
Research Article
Austin J Mult Scler & Neuroimmunol. 2015; 2(4): 1021.
MS Patients’ Performance Profile in the Time- Limited Subtests of the Brief Repeatable Battery of Neuropsychological Tests (BRBNT)
Rosti-Otajärvi E1*, Mäntynen A2, Ruutiainen J3, Huhtala H4 and Hämäläinen P5
¹Department of Neurology and Rehabilitation, Tampere University Hospital, Finland
²Department of Psychology, Seinäjoki Central Hospital, Finland
³Masku Neurological Rehabilitation Centre, Finland
4Tampere University, School of Health Sciences, Finland
5Masku Neurological Rehabilitation Centre, Finland
*Corresponding author: Eija Rosti-Otajärvi, Department of Neurology and Rehabilitation, Tampere University Hospital, PO Box 2000, 33521 Tampere, Finland
Received: October 16, 2015; Accepted: December 22, 2015; Published: December 25, 2015
Abstract
Background: Although the Brief Repeatable Battery of Neuropsychological Tests (BRBNT) is widely used in cognitive screening in multiple sclerosis (MS), the performance profile in it’s’ tests has not been systematically evaluated.
Objective: The aim of the present study was to evaluate the performance profile in the Paced Auditory Serial Addition Test (PASAT), the Symbol Digit Modalities Test (SDMT), and the Controlled Oral Word Association Test (COWAT) in patients with MS, and to find out whether it differs between relapsing and progressive phenotype, as well as whether patient’s mood or cognitive status affects the profiles.
Method: 187 MS patients (136 relapsing-remitting, 51 progressive) underwent neuropsychological assessment with the PASAT, the SDMT, and the COWAT. Ppatients’ performances in these tests were evaluated across the items in three phases (beginning, middle, and end). Cognitive status was determined using the BRBNT composite score, and mood with the Beck Depression Inventory II (BDI-II).
Results: The performance declined from the beginning to the end during all three cognitive tests in both groups. Cognitive status affected the performance profiles. Instead, mood did not.
Conclusion: By recording the course of performance during the tests of the BRBNT, important information on performance stability and possible signs of cognitive fatigue can be collected.
Keywords: Brief Repeatable Battery of Neuropsychological Tests (BRBNT); Cognitive fatigue; Multiple sclerosis; Progressive; Relapsing
Introduction
Different clinical phenotypes, like relapsing-remitting and progressive (primary and secondary), are characteristics of the disease course of multiple sclerosis (MS) [1]. Cognitive deficits are a common manifestation in MS occurring in about 50-60% of patients [2,3]. Cognitive functions most often affected are speed of informationprocessing and memory and learning [2,3]. Deficits in complex attention and executive functions are also relatively frequent, while those in visual perception and verbal skills are more infrequent [2,3]. Clinically significant depression or fatigue may aggravate cognitive symptoms [4]. Most of the evidence at present suggests that cognitive deficits are more frequent and more widespread in progressive than in relapsing form of the disease [5-11].
The Brief Repeatable Battery of Neuropsychological Tests (BRBNT) is a widely used brief neuropsychological battery with reasonable availability and acceptable sensitivity in MS [7,12,13]. The BRBNT includes the Buschke Selective Reminding Test (BSRT) to assess verbal memory, the 10/36 Spatial Recall Test (10/36) to assess visual memory, the Symbol Digit Modalities Test (SDMT) to assess information-processing speed, the Paced Auditory Serial Addition Test to assess attention, information-processing speed, and working memory, and the Controlled Oral Word Association Test (COWAT) to assess semantic fluency [13]. The total scores of single cognitive tests, as well as the composite score are the most frequently used variables in the BRBNT.
