Research Article
Austin J Mult Scler & Neuroimmunol. 2014;1(1): 7.
Experimental Autoimmune Encephalomyelitis: C57BL/6 Mice Show Gender-based Differences in Neuronal and Immune Injury
Tao Yang1, Qi Zheng2, Hui Zhao2, ZiJing Hu3, Lei Wang2* and Yongping Fan1*
1Department of Traditional Chinese Medicine, Capital Medical University, China
2School of Traditional Chinese Medicine, Capital Medical University, China
3School of Basic Medical Sciences, Capital Medical University, China
*Corresponding author: Lei Wang, School of Traditional Chinese Medicine, Capital Medical University, Beijing 100069, China, Tel: +86-010-83911626; Fax: +86-010-83911627; E-mail: tmwangl@ccmu.edu.cn
*Corresponding author: Yongping Fan, Department of Traditional Chinese Medicine, Beijing Tiantan Hospital, Capital Medical University, 100050, China
Received: October 14, 2014; Accepted: December 01, 2014; Published: December 04, 2014
Abstract
Multiple sclerosis (MS) is an inflammatory demyelinating disorder of the central nervous system (CNS). We investigated the role of gender in neuronal and immune injury in experimental autoimmune encephalomyelitis (EAE), using C57BL/6 mice induced with myelin oligodendrocyte glycoprotein (MOG) 35-55 and complete Freund's adjuvant (CFA) supplemented with pertussis toxin (PTX). The body weights and the neurological function scores of mice in different genders were assessed once a day for 40 days. The histopathologic changes in the brains of mice were observed by light microscope with hematoxylin-eosin (H&E) staining. The protein expression of myelin basic protein (MBP) and microtubule-associated protein (MAP)-2 in the brain of mice were measured by Western-blotting (WB) on day 22 post-immunization (PI) and by immunohistochemistry (IHC) on day 40 PI. The Interleukin (IL)-17A and forkhead box P3 (Foxp3) were assessed by IHC on day 22 and 40 PI. In addition to neurological dysfunction and weight loss, decreased protein expression of MBP and MAP-2 was seen. The ratio of IL-17A/Foxp3 was dramatically enhanced in female and male EAE mice, more so in males than females, compared with normal mice, respectively, The mortality and the neurological function scores were significantly increased, In conclusion, EAE results from an imbalance between Th17 and Treg cells, along with the associated demyelination and axonal damage, affecting males more severely.
Keywords: Experimental autoimmune encephalomyelitis; Gender; Myelin basic protein; Microtubule-associated protein; Interleukin-17A; Forkhead box P3
Introduction
Multiple sclerosis (MS) is a common inflammatory demyelinating disease of the central nervous system (CNS), which is characterized by multifocal inflammation, demyelination, and neuronal damage [1- 3]. With different lesions in the brain, the clinical symptoms of MS are complex and varied [4]. Most patients showed impaired vision, numbness, movement disorders and other symptoms. Symptoms accumulate with relapse of MS, severely reducing the patient's quality of life. Increased rate of relapse and severe disability are a major challenge [5]. MS is the most common chronic disabling disease in young adults [2,6-8], with a female-to-male preponderance approaching 2:1 to 3:1 [9-11], and widening gender gap [11]. Our research explores the underlying differences between genders.
Experimental autoimmune encephalomyelitis (EAE) has been proven to be the ideal animal model presenting with similar pathological and clinical manifestations as human MS [12]. Due to the limited evidence available, related to differential incidence between genders, we studied the distinguishing features in EAE [13]. In this study, we compared body weights, neurological function scores, incidence, mortality and latency between the genders. We observed the expression of myelin basic protein (MBP), the microtubule-associated protein (MAP)-2, Interleukin (IL)-17A, and forkhead box P3 (Foxp3) in the brain of mice. A study of the gender-based differences in EAE using a reliable animal model to investigate MS is clinically significant.
Materials and Methods
Animals
Specific pathogen-free grade, 6-8 weeks-old C57BL/6 mice of were purchased from Beijing Weitong Lihua Experimental Animal Tech. Co., Ltd., China [certification NO. SCXK (JING) 2006-0009]. The animals were housed in the Center of Laboratory Animals at Capital Medical University [certification number SYXK (JING) 2010- 0020]. The experiments were approved by the Ethics Committee of Capital Medical University.
