
Special Aricle - Cardiac Magnetic Resonance Imaging
J Mol Biol & Mol Imaging. 2015; 2(2): 1021.
The Prognostic Significance of Late-Gadolinium Enhancement Identified by Cardiac MRI in Association with Renal Function
Dandamudi S1, Slusser JP2, Hodge DO2, Glockner JF3 and Chen HH4*
1The Department of Medicine, Division of Cardiology, Northwestern University Feinberg School of Medicine, USA
2The Division of Biomedical Statistics and Informatics, Mayo Clinic and Foundation, USA
3The Department of Radiology, Mayo Clinic and Foundation, USA
4The Department of Medicine, Division of Cardiovascular Diseases, Mayo Clinic and Foundation, USA
*Corresponding author: Chen HH, The Department of Medicine, Division of Cardiovascular Diseases, Mayo Clinic, 200 First Street SW, Rochester, MN 55905, USA
Received: October 02, 2015; Accepted: October 28, 2015; Published: November 02, 2015
Abstract
Objectives: Cardiac MRI with late-gadolinium enhancement (LGE) provides a noninvasive assessment of myocardial fibrosis. LGE is associated with an increased risk of adverse cardiovascular events. Renal impairment is associated with the development of myocardial fibrosis and cardiac remodeling. However, the prognostic significance of LGE in association with renal function has not been assessed.
Methods: Using the Mayo Clinic Cardiac MRI Database, we collected data from patients who underwent gadolinium contrast cardiac MRI beginning in January 2006 to June 2008 and had a baseline serum creatinine measurement within 30 days of the scan. Patients with a history of congenital heart disease, hypertrophic cardiomyopathy, hemochromatosis, cardiac sarcoidosis, cardiac amyloidosis, myocarditis and arrhythmogenic right ventricular cardiomyopathy were excluded from the study. Each patient’s estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) formula. MRI data for 966 patients were included in the study.
Results: The mean age of the cohort was 55.5 ± 16.7 years with 41% females. The presence of LGE in the total cohort was associated with a lower eGFR (73.2 ± 22.6 mL/min/1.73 m2) as compared to patients without LGE (78.4 ± 22.8 mL/min/1.73 m2, p<0.001), even after adjustment for gender, LVEF and LVEDMi (p=0.006). There were statistically significant associations between the presence of LGE and reduced LVEF (46.8 ± 14.3%, p<0.001), increased LVEDMi (56.6 ± 19.4 g/m2, p=0.015) and elevated plasma B-type natriuretic peptide (BNP) (524.5 ± 655.3 pg/mL, p<0.001) as compared to those subjects without LGE, even after adjustment for age, gender, hypertension, CAD and MI. The presence of LGE was independently associated with increased mortality (HR 1.78, 95% CI (1.21, 2.63); p=0.003), even after adjustment for age, gender and MI (HR 1.61, 95% CI (1.03, 2.51); p=0.0356). Overall mortality was greatest in patients with LGE and eGFR less than 70 mL/min/1.73 m2 (HR 1.38, 95% CI (1.17, 1.63); p<0.001).
Conclusion: This retrospective analysis demonstrates that the presence of late-gadolinium enhancement on cardiac MRI is more commonly associated with renal impairment and markers of adverse ventricular remodeling. In addition, subjects with LGE have an increased risk of mortality and the combination of LGE and renal impairment significantly elevates the overall risk of mortality compared to subjects with neither.
Keywords: Cardiac MRI; Myocardial fibrosis; Late gadolinium enhancement; Renal insufficiency; Prognosis
Abbreviations
LGE: Late Gadolinium Enhancement; LVEF: Left Ventricular Ejection Fraction; LVEDMi: Left Ventricular End Diastolic Mass Index; eGFR: Estimated Glomerular Filtration Rate; CAD: Coronary Artery Disease; MI: Myocardial Infarction; CHF: Congestive Heart Failure; PVD: Peripheral Vascular Disease; BNP: B-Type Natriuretic Peptide
Introduction
Late-gadolinium enhancement (LGE) by cardiac MRI has been shown to provide an accurate non-invasive method of detecting myocardial fibrosis and has been validated against histopathologic examination [1-3]. Over the last several years, many studies have characterized the clinical significance of LGE in various forms of cardiovascular disease. The presence of LGE has an increased association with clinical parameters of structural remodeling, such as diastolic dysfunction, left ventricular hypertrophy (LVH) and increased left ventricular end-diastolic volume [4, 5]. The presence of LGE in the setting of various cardiomyopathies has been shown to have an increased association with adverse clinical outcomes, such as heart failure hospitalizations and arrhythmic events [6-9]. Several important studies have also shown that the LGE in non-ischemic cardiomyopathy is associated with an increased risk of mortality beyond left ventricular ejection fraction [8-12]. These findings have led to the increased use of cardiac MRI as a clinical tool for risk stratification.
