
Case Report
J Mol Biol & Mol Imaging. 2015;2(2): 1020.
False Positive 18F-FDG PET/CT in Cardiac Sarcoidosis
Sibille L*, Chambert B, Collombier L, Kotzki PO and Boudousq V
Department of Nuclear Medicine, Nîmes University Hospital, Place du Pr. Robert Debré, France
*Corresponding author: Sibille L, Department of Nuclear Medicine, Nîmes University Hospital, Place du Pr. Robert Debré 30029 Nîmes cedex 9, France
Received: June 11, 2015; Accepted: July 01, 2015; Published: July 03, 2015
Abstract
Cardiac sarcoidosis is an underdiagnosed disease that may be present in as many as 25% of patients with systemic sarcoidosis. 18F-FDG PET/CT is playing an increasing role in the management of sarcoidosis patient with suspected cardiac involvement. Physiologic FDG uptake by myocardium needs to be suppressed and differential diagnosis must be considered. Here we present a case of false positive 18F-FDG PET/CT due to myocardial ischemia.
Keywords: Cardiac sarcoidosis; PET/CT; False positive; 18F-FDG; Myocardial ischemia
Case Presentation
A 46-year-old non-smoker, non-diabetic woman with newly diagnosed sarcoidosis, presented with atypical chest pain at rest and was referred for a 18FDG PET-CT exam looking for cardiac involvement. She had no prior history of heart disease. Rest electrocardiogram and dobutamine stress echocardiogram were normal. PET-CT revealed hypermetabolic lymph nodes on both sides of the diaphragm, thickening of the peribronchovascular interstitium and large sized antero-septo-apical myocardial uptake (Figure 1). Because of these observations, the patient was diagnosed as having cardiac sarcoidosis. Also, because of the persistent chest pain and the atypical clinical presentation, a coronary angiography was performed. This showed a proximal left anterior descending artery sub occlusion, which lead to myocardial ischemia at rest and was responsible for PET-CT findings (Figure 2).
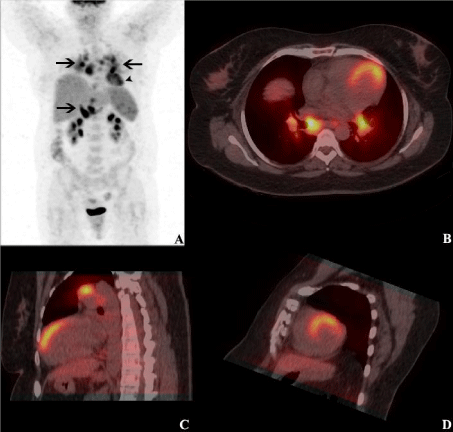
Figure 1: PET/CT fusion images from attenuation-corrected 18F-FDG PET/
CT scans (A, anterior maximum intensity projection PET image; B, horizontal
long axis; C, vertical long axis; and D, short axis) showing hyper metabolic
lymph nodes on both sides of the diaphragm (arrows; SUVmax 12.7) and large
sized antero-septo-apical myocardial uptake (arrowhead; SUVmax 5.8).
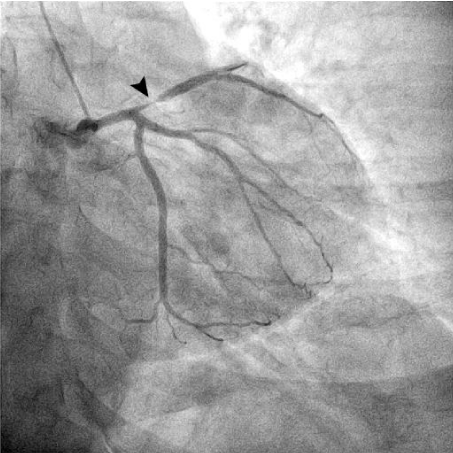
Figure 2: Cardiac catheterization (right anterior oblique angulated view)
showing a proximal left anterior descending artery sub occlusion (arrowhead).