Reagents
Myelin oligodendrocyte glycoprotein peptides (MOG) 35-55 (MEVGWYRSPFSRVVHLYR NGK) were synthesized by Beijing SciLight Biotechnology Co. Ltd. (China) at a purity of >95%. Complete Freund's adjuvant (CFA) and pertussis toxin (PTX) were purchased from Sigma (USA). Rabbit anti-MBP mouse, rabbit anti-MAP-2 mouse, rabbit anti-IL-17A mouse, rabbit anti-Foxp3 mouse were purchased from Abcam (UK). β-actin was purchased from Epitomics (USA). Sheep anti-rabbit IgG and DAB were purchased from Beijing Biosynthesis Biotechnology Co., Ltd. (China). Western-blot kit was purchased from Beijing CoWin Bioscience Co., Ltd. (China).
Induction of EAE animal model
The mice were randomly assigned to four groups: female normal control (F NC) group (n = 12), male normal control (M NC) group (n = 12), female EAE (F EAE) group (n = 15), and male EAE (M EAE) group (n = 15). Each of the EAE mice was injected subcutaneously with 0.2 ml emulsion, containing 50 μg MOG 35-55 in 100 μl of normal saline (NS) and 100 μl of CFA (H37Ra, 4 mg/ml), followed by peritoneal injections of 500 ng of PTX on day 0 and 2 post-immunization (PI) [14]. The mice in NC groups were given NS instead.
Animal observations
Individual animals were examined daily. The body weights, neurological function scores, incidence, mortality and latency data of mice were recorded at the end of experiment. The neurological function scores were defined as reported previously [14]: 0 for no signs, 1 for flaccid tail, 2 for moderate hind-limp paralysis, 3 for complete hind-limp paralysis, 4 for fore-limb paralysis, and 5 deaths.
Sample collection
The mice were sacrificed on day 22 (acute stage) and 40 (remission stage) PI. The four mice out of 15 were anesthetized with an intraperitoneal injection of 10% chloral hydrate (5 ml/kg). The mice were perfused with ice-cold NS (50 ml), followed by slow perfusion with 4% paraformaldehyde through the left ventricle. The brains of mice were removed, fixed, and embedded in paraffin. The tissues were sectioned (thickness, 4μm) for hematoxylin-eosin (H&E) and immunohistochemistry (IHC) staining and detection. The brains of four mice from each group were quickly removed, and immediately frozen in liquid nitrogen, and then kept at -80oC for Western-blotting (WB) detection.
Histopathology
The sections were dewaxed in xylene, dehydrated with gradient alcohol for 5 min each, and stained with Harris hematoxylin for 1 min, followed by eosin for 10 min. The sections were dehydrated in gradient alcohol, permeabilized with xylene, mounted on neutral gum, and observed with light microscopy (Nikon eclipse 80i, Tokyo, Japan).
Immunohistochemical analysis
The paraffin slices were dewaxed in xylene for 15 min, dehydrated in gradient alcohol for 5 min each. The slices were treated with 3% H2O2 for 10 min at room temperature, washed 3 times with PBS (pH7.2) for 5 min each. The slices were pre-treated using heat mediated antigen retrieval with sodium citrate buffer (pH6) for 20 min, washed 3 times with PBS. The slices were incubated with primary antibody [rabbit anti-mouse MBP (1:100), MAP-2 (1:200), IL-17A (1:100) and Foxp3 (1:200)] at 4oC overnight. The slices were later washed 3 times with PBS and incubated with biotin-labeled secondary antibody (sheep anti-rabbit IgG) at 37°C for 30 min, and then washed 3 times with PBS and the colored reaction product was developed using 3,3'-diaminobenzidine tetrahydrochloride (DAB). Finally, the slices were dehydrated and mounted. Each slice was observed microscopically with six high-power fields (x400) randomly selected. The quantitative analysis of immunohistochemical images was carried out with a NIS-Elements BR 3.0 system (Nikon, Japan). Positive results were expressed by integral optical density (IOD).