The relationship between myocardial fibrosis and renal dysfunction has been suggested by endomyocardial biopsy studies in patients with chronic kidney disease [13]. Renal impairment is felt to contribute to the development of myocardial fibrosis through a combination of hemodynamic, neurohormonal and cytokine-mediated processes [13-21]. Small cardiac MRI studies of patients with chronic kidney disease have sought to characterize the patterns of LGE in this population [22, 23]. However, the prognostic association between the presence of LGE and renal function has not been well established. Hence, the objectives of the current study are to determine the association between LGE and renal function and the additive prognostic value of the combination of LGE and renal function.
Materials and Methods
The study protocol was approved by the Mayo Clinic Investigational Review Board and Ethical Committee in January 2011. Using the Mayo Clinic cardiac MRI database, we collected data from patients who underwent gadolinium contrast cardiac MRI from January 2006 to June 2008 and had a baseline serum creatinine measurement within thirty days of the study. These subjects were followed for a mean of 2.28 years and total duration of 6.8 years. The MRI data collected included left ventricular ejection fraction (LVEF), left ventricular end-diastolic mass index (LVEDMi) and the presence of late-gadolinium enhancement. All exams were performed on GE 1.5 Tesla Twin Speed EXCITE or HD systems. After localizing scouts, functional assessment of the ventricles were performed by pre-contrast cine images obtained using ECG-gated steady state free precession imaging. An initial 4 chamber view was obtained from the scout views, then followed by short axis slices (8mm slice thickness, 1mm gap) covering the entire ventricle from base to apex. Scan parameters include TR 3.5ms, TE 1.6ms, bandwidth 125 kHz, flip angle 45, temporal resolution 40ms, matrix 192x160-192. Three short axis slices at the base, mid ventricle, and apex were acquired using a grid tagging ECG-gated gradient echo sequence, with parameters including TR/TE 6.8/3.7 ms, flip angle 30, bandwidth 32 kHz, 8 mm slice thickness, matrix 224x128, tag spacing 7 mm. Contrast enhanced myocardial late enhancement images were obtained approximately 10 minutes after injection of intravenous gadolinium- DTPA (0.2mmol/kg) in the same location used for the short axis cine images using a segmented inversion recovery prepared fast gradient echocardiographic sequence for detection of infarction. Typical scan parameters include TR 6.5ms, TE 3.1ms, inversion time (adjusted for optimal myocardial nulling) 175-225ms, matrix 256x192. Analysis of MRI images: Short axis cine images were used to calculate left ventricular ejection fraction, end diastolic, end systolic volumes, and left ventricular mass based on endocardial contours traced using computer-assisted planimetry at end-diastole and end-systole (Mass Analysis, GE Medical Systems).
Each subject’s estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) formula. Baseline comorbidities were collected using prior documented ICD-9 diagnoses. The characterization of lategadolinium enhancement as secondary to infarction or noninfarction was based on the pattern of fibrosis in association with coronary artery distribution as identified by the interpreting cardiac MRI specialist at the time of the study.
Patients with a history of congenital heart disease, hypertrophic cardiomyopathy, hemochromatosis, cardiac sarcoidosis, cardiac amyloidosis, myocarditis and arrhythmogenic right ventricular cardiomyopathy were excluded from the study to prevent a confounding degree of myocardial fibrosis.
Statistical Analysis
Categorical variables were summarized as percentages and continuous variables as mean ± standard deviation. Univariate and multivariate associations of late-gadolinium enhancement and eGFR were performed with Cox’s proportional hazard modeling. A p-value less than 0.05 was considered significant in this study.
