, to some extent, these studies also suggest limitations by the lack of avidity and the lower affinities of these fragments, compared to the corresponding mAbs [25]. Alternatively, several studies have been describing smaller formats, such as nanobodies (15 kDa) or affibodies (6 kDa) (Figure 1B), conjugated to IRDye 800CW for optical molecular imaging, which in both cases resulted in image acquisition within 30 min or 1 hour after tracer administration.
Nanobodies are the variable domain of a particular sort of antibodies, i.e. the heavy chain antibodies (HcAbs, ~95 kDa) that were discovered in animals from the Camelidae family by Hamers- Casterman in 1993 [26]. The term ‘nanobodies’ was introduced by the Belgian company Ablynx®, but these are also referred to as VHHs (as they are the variable domain of the heavy chain of a heavy chain antibody) or as single domain antibodies (sdAbs) [27]. Although smaller than conventional antibodies, nanobodies bind their targets with similar specificity and with very high affinities (pM - nM). A number of studies have thus far demonstrated the potential of nanobodies conjugated to IRDye 800CW in non-invasive optical imaging and also in the surgical setting, to guide tumor resection or to detect small metastases [19, 20, 28]. Although none of these tracers has to date reached clinical evaluation, this is probably a matter of time, as a nanobody targeting HER2 is currently under clinical evaluation in a first-in human trial for PET imaging of breast cancer [29].
Affibodies are synthetic protein scaffolds that are very stable proteins and have shown potential in preclinical studies [30]. In fact, a radiolabelled affibody targeting HER2 is under clinical evaluation. Thus far, Phase I studies showed that it is safe for use in humans and it is a promising tool for assessing HER2 status of metastatic breast cancer non-invasively [31]. In time, other affibodies will likely be evaluated in humans, possibly also for optical imaging. Even smaller targeting moieties have been investigated, such as ligands (e.g. EGF [30]) or peptides (e.g. RGD [32]). For the former, unwanted activation of signaling cascades upon EGFR binding has limited its exploration for molecular imaging applications. On the other hand, the cyclic RGD peptide conjugated to IRDye 800CW has shown its potential for intraoperative imaging and resection of glioblastomas [32]. Furthermore, this peptide has demonstrated specific binding to alpha-v-beta-3 integrin in cancer patients with PET imaging, providing good contrast at 72 min post-administration [33], and thus its evaluation with optical imaging may be a matter of time.
In general, different formats of tracers have been investigated and the examples here highlighted are only a small part of these, particularly focusing on the near-infrared fluorophore IRDye 800CW, which has recently entered clinical trials. Many other tracers have been and are being investigated thoroughly in the preclinical setting. Although just a small number of these will reach clinical evaluation, only then firm conclusions can be drawn on the value and the application of each format.
Advantages of Small Tracers
Clinically approved mAbs as targeting moieties for optical imaging have recently reached clinical evaluation. In due course the value of these tracers will become clear for non- invasive imaging (e.g. breast cancers, ClinicalTrials.gov: NCT01508572), superficial imaging (e.g. head and neck cancers, ClinicalTrials.gov: NCT01987375), gastro-intestinal tract imaging through endoscopes (ClinicalTrials.gov: NCT02129933, NCT01972373), or intraoperative optical imaging (ClinicalTrials.gov: NCT01987375). Although smaller targeting moieties may need more time to reach this stage, ongoing clinical trials with small radioactive tracers may accelerate optical imaging trials. Preclinical studies clearly indicate several aspects that favor smaller targeting moieties over mAbs: rapid distribution and efficient penetration, combined with rapid clearance of unbound tracers, leading to adequate contrast within minutes or hours after administration instead of days (e.g. [19, 20, 30]). As a result, in the future, earlier and faster diagnosis could be possible and patients could begin their treatments more rapidly after tumor detection. In addition, shorter hospital stays could possibly decrease healthcare costs. Moreover, monitoring the therapeutic efficacy could also be easier and more frequent. Small targeting moieties could be employed that are not competing with therapeutic antibodies, thereby enabling assessment of therapeutic response concomitant with treatment.
Without a doubt, the results obtained with the ongoing optical clinical trials will provide crucial insights into the direction optical molecular imaging should follow in the next decade. These results will clarify whether further clinical trials will be performed with the same mAbs conjugated to IRDye 800CW, or whether other tracers should be considered for optical imaging in patients. Next to these, the outcome of ongoing trials, employing smaller tracers with other imaging modalities, will possibly encourage similar trials with optical imaging. Although preclinical studies clearly show advantages of smaller tracers over mAbs, only when the small formats of tracers have been evaluated in the clinic, it will be clear whether their advantages are also evident in cancer patients. For scientific purposes, the development of new potential tracers for optical imaging can certainly be processed in many different directions, employing all possible formats. Nevertheless, when the goal is to develop a tracer to reach clinical translation, the aspects here discussed are considered of importance to guide the selection of the most promising format of tracer.
References
- Michalski MH, Chen X. Molecular imaging in cancer treatment. Eur J Nucl Med Mol Imaging. 2011; 38: 358-377.
- Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging. 2010; 9: 237-255.
- Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013; 10: 507-518.
- Hellebust A, Richards-Kortum R. Advances in molecular imaging: targeted optical contrast agents for cancer diagnostics. Nanomedicine (Lond). 2012; 7: 429-445.
- Weissleder R. Scaling down imaging: molecular mapping of cancer in mice. Nat Rev Cancer. 2002; 2: 11-18.