Western-Blotting analysis
Protein extraction and quantification were performed according to the procedures of specification. Protein bands were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and subsequently transferred onto nitrocellulose membranes (Millipore, USA). The membranes were blocked with 5% defatted dry milk for 60 min, followed by incubation with primarily anti- MBP antibody (1:1000), anti-MAP-2 antibody (1:2000) and anti β-acting rabbit polyclonal antibody (1:3000) in blocking solution at 4oC overnight. The membranes were washed 4 times (10 min each), followed by incubation with goat anti-rabbit IgG (1:10000) for 60 min. The membranes were then washed 4 times (10 min each) and incubated in electrochemiluminescence (ECL) for 5 min, followed by exposure to Kodak film (Comwin, China) and development with an enhanced chemiluminescent kit (Amersham, UK). The image analysis software ImageQuant TL 2005 (Amersham, Biosciences, Piscataway, NJ) was used to analyze IOD value of each band. The relative expression of the target protein was represented by the IOD ratio, IODMBP/ IODβ-actin, IODMAP-2/ IODβ-actin.
Statistical analysis
Data were expressed as mean ± standard error and analyzed with SPSS software (Version 17.0, Chicago, IL, USA,). The test of normality was conducted by one-way ANOVA with a post-hoc LSD test, or otherwise with a rank-sum test. P values less than 0.05 were considered statistically significant.
Result
Incidence, mortality and latency of EAE mice
The experimental groups developed typical symptoms (limp tail, limb weakness or even death) of F EAE mice after 12.7±3.2 days and M EAE mice after 10.0±1.5 days following immunization, with 100% incidence and a chronic monophasic course. The mortality was 6.3% in F EAE mice, and was significantly higher, up to 20.0% in M EAE mice (p < 0.01). The latency in M EAE group was significantly shorter compared with the F EAE group (p < 0.01, Table 1).
Group
Number (n)
Incidence (%)
Mortality (%)
Latency (days)
F NC
12
0
0
-
F EAE
15
100.0
6.3
12.7±3.2
M NC
12
0
0
-
M EAE
15
100.0
20.0§§
10.0±1.5§§
Table 1: Incidence, mortality and latency of EAE mice ( x̄ ±SE).
Changes in body weights
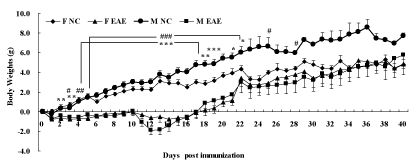
No statistical difference was seen in the changes of body weights of mice before immunization. The body weights decreased significantly in F EAE mice compared with F NC mice on day 2 to day 19 and day 21, 22 (p < 0.05 or p < 0.01 or p < 0.001), respectively. The body weights decreased significantly in M EAE mice compared with M NC mice from day 3 to day 22 and day 25, 28 (p < 0.05 or p < 0.01 or p < 0.001), respectively. The body weights were restored from day 18, and the trend continued. No significant difference was observed until day 40 between the four groups (Figure 1).
Figure 1: Changes in body weights of mice. Mice were weighed daily until day 40 PI. The data were evaluated by body weights, measured in F NC (◆), F EAE (▲), M NC (●), M EAE (■). Note: *p < 0.05, **p < 0.01, ***p < 0.01 vs. F NC; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. M NC.
Neurological function scores
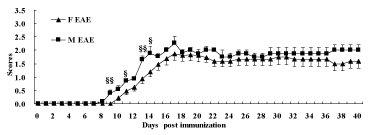
The male mice were attacked initially from day 8, and the female mice from day 10. The symptoms of EAE including flaccid tail, stagger, hind-limb paralysis, fore-limb paralysis and even death appeared successively. The neurological function scores of both F and M EAE mice reached a peak on day 17. The data showed that the scores of M EAE mice on day 9, 11, 13, 14 increased remarkably compared with F EAE mice (p < 0.05 or p < 0.01), declining slightly until day 40 after the peak (Figure 2).
Figure 2: The neurological function scores of mice. Mice were scored daily until day 40 PI. The scores were measured in F NC (◆), F EAE (▲), M NC (●), M EAE (■). Note: §p < 0.05, §§p < 0.01 vs. F EAE.
Pathological changes
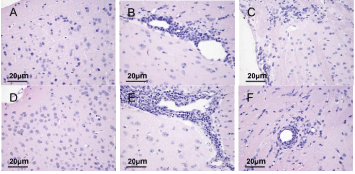
The pathological changes in the brains of mice were observed under a light microscope by H&E staining on day 22 and 40 PI. Results showed a normal neuronal structure in the F NC and M NC groups (Figure 3A, 3D). The cytoplasm and nuclei were clear and the neuroglial cells were scattered. A large number of inflammatory cells were aggregated around small blood vessels to form sleeve-like changes in the F EAE and M EAE groups on day 22 PI (Figure 3B, 3E). Inflammatory cell infiltration was reduced to varying degrees on day 40 PI, but the cell shrinkage and nuclear lysis were visible in white matter sections of mice brains in F EAE and M EAE groups (Figure 3C, 3F).