Results and Discussion
MRI data from a total of 966 patients were included in the study. The mean age of the cohort was 55.5 ± 16.7 years with 41% females. Table 1 outlines the baseline comorbidities of the total cohort. In addition, baseline demographics and comorbidities with respect to the presence or absence of late-gadolinium enhancement on cardiac MRI were also compared. There were statistically significant associations between increased age, male gender, baseline hypertension, coronary artery disease (CAD), myocardial infarction (MI), diabetes, congestive heart failure (CHF), hyperlipidemia and peripheral vascular disease (PVD) in patients with LGE compared to those without (Table 1).
Variable
Overall
(N=966)
Enhancement
DGE (+)
(N=322)
No Enhancement
DGE (-)
(N=644)
P value
DGE (+)
vs.
DGE (-)
Age, years (SD)
55.5 (16.7)
62.4 (14.1)
52.1 (16.8)
<.001
Gender, n (%)
Female
Male
397 (41%)
569 (59%)
100 (31%)
222 (69%)
297 (46%)
347 (54%)
<.001
Baseline Comorbidities
Hypertension, n (%)
456 (47%)
204 (63%)
252 (39%)
<.001
CAD, n (%)
273 (28%)
174 (54%)
99 (15%)
<.001
MI, n (%)
227 (23%)
177 (55%)
50 (8%)
<.001
Diabetes, n (%)
158 (16%)
77 (24%)
81 (13%)
<.001
CHF, n (%)
267 (28%)
111 (34%)
156 (24%)
<.001
Hyperlipidemia, n (%)
387 (40%)
182 (57%)
205 (32%)
<.001
PVD, n (%)
44 (5%)
25 (8%)
19 (3%)
<.001
Values are mean ± SD or n (%). DGE: Delayed Gadolinium Enhancement; CAD: Coronary Artery Disease; MI: Myocardial Infarction; CHF: Congestive Heart Failure; PVD: Peripheral Vascular Disease.
Table 1: Overall Demographic Information.
The presence of LGE in the total cohort was associated with a lower eGFR (73.2 ± 22.6 mL/min/1.73 m2) as compared to patients without LGE (78.4 ± 22.8 mL/min/1.73 m2, p<0.001), even after adjustment for gender, LVEF and LVEDMi (p=0.006) (Figure 1). Furthermore, there were statistically significant associations between the presence of LGE and reduced LVEF (46.8 ± 14.3%, p<0.001), increased LVEDMi (56.6 ± 19.4 g/m2, p=0.015) and elevated plasma B-type natriuretic peptide (BNP) (524.5 ± 655.3 pg/mL, p<0.001) as compared to those subjects without LGE, even after adjustment for age, gender, hypertension, CAD and MI (Figure 1).
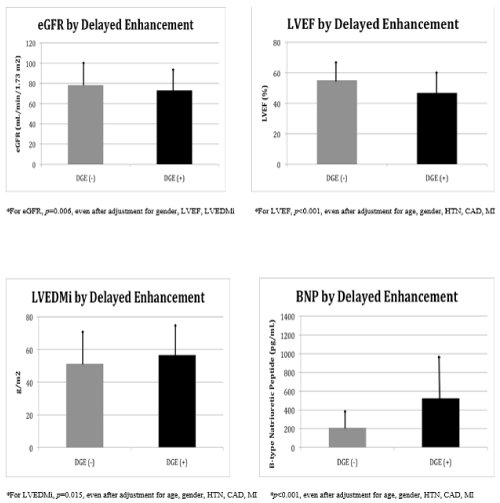
Figure 1: MRI Data by Delayed-Gadolinium Enhancement.
Top left: Comparison of estimated glomerular filtration rate (eGFR) by the presence (DGE+) or absence (DGE-) of delayed gadolinium enhancement.
Top right: Comparison of left ventricular ejection fraction (LVEF) by the presence (DGE+) or absence (DGE-) of delayed gadolinium enhancement.
Bottom left: Comparison of left ventricular end-diastolic mass index (LVEDMi) by the presence (DGE+) or absence (DGE-) of delayed gadolinium enhancement.
Bottom right: Comparison of B-type natriuretic peptide (BNP) levels by the presence (DGE+) or absence (DGE-) of delayed gadolinium enhancement.
The pattern of late enhancement in association with coronary distribution was also assessed in our study. Of the subjects with LGE, 282 (87.6%) were due to infarction, based on the pattern of enhancement in association with coronary distribution, and 40 (12.4%) were due to non-infarction. When comparing infarction with non-infarction enhancement, there were statistically significant associations with CAD, MI and hyperlipidemia in the infarction group, but none with regard to age, gender, other co-morbidities, eGFR, LVEF, LVEDMi or BNP (Table 2).