Fatigue is considered to be one of the most disabling symptoms of MS, greatly impacting quality of life [14]. Assessment of fatigue has typically relied on subjective self-report questionnaires and as such is more frequently seen in progressive than in relapsing form of the disease [15] as well as in patients with higher disability [15,16]. Selfreport assessments of fatigue can be confounded by motor or cognitive impairment, depression as well as lack of universally accepted definition for fatigue. Cognitive fatigue, i.e. temporary decline in sustained cognitive activity, has been reported as a feature of MSrelated cognitive decline [17]. It has been suggested that cognitive fatigue may be evaluated by analyzing the course of performance during a single test or during a neuropsychological assessment offering a more objective way than self-report questionnaires. A decline during cognitive test performance [18-21], slowing response times [22] and increased response time variability [23] among patients with MS has been interpreted as a possible sign of cognitive fatigue. However, declining performance during cognitive tests has been observed also in healthy participants [24]. The performance of patients with MS has been shown to decline during the PASAT, a 3-min-long test with high information-processing and working memory demands [18,19], during the SDMT, a 1.5-min-long information-processing speed task [20], as well as during a 15-min-long computerized test of sustained attention [21]. Sensitivity of the PASAT in detecting performance deterioration has been suggested to vary according to the scoring methods [25,26]. The percent dyad score method, which reflects performance strategy and the degree to which the task has been performed according to the intended demands, has been found to be more sensitive to detect performance deterioration than total correct score [25,26]. The so-called dyad score method involves counting only the total number of times that two correct responses are given in a row (“dyads”) for each PASAT trial. A percent dyad score consequently reflects the percentage of total correct responses accounted for by these dyads [27]. Additionally, so-called fatigue scores for the PASAT have been calculated by subtracting raw scores from the first half of each administration from the raw scores from the second half to describe the change during the performance [25]. There is evidence that patients with MS may show greater slowing of response times [22] and increased response time variability [23] in cognitive tests than healthy controls. The course of performance during cognitive tests has not been compared between relapsing and progressive phenotypes. It is crucial to evaluate the performance profile (course of performance during a test), because temporary decline in cognitive performance whether interpreted as cognitive fatigue or not may be critical in certain activities.
Whether clinical phenotype of the disease, depression, or cognitive status of the patient modulates the performance profile in cognitive tests remains unknown. To the best of our knowledge, despite the comprehensive use of the BRBNT, the performance profile has not been systematically examined in the time-limited tests of the battery. Instead, the total scores of the tests are commonly used. A more specific evaluation of performance during the time-limited tests of the BRBNT, namely the PASAT, the SDMT, and the COWAT, offers information on performance stability and possible signs of deterioration during the tests. Instead, same kind of evaluation is controversial during the BSRT and the 10/36 SRT because they are not time-limited in the same way as the other three tests. In the present study, the performances of patients with MS in the time-limited tests of the BRBNT were evaluated across the items in three phases of each test (beginning, middle, and end). Additionally, the so-called fatigue scores in the tests and the dyad scores in the PASAT were calculated. The aim of the present study was to evaluate the performance profile in MS patients with cognitive complaints in the PASAT, the SDMT, and the COWAT. Specifically, the aims were to find out whether the performance profile differs in relapsing and progressive phenotype of the disease and whether patient’s mood or cognitive status affects the performance profile.
Materials and Methods
Participants
The study population consisted of a pooled sample of MS patients from two previous study samples. The common inclusion criteria were clinically definite [28] MS, the Expanded Disability Status Scale (EDSS) [29] between 0 and 8, and subjective cognitive complaints (identified either with the Multiple Sclerosis Neuropsychological Questionnaire–Patient (MSNQ-P) or interview), and age 18-62. Patients with a history of alcohol or drug abuse, psychiatric disorder, acute relapses, neurological disease other than MS, or severe overall cognitive impairment were excluded. A total of 187 patients with clinically definite [28] relapsing [RRMS (n = 136)] or progressive [SPMS (n = 25), PPMS (n = 26)] MS were included. All patients provided written informed consent, and the study protocol was approved by the Ethics Committee of Seinäjoki Central Hospital, Tampere University Hospital, and Turku University Hospital.
Outcome measures
Cognitive performance was evaluated with the BRBNT including the BSRT, the 10/36, the SDMT, the PASAT (two and three second interstimulus versions; PASAT-2 and PASAT-3, respectively), and the COWAT [13].
The analysed variables for the PASAT were the number of correct (max 60), dyad scores (max 59) and percent dyad scores. The percent dyad score is the proportion of the total correct responses accounted for by the dyads (two correct consecutive answers), and it was calculated using the following formula: (1–(total correct score– dyad score)/total correct score) x 100. To evaluate the performance profile (change during performance) in cognitive tests, patients’ performance in the PASAT-2 and -3 (raw and dyad score), SDMT, and COWAT was evaluated across the items in three phases. The phases refer to the performance during the first 20 calculations (1- 20; beginning), second 20 calculations (21-40; middle), and last 20 calculations (41-60; end) in the PASAT, and performance during the first 30 sec. (0-30; beginning), second 30 sec. (31-60; middle), and last 30 sec. (61-90; end) in the SDMT and the COWAT. Additionally, fatigue scores were calculated according to Walker et al. [25]. For the PASAT, raw scores from the first half of each administration were subtracted from raw scores from the second half. For the SDMT and the COWAT, scores from the first part (0-30 sec) were subtracted from scores from the last part (61-90 sec). Negative difference scores were suggestive of cognitive fatigue [25].