Figure 3: Observation of pathological changes in the brains of mice under the light microscope with H&E staining (magnification, x400). A, D Normal structure in the F NC and M NC groups. B, E A large amount of inflammatory cell infiltration in F EAE and M EAE groups on day 22 PI. C, F Cell shrinkage and nuclear lysis in the F NC and M NC groups on day 40 PI.
Protein expression of MBP and MAP-2
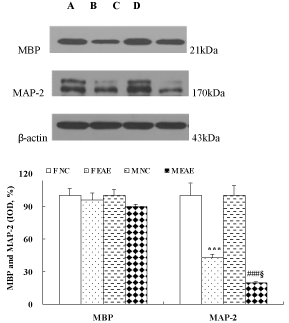
The protein expression of MBP and MAP-2 in the brains of mice was observed with WB method on day 22 PI. The data showed no difference in MBP expression between the F NC, F EAE, M NC and M EAE mice. The MAP-2 in the brains of mice was significantly decreased in F EAE and M EAE mice compared with the F NC and M NC mice, respectively (p < 0.001). In addition, MAP-2 was apparently decreased in M EAE mice compared with the F EAE mice (p < 0.05, Figure 4).
Figure 4: MBP and MAP-2 protein expression in the brains of mice on day 22 PI. Lines A, B, C and D showed the expression of MBP or MAP-2 in the brain of mice in F NC, F EAE, M NC, M EAE groups, respectively. The protein expression of MBP and MAP-2 was assessed by Western-blot. Mean ± SE are depicted (n = 4 per group). Note: ***p < 0.001 vs. F NC; ###p < 0.001 vs. M NC; §p < 0.05 vs. F EAE.
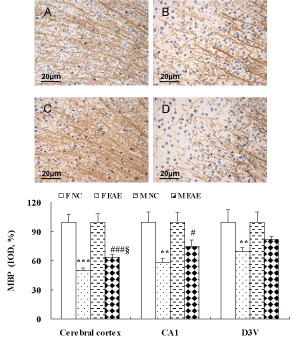
The protein expression of MBP and MAP-2 in the brains of mice was observed with IHC on day 40 PI. The protein expression of MBP in the cerebral cortex, hippocampal CA1 and dorsal 3rd ventricle (D3V) region was significantly decreased in F EAE mice compared with the F NC mice (p < 0.01 or p < 0.001). MBP in the cerebral cortex and hippocampal CA1 region was significantly decreased in M EAE mice compared with the M NC mice (p < 0.05 or p < 0.001). MBP in the cerebral cortex was significantly decreased in F EAE mice compared with the M EAE mice (p < 0.05, Figure 5).
Figure 5: MBP protein expression in the mouse brain. A to D showed the MBP expression in the cerebral cortex of mice in F NC, F EAE, M NC and M EAE groups, respectively (magnification, x400). The protein expression of MBP was assessed by IHC on day 40 PI. Means ± SE are depicted (n = 4 per group). Note: **p < 0.01, ***p < 0.001 vs. F NC; #p < 0.05, ###p < 0.001 vs. M NC; §p < 0.05 vs. F EAE.
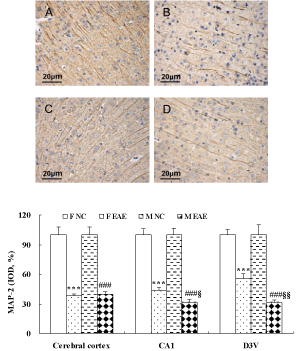
The protein expression of MAP-2 in the cerebral cortex, hippocampal CA1 and D3V region was significantly decreased in F EAE and M EAE mice compared with the F and M NC mice, respectively (p < 0.001). MAP-2 in the hippocampal CA1 and D3V region was significantly decreased in M EAE mice compared with the F EAE mice (p < 0.05 or p < 0.01, Figure 6).