Discussion
In our case cardiac catheterization (right anterior oblique angulated view) was performed because of persistent chest pain, which is an atypical clinical presentation for cardiac sarcoidosis as well as palpitations, syncope, bradycardia, peripheral edema and dyspnea [1,2]. Patient has not reported any chest pain symptoms since angioplasty was performed. Cardiac involvement in patients with systemic sarcoidosis is probably underestimated. Autopsy studies show a prevalence of cardiac involvement in sarcoidosis in more than 25% of cases [3], although clinical evidence of myocardial involvement is found in only 5% of patients with systemic sarcoidosis. Clinical manifestations include conduction disturbances, arrhythmia, congestive cardiac failure, and sudden cardiac death [4]. Early diagnosis is challenging and requires different imaging techniques [5]. Revised guidelines of the “Japanese society of sarcoidosis and other granulomatous disorders” provide recommendations for the diagnosis of this potentially life-threatening disease [6]. These guidelines do not include recent emerging techniques such as 18F-FDG PET/CT even if this is a promising tool for early disease detection as well as for therapy monitoring and image-guided biopsy [7,8]. However, 18F-FDG PET/CT must be performed following specific patient preparation protocols [4,7,9] (long fasting [10], highfat low-carbohydrate diet [11], use of unfractionated heparin [12]) to suppress cardiac physiological 18F-FDG uptake and avoid false positive results. Focal or patchy 18F-FDG uptakes are consistent with a diagnosis of cardiac sarcoidosis whereas the diffuse myocardial uptake pattern or the focally increased lateral-wall uptake pattern are likely to represent normal variations [13]. The present clinical case shows another pitfall in interpreting cardiac 18F-FDG PET/CT exam in patient with sarcoidosis. In spite of a well-done cardiac preparation, intense myocardial uptake can be seen in ischemic tissue because of the preferential use of glycolysis under anaerobic conditions [14,15]. In our case the imaging protocol did not include myocardial perfusion imaging since dobutamine stress echocardiogram had already been done in order to exclude coronary macroangiopathy or to detect other cardiomyopathies [16].
Conclusion
We would like to emphasize the importance of ruling out coronary artery disease before interpreting cardiac 18F-FDG PET/CT exam in patients with sarcoidosis, especially in case of atypical chest pain.
References
- Okura Y, Dec GW, Hare JM, Kodama M, Berry GJ, Tazelaar HD, et al. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003; 41: 322-329.
- Kim JS, Judson MA, Donnino R, Gold M, Cooper LT Jr, Prystowsky EN, et al. Cardiac sarcoidosis. Am Heart J. 2009; 157: 9-21.
- Iwai K, Sekiguti M, Hosoda Y, DeRemee RA, Tazelaar HD, Sharma OP, et al. Racial difference in cardiac sarcoidosis incidence observed at autopsy. Sarcoidosis. 1994; 11: 26-31.
- Schatka I, Bengel FM. Advanced imaging of cardiac sarcoidosis. J Nucl Med. 2014; 55: 99-106.
- Doughan AR, Williams BR. Cardiac sarcoidosis. Heart. 2006; 92: 282-288.
- Soejima K, Yada H. The work-up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol. 2009; 20: 578-583.
- Mc Ardle BA, Leung E, Ohira H, Cocker MS, deKemp RA, DaSilva J, et al. The role of F(18)-fluorodeoxyglucose positron emission tomography in guiding diagnosis and management in patients with known or suspected cardiac sarcoidosis. J Nucl Cardiol. 2013; 20: 297-306.
- Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med. 2012; 53: 241-248.
- Ishida Y, Yoshinaga K, Miyagawa M, Moroi M, Kondoh C, Kiso K, et al. Recommendations for (18)F-fluorodeoxyglucose positron emission tomography imaging for cardiac sarcoidosis: Japanese Society of Nuclear Cardiology recommendations. Ann Nucl Med. 2014; 28: 393–403.
- Ambrosini V, Zompatori M, Fasano L, Nanni C, Nava S, Rubello D, et al. (18)F-FDG PET/CT for the assessment of disease extension and activity in patients with sarcoidosis: results of a preliminary prospective study. Clin Nucl Med. 2013; 38: e171-177.
- Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol. 2011; 18: 926-936.
- Ito K, Morooka M, Okazaki O, Minaminoto R, Kubota K, Hiroe M. Efficacy of heparin loading during an 18F-FDG PET/CT examination to search for cardiac sarcoidosis activity. Clin Nucl Med. 2013; 38: 128-130.
- Ishimaru S, Tsujino I, Takei T, Tsukamoto E, Sakaue S, Kamigaki M, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005; 26: 1538-1543.
- Mariano-Goulart D, Ilonca D, Bourdon A. Diagnosis of silent myocardial ischemia during the staging of HIV-associated lymphoma with FDG PET/CT. Clin Nucl Med. 2009; 34: 731-733.
- Vilain D, Bochet J, Le Stanc E, Wattel C, Hameg A, Tainturier C. Unsuspected hibernating myocardium detected by routine oncology (18)F-FDG PET/CT. Eur J Nucl Med Mol Imaging. 2010; 37: 409.
- Agarwal A, Sulemanjee NZ, Cheema O, Downey FX, Tajik AJ. Cardiac sarcoid: a chameleon masquerading as hypertrophic cardiomyopathy and dilated cardiomyopathy in the same patient. Echocardiography. 2014; 31: E138-141.