In order to determine the overall cognitive status of the patients, a composite score (a single Z-score from all BRBNT subtests) was calculated using the formula suggested by Sepulcre et al. [7]. This composite score has been widely used to describe the overall cognitive performance of patients with MS. To obtain Z-scores for each cognitive domain in the BRBNT, a reference group of 24 healthy controls was used [30]. Patients who failed on at least 33% (3/9) of the tests of the BRBNT were classified as cognitively impaired [6,31]. Patients were considered to have failed a particular cognitive test if they scored below the 5th percentile for healthy controls. Self-perceived depressive symptoms were evaluated with the Beck Depression Inventory II (BDI-II) [32]. The score ≥14 was used as a cut-off in the BDI-II for classifying patients as depressed.
Statistical analyses
In comparisons between the two groups (comparisons in demographic and clinical characteristics, as well as in fatigue scores between the groups), the Chi-Square tests for nominal, Mann Whitney U-tests for non-normally and Student’s t-tests for normally distributed variables were used. In order to investigate the change during cognitive test performance, a repeated measures analysis of variance (ANOVA) was conducted to assess possible differences over time (tests divided into three phases) and possible differences between groups (relapsing vs. progressive; depressed vs. nondepressed; cognitively intact vs. impaired) as well as the interaction between time and group. When compared groups differed in terms of demographic factors adjustments for these were made.
Results
Demographic and clinical characteristics
Patients in the relapsing group were younger than patients in the progressive group. Education or gender distribution did not differ between the groups. Patients in the relapsing group had lower Expanded Disability Status Scale (EDSS) scores and shorter disease duration than patients in the progressive group. The SDMT performance was better in the relapsing than in the progressive group. Otherwise cognitive performance or mood did not differ between the groups (Table 1).
Descriptive variables
Relapsing (n = 136)
Progressive (n = 51)
P
P after adjustment by age
Mean
SD
Mean
SD
Demographics
Age in years
43.9
8.8
50.2
8.1
<0.001
Sex, female / male
100 / 36
35 / 16
0.505
Education in years
13.8
2.5
13
2.8
0.11
Clinical
EDSS, n / %
0-4
102 / 75.0
9 / 17.7
4.5 – 6.0
27 / 19.8
20 / 39.2
≥ 6.5
7 / 5.2
22 / 43.1
<0.001
Duration since MS diagnosis in years
9.2
6.6
13.7
7.8
<0.001
Cognitive performance
BRBNT (composite z-score)
-0.8
1.1
-1.1
1.3
0.062
0.488
PASAT-3 (total score)
39.8
12.2
39.2
14.5
0.776
0.801
PASAT-3-dyad score (total score)
27.8
16.1
27.5
19.5
0.919
0.688
Percent dyad score PASAT-3
62.9
24.9
59.3
32.6
0.964
0.794
PASAT-2 (total score)
28.2
9.6
27.9
11.1
0.888
0.877
PASAT-2-dyad score (total score)
13.6
11
12.6
13.5
0.21
0.986
Percent dyad score PASAT-2
41.7
23.4
37.2
27.7
0.265
0.591
SDMT (total score)
45.1
9.8
38.1
10.6
<0.001
0.004
COWAT (total score)
23.3
7.2
22.1
7.6
0.321
0.621
Mood
BDI-II (total score)
11.2
6.7
12.6
7.7
0.632
EDSS: Expanded Disability Status Scale; BRBNT: Brief Repeatable Battery of Neuropsychological Tests; PASAT-3: Paced Auditory Serial Addition Test (three-second interstimulus version); PASAT-2: Paced Auditory Serial Addition Test (two-second interstimulus version); SDMT: Symbol Digit Modalities Test; COWAT: Controlled Oral Word Association Test; BDI-II: Beck Depression Inventory II.
Bold values are indicating significance at P < 0.05
`
Table 1: Demographic and clinical characteristics of the study population as well as the raw scores (mean, SD) and comparisons of variables in relapsing and progressive groups.