Figure 6: MAP-2 expression in the mouse brain. A to D showed the expression of MAP-2 in the cerebral cortex of mice in F NC, F EAE, M NC and M EAE, respectively (magnification, x400). The expression of MAP-2 protein was assessed by IHC on day 40 PI. Mean ± SE are depicted (n = 4 per group). Note: ***p < 0.001 vs. F NC; ###p < 0.001 vs. M NC, §§p < 0.01 vs. F EAE.
Protein expression of IL-17A and Foxp3 on day 22 and 40 PI
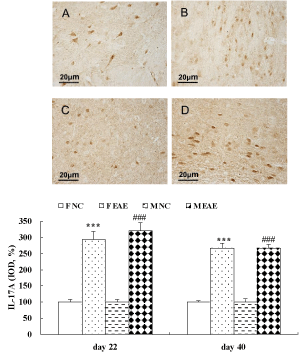
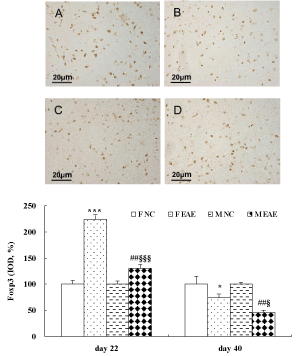
The data showed that the protein expression of IL-17A in the brain of mice was significantly increased in the F EAE and M EAE mice compared with the F and M NC mice on day 22 and 40, respectively (p < 0.001, Figure 7). The protein expression of Foxp3 in the mouse brains was significantly increased in the F EAE and M EAE mice compared with the F NC and M NC mice on day 22 PI (p < 0.01 or p < 0.001), but it was significantly decreased in M EAE mice compared with F EAE mice (p < 0.001). Foxp3 was significantly decreased in the F EAE and M EAE mice compared with F NC and M NC mice on day 40, respectively (p < 0.05 or p < 0.01), and it was also markedly reduced in M EAE mice compared with the F EAE mice on day 40 (p < 0.05, Figure 8).
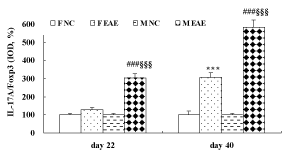
Figure 7: Expression of IL-17A protein in the mouse brain. A to D showed the expressions of IL-17A in the mouse brain in F NC, FE EAE, M NC and M EAE groups on day 40, respectively, (magnification, x400). The protein expression of IL-17A was assessed by IHC on day 22 and 40 PI. Mean ± SE are depicted (n = 4 per group). Note: ***p < 0.001 vs. F NC, ###p < 0.001 vs. M NC.
Figure 8: Expression of Foxp3 protein in the mouse brain. A to D showed the expressions of Foxp3 in the brains of mice in F NC, F EAE, M NC and M EAE on day 40, respectively (magnification, x400). The Foxp3 protein expression was assessed by IHC on day 22 and 40 PI. Mean ± SE are depicted (n = 4 per group). Note: *p < 0.05, ***p < 0.001, vs. F NC, # p < 0.01 vs. M NC, §p < 0.05, §§§p < 0.001 vs. F EAE.
Furthermore, the ratio of IL-17A/Foxp3 indicated no difference between the F EAE mice and F NC mice but was statistically significant between M EAE mice and M NC mice (p < 0.001) on day 22 PI. The ratio was significantly increased in the F EAE and M EAE mice compared with F NC and M NC mice on day 40, respectively (p < 0.001). It was also markedly increased in M EAE mice compared with F EAE mice on day 22 and 40 PI (p < 0.001, Figure 9).
Figure 9: The ratio of IL-17A/Foxp3 in the brain of mice. Means ± SE are depicted (n = 4 per group). Note: ***p < 0.001 vs. F NC, ###p < 0.01 vs. M NC, §§§p < 0.001 vs. F EAE.
Finally, the cytokine levels in the brains of mice were analyzed on day 22 and 40 PI. The data showed that the protein expression of IL- 17A was significantly decreased on day 40 PI compared with day 22 PI in the M EAE mice (p < 0.05) and no difference was seen between day 22 and 40 PI in the F EAE mice. Foxp3 was significantly decreased on day 40 PI compared with day 22 PI in the F EAE and M EAE mice, respectively (p < 0.001, Figure 10).