Variable
Infarction
(N=282)
Non-Infarction
(N=40)
P-value
Age, years (SD)
63.1 (13.4)
57.4 (17.5)
0.07
Gender, n (%)
Female
Male
85 (30%)
197 (70%)
15 (38%)
25 (63%)
0.35
Baseline Comorbidities
Hypertension, n (%)
184 (65%)
20 (50%)
0.06
CAD, n (%)
164 (58%)
10 (25%)
<.001
MI, n (%)
175 (62%)
2 (5%)
<.001
Diabetes, n (%)
68 (24%)
9 (23%)
0.82
CHF, n (%)
97 (34%)
14 (35%)
0.94
Hyperlipidemia, n (%)
171 (61%)
11 (28%)
<.001
PVD, n (%)
24 (9%)
1 (3%)
0.18
MRI Data
LVEF, % (SD)
46.9 (14.0)
46.0 (16.6)
0.73
LVEDMi, g/m2 (SD)
55.8 (16.6)
62.7 (32.7)
0.63
BNP, pg/mL (SD)
550.8 (667.6)
396.2 (526.0)
0.29
eGFR, mL/min/1.73 m2 (SD)
73.3 (22.3)
72.1 (25.3)
0.55
Values are mean ± SD or n (%). DGE: Delayed Gadolinium Enhancement; CAD: Coronary Artery Disease; MI: Myocardial Infarction; CHF: Congestive Heart Failure; PVD: Peripheral Vascular Disease; LVEF: Left Ventricular Ejection Fraction; LVEDMi: Left Ventricular End Diastolic Mass Index; BNP: B-type Natriuretic Peptide; eGFR: Estimated Glomerular Filtration Rate.
Table 2: Demographic and MRI Data by Presence of Infarction.
When stratifying patients by eGFR greater than or less than 70 mL/ min/1.73 m2, a history of increased age, male gender, hypertension, CAD, MI, diabetes, CHF and hyperlipidemia was statistically more likely in patients with lower eGFR (Table 3). Patients with an eGFR less than 70 mL/min/1.73 m2 also had reduced LVEF (49.9 ± 15.5%, p<0.001), increased presence of LGE (n=156, p<0.001) and increased BNP (447.4 ± 639.7 pg/mL, p<0.001) when compared with patients with an eGFR greater than 70 mL/min/1.73 m2, even after adjustment for age, gender, hypertension, CAD, MI and diabetes (Table 3).
Variable
eGFR<70
(N=372)
eGFR>70
(N=594)
P-value
Age, years (SD)
63.3 (13.1)
50.7 (16.9)
<.001
Gender, n (%)
Female
Male
183 (49%)
189 (51%)
214 (36%)
380 (64%)
<.001
Baseline Comorbidities
Hypertension, n (%)
219 (59%)
237 (40%)
<.001
CAD, n (%)
132 (35%)
141 (24%)
<.001
MI, n (%)
101 (27%)
126 (21%)
0.034
Diabetes, n (%)
78 (21%)
80 (13%)
0.002
CHF, n (%)
142 (38%)
125 (21%)
<.001
Hyperlipidemia, n (%)
179 (48%)
208 (35%)
<.001
PVD, n (%)
22 (6%)
22 (4%)
0.11
MRI Data
LVEF, % (SD)
49.9 (15.5)
53.9 (13.1)
<.001*
LVEDMi, g/m2 (SD)
54.1 (10.8)
52.3 (18.5)
0.18
DGE, n (%)
156 (42%)
166 (28%)
<.001*
BNP, pg/mL (SD)
447.4 (639.7)
193.7 (345.1)
<.001*
Values are mean ± SD or n (%). eGFR: Estimated Glomerular Filtration Rate; CAD: Coronary Artery Disease; MI: Myocardial Infarction; CHF: Congestive Heart Failure; PVD: Peripheral Vascular Disease; LVEF: Left Ventricular Ejection Fraction; LVEDMi: Left Ventricular End Diastolic Mass Index; DGE: Delayed Gadolinium Enhancement; BNP: B-type Natriuretic Peptide. (*) adjusted for age, gender, hypertension, CAD, diabetes and MI.