Cognitive performance profile in the relapsing and the progressive group
Table 2 summarizes the comparisons within and between relapsing and progressive groups over time in the PASAT-3 (raw and dyad scores), the PASAT-2 (raw and dyad scores), the SDMT, and the COWAT. Interaction effect between time and group was not significant in the PASAT or in the SDMT, indicating that the relapsing and progressive groups did not differ. Neither did fatigue scores differ between the groups in the PASAT and in the SDMT. A significant time effect was observed in both groups indicating a decline in performance during all four tests. The responding profile across the PASAT-3’s 60 items in the relapsing and the progressive group is shown in Figure 1. In the COWAT, time and group interaction effect was significant and fatigue scores differed between the relapsing and the progressive group. The performance of the relapsing group declined more than the performance of the progressive group.
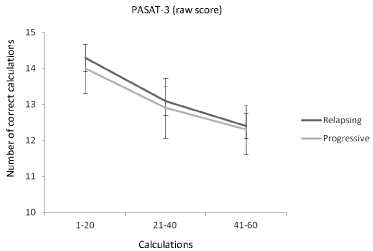
Figure 1: The mean number of items correct (SEM) during the three phases
(calculations 1-20, 21-40, 41-60) of the PASAT-3 in the relapsing and the
progressive group. Number of correct responses declined from the beginning
to the end of the test in both groups.
After adjustment by age
Phase
Time x group interaction
Time
Group
Time x group interaction
Time
Group
1
2
3
Mean
SD
Mean
SD
Mean
SD
PASAT-3
Relapsing
14.3
4.4
13.1
4.6
12.4
4.1
Progressive
14
4.9
12.9
5.3
12.3
4.3
0.815
<0.001
0.762
0.986
0.01
0.814
PASAT-3-dyad
Relapsing
10.3
5.8
9.3
6
8.1
5.5
0.531
<0.001
0.937
0.772
0.052
0.666
Progressive
9.9
6.7
9.3
6.9
8.4
6.6
PASAT-2
Relapsing
10.8
3.9
8.9
3.4
8.5
3.4
Progressive
10.7
4.2
8.5
4.1
8.7
3.9
0.512
<0.001
0.859
0.597
0.001
0.898
PASAT-2-dyad
Relapsing
5.9
4.6
3.9
3.7
3.7
3.7
Progressive
5.5
5.1
3.6
4.6
3.5
4.6
0.909
<0.001
0.637
0.924
0.005
0.985
SDMT
Relapsing
16.2
3
14.4
3.6
14.5
3.9
Progressive
14
3.9
11.9
3.4
12.1
4
0.665
<0.001
<0.001
0.833
0.319
0.003
COWAT
Relapsing
12.6
3.5
6.2
3.2
4.5
2.8
Progressive
11.7
3.4
5.4
3.2
5
3.6
0.046
<0.001
0.312
0.057
<0.001
0.611
P (difference between the groups)
P (after adjustment by age)
Fatigue scores
PASAT-3
Relapsing
-2.1
3.8
Progressive
-1.7
3.2
0.431
0.69
PASAT-2
Relapsing
-2
3.5
Progressive
-2.2
3.5
0.924
0.567
SDMT
Relapsing
-1.7
2.3
Progressive
-1.9
2.9
0.574
0.72
COWAT
Relapsing
-8
3.8
Progressive
-6.7
4.8
0.046
0.063
PASAT-3: Paced Auditory Serial Addition Test (three-second interstimulus version); PASAT-2: Paced Auditory Serial Addition Test (two-second interstimulus version); SDMT: Symbol Digit Modalities Test; COWAT: Controlled Oral Word Association Test.
Bold values are indicating significance at P < 0.05
Table 2: Firstly, the raw scores (mean, SD) in three phases in the PASAT, SDMT, and COWAT in the relapsing (n = 136) and the progressive (n = 51) group, comparisons of changes in raw scores within the two groups, as well as comparisons of the changes between the two groups. Secondly, the fatigue scores in the tests.
The effects of depressive symptoms on performance profile
The same analyses were repeated between depressed (n = 72) and non-depressed (n = 112) patients. The groups did not differ in terms of demographic factors (age, education years, and gender). Interaction effects between time and group were not significant, indicating that the depressed and non-depressed patients did not differ. Neither did fatigue scores differ between the groups in any of the cognitive tests. A significant time effect was observed in depressed and nondepressed patients indicating a decline in performance during the PASAT-3 (raw and dyad score), the PASAT-2 (raw and dyad score), the SDMT, and the COWAT.