- Korb ML, Hartman YE, Kovar J, Zinn KR, Bland KI, Rosenthal EL. Use of monoclonal antibody-IRDye800CW bioconjugates in the resection of breast cancer. J Surg Res. 2014; 188: 119-128.
- Zhang L, Zhao D. Liposomal encapsulation enhances in vivo near infrared imaging of exposed phosphatidylserine in a mouse glioma model. Molecules. 2013; 18: 14613-14628.
- Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998; 95: 4607-4612.
- Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004; 303: 1818-1822.
- Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000; 65: 271-284.
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001; 41: 189-207.
- Schmidt MM, Wittrup KD. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther. 2009; 8: 2861-2871.
- Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol. 2001; 281: F579-596.
- Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond). 2008; 3: 703-717.
- Kijanka M, Dorresteijn B, Oliveira S, van Bergen en Henegouwen PM. Nanobody-based cancer therapy of solid tumors. Nanomedicine (Lond). 2015; 10: 161-174.
- Mishani E, Hagooly A. Strategies for molecular imaging of epidermal growth factor receptor tyrosine kinase in cancer. J Nucl Med. 2009; 50: 1199-1202.
- Zhao H, Cui K, Muschenborn A, Wong ST. Progress of engineered antibody-targeted molecular imaging for solid tumors (Review). Mol Med Rep. 2008; 1: 131-134.
- Kijanka M, Warnders FJ, El Khattabi M, Lub-de Hooge M, van Dam GM, Ntziachristos V, et al. Rapid optical imaging of human breast tumour xenografts using anti-HER2 VHHs site-directly conjugated to IRDye 800CW for image-guided surgery. Eur J Nucl Med Mol Imaging. 2013; 40: 1718-1729.
- Kijanka M, Warnders FJ, El Khattabi M, Lub-de Hooge M, van Dam GM, Ntziachristos V, et al. Rapid optical imaging of human breast tumour xenografts using anti-HER2 VHHs site-directly conjugated to IRDye 800CW for image-guided surgery. Eur J Nucl Med Mol Imaging. 2013; 40: 1718-1729.
- S. Oliveira, G.A. van Dongen, M. Stigter-van Walsum, R.C. Roovers, J.C. Stam, W. Mali, et al. Rapid visualization of human tumor xenografts through optical imaging with a near-infrared fluorescent anti-epidermal growth factor receptor nanobody. Mol Imaging 2012; 11: 33-46.
- A.G. Terwisscha van Scheltinga, G.M. van Dam, W.B. Nagengast, V. Ntziachristos, H. Hollema, J.L. Herek, et al. Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J Nucl Med 2011; 52: 1778-1785.
- Gong H, Kovar JL, Cheung L, Rosenthal EL, Olive DM. A comparative study of affibody, panitumumab, and EGF for near-infrared fluorescence imaging of EGFR- and EGFRvIII-expressing tumors. Cancer Biol Ther. 2014; 15: 185-193.
- Sarma VR, Silverton EW, Davies DR, Terry WD. The three-dimensional structure at 6 A resolution of a human gamma Gl immunoglobulin molecule. J Biol Chem. 1971; 246: 3753-3759.
- Fujimori K, Covell DG, Fletcher JE, Weinstein JN. A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. J Nucl Med. 1990; 31: 1191-1198.
- Batra SK, Jain M, Wittel UA, Chauhan SC, Colcher D. Pharmacokinetics and biodistribution of genetically engineered antibodies. Curr Opin Biotechnol. 2002; 13: 603-608.
- Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993; 363: 446-448.
- Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013; 82: 775-797.
- P.B. van Driel, J.R. van der Vorst, F.P. Verbeek, S. Oliveira, T.J. Snoeks, S. Keereweer, et al. Intraoperative fluorescence delineation of head and neck cancer with a fluorescent anti-epidermal growth factor receptor nanobody. Int J Cancer 2014; 134: 2663-2673.
- Xavier C, Vaneycken I, D'huyvetter M, Heemskerk J, Keyaerts M, Vincke C, et al. Synthesis, preclinical validation, dosimetry, and toxicity of 68Ga-NOTA-anti-HER2 Nanobodies for iPET imaging of HER2 receptor expression in cancer. J Nucl Med. 2013; 54: 776-784.
- H. Gong, J. Kovar, G. Little, H. Chen, D.M. Olive. In Vivo Imaging of Xenograft Tumors Using an Epidermal Growth Factor Receptor-Specific Affibody Molecule Labeled with a Near-infrared Fluorophore. Neoplasia (New York, N.Y.) 2009; 12: 139-149.
- J. Sorensen, D. Sandberg, M. Sandstrom, A. Wennborg, J. Feldwisch, V. Tolmachev, G. et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY- 025 affibody molecule. J Nucl Med 2014; 55: 730-735.
- Huang R, Vider J, Kovar JL, Olive DM, Mellinghoff IK, Mayer-Kuckuk P, et al. Integrin αvβ3-targeted IRDye 800CW near-infrared imaging of glioblastoma. Clin Cancer Res. 2012; 18: 5731-5740.
- Beer AJ, Haubner R, Goebel M, Luderschmidt S, Spilker ME, Wester HJ, et al. Biodistribution and pharmacokinetics of the alphavbeta3-selective tracer 18F-galacto-RGD in cancer patients. J Nucl Med. 2005; 46: 1333-1341.