Table 3: Demographic and MRI Data by eGFR Category.
By univariate and multivariate analyses of the overall cohort, the presence of LGE was noted to be independently associated with increased mortality (HR 1.78, 95% CI (1.21, 2.63); p=0.003), even after adjustment for age, gender and MI (HR 1.61, 95% CI (1.03, 2.51); p=0.0356) (Figure 2). When stratifying survival rates based on combined eGFR and LGE status, overall mortality was greatest in patients with LGE and eGFR less than 70 mL/min/1.73 m2 (HR 1.38, 95% CI (1.17, 1.63); p<0.001) (Figure 3). Of note, in patients with eGFR greater than or equal to 70 mL/min/1.73 m2, the presence of LGE was not associated with an increased risk of mortality compared to patients without LGE (HR 1.43, 95% CI (0.77, 2.65); p=0.26). In contrast, in patients with eGFR less than 70 mL/min/1.73 m2, the presence of enhancement was associated with an increased risk of mortality compared to patients without LGE (HR 1.80, 95% CI (1.07, 3.04); p=0.026).
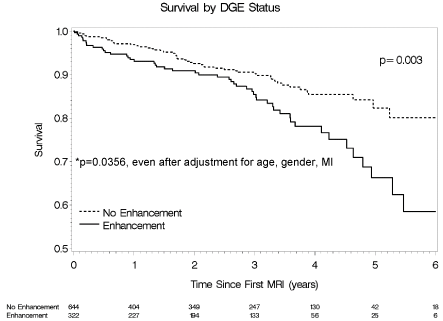
Figure 2: Survival Rate by DGE Status.
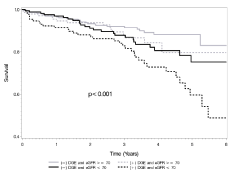
Figure 3: Survival Rate by DGE Status and eGFR.
p-value is for trend.
In the current study, we determined that the presence of lategadolinium enhancement on cardiac MRI is more commonly associated with renal impairment and markers of adverse ventricular remodeling, including elevated BNP, reduced LVEF and increased LVEDMi. In addition, subjects with impairment of renal function were more likely to have LGE along with increased BNP and reduced LVEF. Lastly, our results confirm that subjects with LGE have an increased risk of mortality and the combination of LGE and renal dysfunction significantly elevates the overall mortality risk compared to subjects with neither.
The results of this study correlate with prior studies which established the role of LGE as a predictor of adverse outcomes. However, we extend this risk association to demonstrate that the combined presence of LGE andrenal dysfunction corresponds to an increased risk of death above LGE alone. In fact,LGE was not predictive of an increased risk of mortality in subjects with eGFR >70 mL/min/1.73 m2.
The association between renal impairment and adverse cardiac remodeling and increased mortality seen in this study is also consistent with previous studies which show evidence of a cardiovascular disease process in the early stages of chronic kidney disease. In a cross-sectional echocardiographic study of over 3,000 patients, Park et al. reported a 32% prevalence of LVH in patients with eGFR >60 mL/min/1.73 m2 [24]. Compared with healthy controls, Edwards et al. showed that patients with stage II CKD (eGFR 60-89 mL/ min/1.73 m2) had reduced aortic distensibility and increased arterial and ventricular systolic and diastolic stiffness [25]. A meta-analysis of general population cohorts analyzing over 100,000 participants showed an increased risk of mortality associated with eGFR <75 mL/ min/1.73 m2 [26].
Prior to our current study, the previous largest analysis of LGE in association with renal function was performed Schietinger et al. in 2008. They sought to characterize the presence of LGE by cardiac MRI in hemodialysis patients at high risk for cardiovascular events. Twenty-four patients were included in the study and 79% had evidence of LGE. Patients were followed for an average of one year with five deaths in the LGE group and two deaths in the non- LGE group [23]. With over 900 patients included in our cohort, the current study makes a significant contribution towards advancing our understanding of relationship between LGE and renal function.
Important limitations of this study include its retrospective nature with an inability to account for all confounding variables. In addition, the inclusion of baseline comorbidities relies on appropriate ICD-9 documentation with an inability to completely exclude asymptomatic or undocumented disease and co-morbidities.