The effects of cognitive status on performance profile
The same analyses were repeated between cognitively intact (n = 90) and impaired (n = 97) patients (Table 3). The groups differed in age (cognitively intact mean 44.1 SD (8.8) vs. cognitively impaired mean 47.0 SD (9.1), p = 0.029), education years (cognitively intact 14.4 (2.4) vs. cognitively impaired 12.8 (2.5), p < 0.001), and gender (female/male: cognitively intact 71/19 vs. cognitively impaired 64/33, p = 0.049). A significant time and group interaction effect was observed in the PASAT-3 (raw and dyad score), the PASAT-2 (raw and dyad score), and the SDMT indicating that the cognitively intact and impaired patients differed in their performance profile in these tests. During the PASAT-3 (raw and dyad score) and the PASAT-2 (raw and dyad score) the performance of cognitively intact patients declined more than that of cognitively impaired patients (Figure 2). During the SDMT, instead, the performance of cognitively impaired patients declined more than that of cognitively intact patients (Figure 3). Fatigue scores were significant for the PASAT-2 and the SDMT. Time effects were significant for all four tests. Results did not significantly change after adjustment by age, or after adjustment by age, education years, and gender.
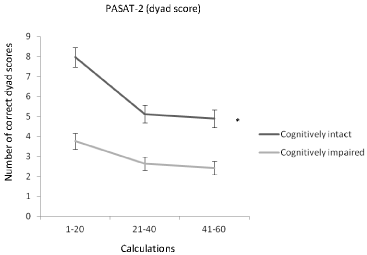
Figure 2: The mean number of dyad scores correct (SEM) during the three
phases (calculations 1-20, 21-40, 41-60) of the PASAT-2 in cognitively intact
and impaired patients. Number of correct dyad-scores declined from the
beginning to the end of the test more in cognitively intact than in cognitively
impaired patients.
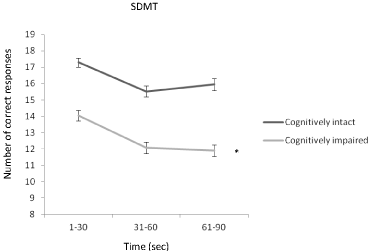
Figure 3: The mean number of responses correct (SEM) during the three
phases (0-30, 31-60, 61-90 sec) of the SDMT in cognitively intact and
impaired patients. Number of correct responses declined from the beginning
to the end of the test more in cognitively impaired than in cognitively intact
patients.
After adjustment by age
Phase
Time x group interaction
Time
Group
Time x group interaction
Time
Group
1
2
3
Mean
SD
Mean
SD
Mean
SD
PASAT-3
Cognitively intact
16.5
2.9
15.4
3.1
14.3
3.4
Cognitively impaired
12.1
4.7
10.8
4.9
10.6
4.3
0.039
<0.001
<0.001
0.049
0.007
<0.001
PASAT-3-dyad
Cognitively intact
13.2
4.7
12.4
4.8
10.5
5.1
Cognitively impaired
7.4
5.9
6.4
6
6
5.5
0.008
<0.001
<0.001
0.013
0.037
<0.001
PASAT-2
Cognitively intact
12.7
3.5
10.2
3.2
9.8
3.4
Cognitively impaired
9
3.5
7.5
3.4
7.4
3.3
0.004
<0.001
<0.001
0.008
0.001
<0.001
PASAT-2-dyad
Cognitively intact
8
4.6
5.1
4.2
4.9
4.2
Cognitively impaired
3.8
3.9
2.6
3.4
2.4
3.3
<0.001
<0.001
<0.001
<0.001
0.014
<0.001
SDMT
Cognitively intact
17.3
2.7
15.5
3.1
15.9
3.4
Cognitively impaired
14
3.2
12.1
3.4
11.9
3.6
0.049
<0.001
<0.001
0.055
0.246
<0.001
COWAT
Cognitively intact
13.5
3.3
6.7
3.4
5.2
3.4
Cognitively impaired
11.3
3.2
5.4
2.9
4.1
2.6
0.089
<0.001
<0.001
0.111
<0.001
<0.001
P (difference between the groups)
P (after adjustment by age)
Fatigue scores
PASAT-3
Cognitively intact
-2.4
3.5
Cognitively impaired
-1.6
3.7
0.118
0.169
PASAT-2
Cognitively intact
-2.7
3.6
Cognitively impaired
-1.5
3.3
0.018
0.033
SDMT
Cognitively intact
-1.3
2.3
Cognitively impaired
-2.1
2.6
0.03
0.037
COWAT
Cognitively intact
-8.3
4.8
Cognitively impaired
-7.1
3.4
0.063
0.074
PASAT-3: Paced Auditory Serial Addition Test (three-second interstimulus version); PASAT-2: Paced Auditory Serial Addition Test (two-second interstimulus version); SDMT: Symbol Digit Modalities Test; COWAT: Controlled Oral Word Association Test.