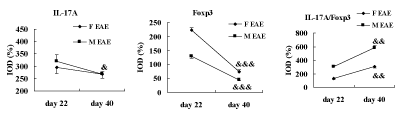
Figure 10: Cytokine levels in the mouse brain. The ratio of IL-17A/Foxp3 was apparently increased on day 40 PI compared with day 22 PI in the F EAE and M EAE mice, respectively. Means ± SE are depicted (n = 4 per group). Note: &p < 0.05, &&&p < 0.001 vs. day 22 PI.
Discussion
MS is characterized by local inflammation surrounding the white matter in CNS, inducing demyelination, gliosis and neuronal destruction mediated by T cells, especially in young adults [1,2,15]. It affects women 2 to 3 times more frequently than men [10], the ratio increasing to 4 to 1, in a recent Canadian study [11]. Our results showed that the neurological function scores of the male EAE showed a zigzag increase in the acute phase compared with the female EAE. The male EAE neurological dysfunction appeared on day 8 PI while the female on day 10 PI.
Although the MBP (a major component of myelin sheath [16,17]) of EAE in WB was not considerably different from the NC mice, the IHC showed EAE mice were deficient in MBP in the white matter. It is plausible that the myelin sheath was damaged in some parts of EAE but no difference between genders was observed. MBP is closely related to the vulnerability of white matter and hippocampus [18]. MBP staining appears to overlie NFH staining, which is lost at higher resolution [19]. MAP-2 is an important marker, and a cytoskeletal protein involved in neuronal plasticity [20]. It is mainly expressed in neuronal cell bodies, dendrites, dendritic spines and postsynaptic dense area in CNS [20]. MAP-2, playing a role in the growth of nervous system, was also detected by WB and IHC. The low expression of MAP-2 is related to axon and dendrite damage [21,22]. We detected MAP-2 in the white matter of mice. The results showed that MAP-2 in the cerebral cortex, hippocampal CA1 and D3V region was significantly decreased in EAE compared with the NC mice. Furthermore, MAP-2 was expressed higher in F EAE than M EAE, which suggested that the male mice had more serious axon damage than female mice. EAE has been attributed to the imbalance of Th1/ Th2 [23-25]. Recent research showed that the imbalance in Th17/Treg plays a critical role in the EAE [26]. Furthermore, the pathogenesis of EAE/MS involves aberrant activation of autoreactive T lymphocytes in the peripheral access to the brain followed by T cell recruitment [15]. IL-17, a cytokine produced by Th17 cells, plays a crucial role in the development of EAE [27-29]. The ablation of ACT1 ameliorates the EAE in mice [30]. ACT1 is a key adaptor molecule mediating IL-17 signaling, not affecting the Th1 cell-mediated EAE. The IL-17 family contains IL-17A, IL-17B, IL-17C, IL-17D, IL-17E [31,32]. The IL-17A is the key player in IL-17-induced autoimmunity [31-33]. The results showed that the IL-17A was overexpressed on the day 22, 40 PI in EAE mice, without any difference between genders. No significant differences were seen clinically in any cytokines between men and women with MS [34]. Foxp3 mRNA is expressed by T regulatory cells (Treg) [35], with low expression of Foxp3 decreasing the function of Treg [36,37]. Foxp3+ Treg has the ability to control the autoimmune responses and inhibits Th17 [26,38]. The overexpression of Foxp3+ inhibits the IL17-induced immune damage [26]. The data showed that Foxp3 was increased in EAE mice on day 22 PI and decreased in M EAE mice compared with F EAE mice on day 22, 40 PI. EAE mice overexpressed IL-17A and Foxp3 compared with NC mice, which demonstrates the inflammation due to EAE in the CNS. We also found a higher IL-17A/ Foxp3 ratio in male EAE than female EAE on day 22, 40 PI, implying that the higher neurological function scores associated with EAE in males caused more serious inflammation, although no differences were observed in the IL-17A expression between genders. The Th17 cell lineage characteristically produces high levels of IL-17 [39], while the Treg cells expressing the Foxp3 transcription factor are essential for immune regulation [40]. All of above suggest that Th17 or Treg alone have a limited role in the measurement of immunity. The ratio of Th17 and Treg may reflect the true measure of immunity. As the results showed, the ratio of IL-17A/ Foxp3 can be used as a measuring index of the degree of inflammation, of the underlying pathology mediating inflammation in EAE which may be related to the imbalance in Th17/Treg levels. The severe neurological deficiency in males and the high ratio of IL-17A/ Foxp3 indicate that inflammation results in demyelination, suggesting a role for injury due to inflammation in the differential morbidity of males and females. A number of studies showed that estrogen has protective role in inflammatory process [41]. It was also shown to be protective in EAE [42,43]. Pregnancy is consistently associated with a reduced relapse rate [44,45]. Our research found that the female EAE mice weighed lower compared with male EAE mice, probably related to high levels of estrogen.