Conclusion
This retrospective analysis demonstrates that the presence of late-gadolinium enhancement on cardiac MRI is more commonly associated with renal impairment and markers of adverse ventricular remodeling. In addition, subjects with LGE have an increased risk of mortality and the combination of LGE andrenal impairment significantly elevates the overall risk of mortality compared to subjects with neither.
References
- Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, et al. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004; 109: 1250 –1258.
- Rehwald WG, Fieno DS, Chen EL, Kim RJ, Judd RM. Myocardial magnetic resonance imaging contrast agent concentrations after reversible and irreversible ischemic injury. Circulation. 2002; 105: 224 –229.
- Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005; 26: 1461-1474.
- Moreo A, Ambrosio G, De Chiara B, Pu M, Tran T, et al. Influence of myocardial fibrosis on left ventricular diastolic function: noninvasive assessment by cardiac magnetic resonance and echo. Circ Cardiovasc Imaging. 2009; 2: 437-443.
- Rudolph A, Abdel-Aty H, Bohl S, Boyé P, Zagrosek A, Dietz R, et al. Noninvasive detection of fibrosis applying contrast-enhanced cardiac magnetic resonance in different forms of left ventricular hypertrophy relation to remodeling. J Am Coll Cardiol. 2009; 20: 284-291.
- Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013; 6: 501-511.
- Dawson DK, Hawlisch K, Prescott G, Roussin I, Di Pietro E, Deac M, et al. Prognostic role of CMR in patients presenting with ventricular arrhythmias. JACC Cardiovasc Imaging. 2013; 6: 335-344.
- Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013; 309: 896-908.
- Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008; 51: 2414-2421.
- O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010; 56: 867-874.
- Lehrke S, Lossnitzer D, Schöb M, Steen H, Merten C, Kemmling H, et al. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart. 2011; 97: 727-732.
- Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006; 48: 1977-1985.
- Díez J, Laviades C. [Hypertensive heart disease in the patient with chronic kidney disease]. Nefrologia. 2008; 28: 135-142.
- Díez J, González A, López B, Querejeta R. Mechanisms of disease: pathologic structural remodeling is more than adaptive hypertrophy in hypertensive heart disease. Nat Clin Pract Cardiovasc Med. 2005; 2: 209-216.
- Laviades C, Varo N, Díez J. Transforming growth factor beta in hypertensives with cardiorenal damage. Hypertension. 2000; 36: 517-522.
- Ortega O, Gallar P, Muoz M, Rodríguez I, Carreo A, Ortiz M, et al. Association between C-reactive protein levels and N-terminal pro-B-type natriuretic peptide in pre-dialysis patients. Nephron Clin Pract. 2004; 97: c125-130.
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE . The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am J Kidney Dis. 1996; 28: 53-61.
- Strozecki P, Adamowicz A, Nartowicz E, Odrowaz-Sypniewska G, Wlodarczyk Z, Manitius J. Parathormone, calcium, phosphorus, and left ventricular structure and function in normotensive hemodialysis patients. Ren Fail. 2001; 23: 115-126.
- Vlahakos DV, Hahalis G, Vassilakos P, Marathias KP, Geroulanos S. Relationship between left ventricular hypertrophy and plasma renin activity in chronic hemodialysis patients. J Am Soc Nephrol. 1997; 8: 1764-1770.
- Sato A, Funder JW, Saruta T. Involvement of aldosterone in left ventricular hypertrophy of patients with end-stage renal failure treated with hemodialysis. Am J Hypertens. 1999; 12: 867-873.
- Martin FL, McKie PM, Cataliotti A, Sangaralingham SJ, Korinek J, Huntley BK, et al. Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: a kidney-heart connection. Am J Physiol Regul Integr Comp Physiol. 2012; 302: R292-299.
- Mark PB, Johnston N, Groenning BA, Foster JE, Blyth KG, Martin TN, et al. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006; 69: 1839-1845.
- Schietinger BJ, Brammer GM, Wang H, Christopher JM, Kwon KW, Mangrum AJ, et al. Patterns of late gadolinium enhancement in chronic hemodialysis patients. JACC Cardiovasc Imaging. 2008; 1: 450-456.
- Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012; 23: 1725-1734.
- Edwards, NC, Ferro CJ, Townend JN, Steeds RP. Aortic distensibility and arterial-ventricular coupling in early chronic kidney disease: a pattern resembling heart failure with preserved ejection fraction. Heart. 2008; 94: 1038-1043.
- Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010; 375: 2073-2081.