Bold values are indicating significance at P < 0.05
Table 3: Firstly, the raw scores (mean, SD) in three phases in the PASAT, SDMT, and COWAT in the cognitively intact (n = 90) and impaired (n = 97) groups, comparisons of changes in raw scores within the two groups, as well as comparisons of the changes between the two groups. Secondly, the fatigue scores in the tests.
Discussion
The present study evaluates the performance profile during the time-limited tests of the BRBNT. A decline during the PASAT, the SDMT, and the COWAT was observed among MS patients independently of the clinical phenotype of the disease. Cognitive status of the patients affected the performance profiles. Instead, mood did not.
The performance declined from the beginning to the end during the PASAT, the SDMT, and the COWAT in the relapsing and the progressive group. The present sample did not include healthy controls. Therefore, MS-specific factors could not be separated from the performance profile observed in a neurologically healthy population. Especially in the COWAT, the declining trend in performance profile can be observed also in the healthy population [24]. In the PASAT [18,19] and the SDMT [20], patients with MS have been found to decline more during the task than healthy controls. Relapsing and progressive groups did not significantly differ in their performance profile during the PASAT and the SDMT in the present study. Neither time and group interaction effects nor fatigue scores were significant. The performance of the relapsing group declined more than that of the progressive group in the COWAT. Our findings are in line with previous ones, in which response time variability has not been found to differ between relapsing and progressive patients [23]. In the present sample, the progressive group had longer disease duration and more severe disease than the relapsing group. Thus, these clinical characteristics did not affect the observed declining performance profile. Previously Bailey et al. [33] found only limited evidence of change in the n-back task performed in the beginning and in the end of a one-hour session in patients with advanced MS (EDSS mean 7.7 SD (0.4)). They speculated that cognitive fatigue may play a minor role in relation to other symptom characteristics in the advanced disease stage. The differences in the used methods probably, at least partly, explain the contradictory findings between the study of Bailey et al. [33] and the present study.
Patient-reported depressive symptoms did not essentially affect the performance profile observed in the PASAT, the SDMT, and the COWAT. Significant relationships between depressive symptoms and subjective fatigue have been observed in MS [16,34-36]. No decline in PASAT performance has been observed either in repeated evaluations [37] or during the administration [19] in moderately to mildly depressed patients with MS. In the present sample, subjective depressive symptoms were mild. The present preliminary findings together with the previous ones suggest that mild to moderate depressive symptoms do not significantly affect the performance profile during a cognitive test in MS. Further studies are needed to verify these findings and to evaluate the effects of more severe depressive symptoms on the profile.
Cognitive status of the patients affected the performance profile observed in the PASAT and in the SDMT, but not in the COWAT. The results in the PASAT and the SDMT were, however, not consistent with each other. Cognitively intact patients declined more than cognitively impaired patients during the PASAT (PASAT-3 and -2, raw and dyad scores). Time and group interaction effects were significant for all PASAT variables and fatigue scores for the PASAT-2. Instead, cognitively impaired patients declined more than cognitively intact patients during the SDMT. Both time and group interaction effects and fatigue scores were significant. The contradictory findings may at least partly relate to the differences in the test characteristics. Especially the PASAT is a highly multifactorial test requiring sustained and divided attention, information processing speed, and preserved working memory [24]. The SDMT is mainly a measure of processing speed, complex scanning, and visual tracking [24]. It could be speculated that cognitively intact patients with less progression of the disease can maintain their better performance level during a simpler task, but no longer during a test with high working memory demands. These speculations are, however, preliminary and need further verification.
The percent dyad score/dyad score method in the PASAT has been found to be more sensitive to detect performance deterioration in MS [25,26], and to better discriminate between relapsing and progressive patients [27] than the standard scoring method. In the present study, the decline in the performance profiles of relapsing and progressive groups was observed in the PASAT-3 and the PASAT-2 using both standard and dyad score methods. Neither the standard nor the dyad score method discriminated the performance profile between the relapsing and the progressive group. However, the dyad score method discriminated between the cognitively intact and impaired patients slightly better than the standard score method when inspecting the significance levels. Therefore, in line with previous findings, our findings suggest that the dyad score method may be slightly more sensitive to show performance deterioration during the test than recording the correct responses.