Finally, the results described in this study introduce gender differences in EAE, immunologically, with males showing higher immune reaction compared with females. However, the general pathological changes are not significantly different. In spite of the indispensable role played by estrogen in EAE gender [46-48], the higher neurological dysfunction in males is attributed to imbalance in Th17/Treg levels.
Acknowledgment
This work was supported by the National Natural Science Foundation (81072765; 81173237; 81273742; 81473640), the Beijing Natural Science Foundation (7142053), the Scientific Research Key Program of Beijing Municipal Commission of Education (KZ201310025023) and the Program of Changcheng Scholars for the Importation and Development of High-Caliber Talents Project of Beijing Municipal Institutions (CIT&TCD20140329).
References
- Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006; 129: 1953-1971.
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008; 372: 1502-1517.
- Herz J, Zipp F, Siffrin V. Neurodegeneration in autoimmune CNS inflammation. Exp Neurol. 2010; 225: 9-17.
- Sloane E, Ledeboer A, Seibert W, Coats B, van Strien M, Maier SF, et al. Anti-inflammatory cytokine gene therapy decreases sensory and motor dysfunction in experimental Multiple Sclerosis: MOG-EAE behavioral and anatomical symptom treatment with cytokine gene therapy. Brain Behav Immun. 2009; 23: 92-100.
- Scalfari A, Neuhaus A, Daumer M, Ebers GC, Muraro PA. Age and disability accumulation in multiple sclerosis. Neurology. 2011; 77: 1246-1252.
- Kantarci OH, Weinshenker BG. Natural history of multiple sclerosis. Neurol Clin. 2005; 23: 17-38, v.
- Sellner J, Kraus J, Awad A, Milo R, Hemmer B, Stüve O. The increasing incidence and prevalence of female multiple sclerosis--a critical analysis of potential environmental factors. Autoimmun Rev. 2011; 10: 495-502.
- Tsang BK, Macdonell R. Multiple sclerosis- diagnosis, management and prognosis. Aust Fam Physician. 2011; 40: 948-955.
- Duquette P, Pleines J, Girard M, Charest L, Senecal-Quevillon M, Masse C. The increased susceptibility of women to multiple sclerosis. Can J Neurol Sci. 1992; 19: 466-471.
- Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001; 2: 777-780.
- Orton SM, Herrera BM, Yee IM, Valdar W, Ramagopalan SV, Sadovnick AD, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 2006; 5: 932-936.
- van der Star BJ, Vogel DY, Kipp M, Puentes F, Baker D, Amor S. In vitro and in vivo models of multiple sclerosis. CNS Neurol Disord Drug Targets. 2012; 11: 570-588.
- Teuscher C, Bunn JY, Fillmore PD, Butterfield RJ, Zachary JF, Blankenhorn EP. Gender, age, and season at immunization uniquely influence the genetic control of susceptibility to histopathological lesions and clinical signs of experimental allergic encephalomyelitis: implications for the genetics of multiple sclerosis. Am J Pathol. 2004; 165: 1593-1602.
- Kono DH, Urban JL, Horvath SJ, Ando DG, Saavedra RA, Hood L. Two minor determinants of myelin basic protein induce experimental allergic encephalomyelitis in SJL/J mice. J Exp Med. 1988; 168: 213-227.
- Piccio L, Rossi B, Scarpini E, Laudanna C, Giagulli C, Issekutz AC, et al. Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric G(i)-linked receptors. J Immunol. 2002; 168: 1940-1949.
- Latasa MJ, Ituero M, Moran-Gonzalez A, Aranda A, Cosgaya JM. Retinoic acid regulates myelin formation in the peripheral nervous system. Glia. 2010; 58: 1451-1464.
- Chen Y, Yi Q, Liu G, Shen X, Xuan L, Tian Y. Cerebral white matter injury and damage to myelin sheath following whole-brain ischemia. Brain Res. 2013; 1495: 11-17.