Cognitive fatigue, either self-reported or objectively observed as a decline in sustained cognitive activity, has been considered as a feature of neuropsychological symptom sequelae in MS [17]. Assessment methods to verify decline during cognitive tests should be developed. Systematic recording of performance during cognitive tests offers a possibility to evaluate performance profile and observe signs of cognitive fatigue. In the lack of healthy controls, further studies are needed to determine the level of decrement possibly referring to cognitive fatigue. In the present study, the performance profile of MS patients during the PASAT, the SDMT, and the COWAT was evaluated. The declining performance profile was observed in all tests independently of the clinical phenotype or depressive symptoms of the patients.
The limitations of the present study should be considered. The main limitation is the fact that the study sample did not include healthy controls and it should be taken into account when interpreting the results of the present study. In the absence of a group of healthy controls, it is impossible to evaluate whether the observed phenomenon is MS specific. Further studies with healthy controls are needed to verify our preliminary findings. The present study excluded patients with dementia. Additionally, the results of the present study concern only patients with subjective cognitive complaints. Therefore, we are unable to generalize our findings to patients with severe cognitive decline or without subjective cognitive complaints. The study did not examine the performance profile in the memory and learning tests of the BRBNT (BSRT, 10/36), because they are not time-limited in the same way as the other three tests and the course of performance in these tests is especially prone to learning effects. Sample sizes in the relapsing and progressive groups were different, the relapsing group being larger than the progressive one. We classified our patients into depressed and non-depressed and cognitively impaired and intact by using cut-off points and criteria described previously [6,31,32]. However, as every classification is artificial and imperfect, the findings should be considered preliminary and should be interpreted with caution.
Conclusion
A decline in the performance profile suggestive as possible sign of objective cognitive fatigue was demonstrated in the PASAT, the SDMT, and the COWAT in relapsing and progressive MS patients. The examination of the performance profile across the items in the tests of the BRBNT is recommended in addition to the use of standard scoring method. The procedure offers a possibility to evaluate performance stability, and observe whether test performances deteriorate towards the end of the tests.
Acknowledgement
We thank all our patients for their time and interest in this study. The study was funded by the MS Association of Finland, which is gratefully acknowledged.
References
- Rice CM, Cottrell D, Wilkins A, Scolding NJ. Primary progressive multiple sclerosis: progress and challenges. J Neurol Neurosurg Psychiatry. 2013; 84: 1100-1106.
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008; 7: 1139-1151.
- Langdon DW. Cognition in multiple sclerosis. Curr Opin Neurol. 2011; 24: 244-249.
- Rogers JM, Panegyres PK. Cognitive impairment in multiple sclerosis: evidence-based analysis and recommendations. J Clin Neurosci. 2007; 14: 919-927.
- Huijbregts SCJ, Kalkers NF, de Sonneville LMJ, de Groot V, Reuling IEW, Polman CH. Differences in cognitive impairment of relapsing remitting, secondary, and primary progressive MS. Neurology. 2004; 63: 335-339.
- Potagas C, Giogkaraki E, Koutsis G, Mandellos D, Tsirempolou E, Sfagos C, et al. Cognitive impairment in different MS subtypes and clinically isolated syndromes. J Neurol Sci. 2008; 267: 100-106.
- Sepulcre J, Vanotti S, Hernández R, Sandoval G, Cáceres F, Garcea O, et al. Cognitive impairment in patients with multiple sclerosis using the Brief Repeatable Battery-Neuropsychology test. Mult Scler. 2006; 12: 187-195.
- Rodrigues D, Paes RA, Vasconcelos CC, Landeira-Fernandez J, Alvarenga RM. Different cognitive profiles of Brazilian patients with relapsing-remitting and primary progressive multiple sclerosis. Arq Neuropsiquiatr. 2011; 69: 590-595.
- Lynch SG, Parmenter BA, Denney DR. The association between cognitive impairment and physical disability in multiple sclerosis. Mult Scler. 2005; 11: 469-476.
- Gaudino EA, Chiaravalloti ND, DeLuca J, Diamond BJ. A comparison of memory performance in relapsing-remitting, primary progressive and secondary progressive multiple sclerosis. Neuropsych Neuropsychol Behav Neurol. 2001; 14: 32-44.
- Ruet A, Deloire M, Charré-Morin J, Hamel D, Brochet B. Cognitive impairment differs between primary progressive and relapsing-remitting MS. Neurology. 2013; 80: 1501-1508.
- Solari A, Mancuso L, Motta A, Mendozzi L, Serrati C. Comparison of two brief neuropsychological batteries in people with multiple sclerosis. Mult Scler. 2002; 8: 169-176.
- Rao SM. A manual for the brief repeatable battery of neuropsychological tests in multiple sclerosis. National Multiple Sclerosis Society. 1990.
- Induruwa I, Constantinescu CS, Gran B. Fatigue in multiple sclerosis - a brief review. J Neurol Sci. 2012; 323: 9-15.
- Mills RJ, Young CA. The relationship between fatigue and other clinical features of multiple sclerosis. Mult Scler. 2011; 17: 604-612.
- Pittion-Vouyovitch S, Debouverie M, Guillemin F, Vandenberghe N, Anxionnat R, Vespignani H. Fatigue in multiple sclerosis is related to disability, depression and quality of life. J Neurol Sci. 2006; 243: 39-45.
- DeLuca J. Fatigue, cognition and mental effort. In: DeLuca J, editor. Fatigue as a window to the brain. Massachusetts Institute of Technology. 2005: 37-58.
- Rosti E, Hämäläinen P, Koivisto K, Hokkanen L. The PASAT performance among patients with multiple sclerosis: analyses of responding patterns using different scoring methods. Mult Scler. 2006; 12: 586-593.
- Schwid SR, Tyler CM, Scheid EA, Weinstein A, Goodman AD, McDermott MP. Cognitive fatigue during a test requiring sustained attention: a pilot study. Mult Scler. 2003; 9: 503-508.
- Holtzer R, Foley F, D'Orio V, Spat J, Shuman M, Wang C. Learning and cognitive fatigue trajectories in multiple sclerosis defined using a burst measurement design. Mult Scler. 2013; 19: 1518-1525.
- Kujala P, Portin R, Revonsuo A, Ruutiainen J. Attention related performance in two cognitively different subgroups of patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 1995; 59: 77-82.
- Krupp LB, Elkins LE. Fatigue and declines in cognitive functioning in multiple sclerosis. Neurology. 2000; 55: 934-939.
- Bruce JM, Bruce AS, Arnett PA. Response variability is associated with self-reported cognitive fatigue in multiple sclerosis. Neuropsychology. 2010; 24: 77-83.
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological assessment. Oxford University Press. 2004.
- Walker LA, Berard JA, Berrigan LI, Rees LM, Freedman MS. Detecting cognitive fatigue in multiple sclerosis: method matters. J Neurol Sci. 2012; 316: 86-92.
- Bryant D, Chiaravalloti N, DeLuca J. Objective measurement of cognitive fatigue in multiple sclerosis. Rehabil Psychol. 2004; 49: 114-122.
- Snyder P, Cappelleri JC, Archibald CJ, Fisk JD. Improved detection of different information-processing speed deficits between two disease-course types of multiple sclerosis. Neuropsychology. 2001; 15: 617-625.
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983; 13: 227-231.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983; 33: 1444-1452.
- Huolman S, Hämäläinen P, Vorobyev V, Ruutiainen J, Parkkola R, Laine T, et al. The effects of rivastigmine on processing speed and brain activation in patients with multiple sclerosis and subjective cognitive fatigue. Mult Scler. 2011; 17: 1351-1361.
- Basso MR, Beason-Hazen S, Lynn J, Rammohan K, Bornstein RA. Screening for cognitive dysfunction in multiple sclerosis. Arch Neurol. 1996; 53: 980-984.
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961; 4: 561-571.
- Bailey A, Channon S, Beaumont JG. The relationship between subjective fatigue and cognitive fatigue in advanced multiple sclerosis. Mult Scler. 2007; 13: 73-80.
- Penner IK, Bechtel N, Raselli C, Stöcklin M, Opwis K, Kappos L, et al. Fatigue in multiple sclerosis: relation to depression, physical impairment, personality and action control. Mult Scler. 2007; 13: 1161-1167.
- Wood B, van der Mei IA, Ponsonby AL, Pittas F, Quinn S, Dwyer T, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler. 2013; 19: 217-224.
- Koch M, Mostert J, Heerings M, Uyttenboogaart M, De Keyser J. Fatigue, depression and disability accumulation in multiple sclerosis: a cross-sectional study. Eur J Neurol. 2009; 16: 348-352.
- Johnson SK, Lange G, DeLuca J, Korn LR, Natelson B. The effects of fatigue on neuropsychological performance in patients with chronic fatigue syndrome, multiple sclerosis, and depression. Appl Neuropsychol. 1997; 4: 145-153.