- Kapadia M, Sakic B. Autoimmune and inflammatory mechanisms of CNS damage. Prog Neurobiol. 2011; 95: 301-333.
- McLaurin JA, Yong VW. Oligodendrocytes and myelin. Neurol Clin. 1995; 13: 23-49.
- Zhang J, Li Y, Lu M, Cui Y, Chen J, Noffsinger L. Bone marrow stromal cells reduce axonal loss in experimental autoimmune encephalomyelitis mice. J Neurosci Res. 2006; 84: 587-595.
- Mews I, Bergmann M, Bunkowski S, Gullotta F, Brück W. Oligodendrocyte and axon pathology in clinically silent multiple sclerosis lesions. Mult Scler. 1998; 4: 55-62.
- Lovas G, Szilágyi N, Majtényi K, Palkovits M, Komoly S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain. 2000; 123: 308-317.
- Charlton B, Lafferty KJ. The Th1/Th2 balance in autoimmunity. Curr Opin Immunol. 1995; 7: 793-798.
- Laman JD, Thompson EJ, Kappos L. Balancing the Th1/Th2 concept in multiple sclerosis. Immunol Today. 1998; 19: 489-490.
- Monteiro de Castro G, Eduarda Zanin M, Ventura-Oliveira D, Aparecida Vilella C, Ashimine R, de Lima Zollner R. Th1 and Th2 cytokine immunomodulation by gangliosides in experimental autoimmune encephalomyelitis. Cytokine. 2004; 26: 155-163.
- Petro TM. Regulatory role of resveratrol on Th17 in autoimmune disease. Int Immunopharmacol. 2011; 11: 310-318.
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007; 448: 480-483.
- Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008; 118: 597-607.
- Caruso R, Botti E, Sarra M, Esposito M, Stolfi C, Diluvio L, et al. Involvement of interleukin-21 in the epidermal hyperplasia of psoriasis. Nat Med. 2009; 15: 1013-1015.
- Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010; 32: 414-425.
- Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002; 71: 1-8.
- Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang SK. IL-17 cytokine family. J Allergy Clin Immunol. 2004; 114: 1265-1273.
- Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008; 205: 1063-1075.
- Eikelenboom MJ, Killestein J, Uitdehaag BM, Polman CH. Sex differences in proinflammatory cytokine profiles of progressive patients in multiple sclerosis. Mult Scler. 2005; 11: 520-523.
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012; 30: 531-564.
- Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007; 445: 766-770.
- Irony-Tur-Sinai M, Grigoriadis N, Tsiantoulas D, Touloumi O, Abramsky O, Brenner T. Immunomodulation of EAE by alpha-fetoprotein involves elevation of immune cell apoptosis markers and the transcription factor FoxP3. J Neurol Sci. 2009; 279: 80-87.
- Shalev I, Schmelzle M, Robson SC, Levy G. Making sense of regulatory T cell suppressive function. Semin Immunol. 2011; 23: 282-292.
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005; 6: 1123-1132.
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012; 30: 531-564.
- Vegeto E, Ciana P, Maggi A. Estrogen and inflammation: hormone generous action spreads to the brain. Mol Psychiatry. 2002; 7: 236-238.
- Palaszynski KM, Liu H, Loo KK, Voskuhl RR. Estriol treatment ameliorates disease in males with experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol. 2004; 149: 84-89.
- Morales LB, Loo KK, Liu HB, Peterson C, Tiwari-Woodruff S, Voskuhl RR. Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. J Neurosci. 2006; 26: 6823-6833.
- Borchers AT, Naguwa SM, Keen CL, Gershwin ME. The implications of autoimmunity and pregnancy. J Autoimmun. 2010; 34: J287-299.
- Coyle PK. Pregnancy and multiple sclerosis. Neurol Clin. 2012; 30: 877-888.
- Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005; 170: 85-92.
- Gold SM, Voskuhl RR. Estrogen and testosterone therapies in multiple sclerosis. Prog Brain Res. 2009; 175: 239-251.
- MacKenzie-Graham AJ, Rinek GA, Avedisian A, Morales LB, Umeda E, Boulat B, et al. Estrogen treatment prevents gray matter atrophy in experimental autoimmune encephalomyelitis. J Neurosci Res. 2012; 90: 1310-1323.