
Research Article
Austin J Musculoskelet Disord. 2014;1(1): 1006.
LCADL Dyspnea Scale and Physical Activity in COPD Patients
Carpes MF1, Castro AAM1, Simon KM2, Porto EF3, Fleig Mayer A4
1Physical Therapy Department at the Federal University of Pampa – UNIPAMPA – Rio Grande do Sul, Brazil
2Physical Therapy
3Physical Therapy Department at the Adventist University – UNASP – São Paulo, Brazil
4Physical Therapy Department at the University of the State of Santa Catarina – UDESC – Santa Catarina, Brazil
*Corresponding author: Marta Fioravanti Carpes, Federal University of Pampa, BR 472 - Km 592, PO Box 118, Uruguaiana, Rio Grande do Sul, Brazil
Received: August 03, 2014; Accepted: August 22, 2014; Published: August 25, 2014
Abstract
Introduction: Chronic obstructive pulmonary disease (COPD) patients commonly present dyspnea as a limiting factor to activities of daily living (ADL) accomplishment. However, it might be possible that this limitation may be decreased with regular aerobic physical activity. The London Chest Activity Daily Living (LCADL) scale assesses COPD patient’s dyspnea during ADL accomplishment.
Objectives: Analyze if there is an association between the LCADL dyspnea scale and aerobic physical activity in COPD patients.
Methods: The overall LCADL score was calculated summing the five domain scores, Body Mass Index (BDI) and airflow limitation (FEV1). Patients were grouped according to their current physical activity profile and were classified as Physically Active (PA) and Physically Inactive (PI).
Results: Out of 38 patients, 15 (39.47%) were considered physically active (PA) and 23 (60.53%) physically inactive (PI). PA patients presented mean values for the BMI of 24.5 ± 4.2, for FEV1 of 40.7 ± 11.7, for LCADL total score of 21.0 ± 13.6 and LACDL predicted value (%) of 35.5 ± 16.7. PI patients presented mean values for BMI of 23.5 ± 4.4, for FEV1 of 33.7 ± 11.4, for LCADL total score of 32.8 ± 15.1 and LACDL predicted value (%) of 53.14 ± 19.5. LCADL score was worse in the PI than in the PA patients (p=0.015).
Conclusion: Physical inactivity contributes to higher scores in the LCADL scale which is associated to higher scores of dyspnea perception during activities of daily living accomplishment.
Keywords: COPD; Physical activity; Dyspnea; Activities of daily living
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a preventable and treatable respiratory impairment characterized by non-reversible airflow obstruction. Airflow obstruction is progressive and it is associated with an abnormal inflammatory response in the lungs due to toxic gases and particles, especially caused by tobacco use [1].
Chronic pulmonary obstruction leads to pulmonary mechanics changes [2] that contribute to dynamic hyperinflation and dyspnea in COPD patients [3-10]. Specially, this mechanism can be seen during high-intensity exercise tests or even during simple activities of daily living [8-24]. Therefore, patients with increasing dyspnea [18] present lower levels of physical activity performed [24].
The London Chest Activity Daily Living (LCADL) scale is frequently used to analyze dyspnea limitation during exercises and Activities of Daily Living (ADL) accomplishment in COPD patients [25]. Comprehensive exercise training has already been proven to diminish referred dyspnea during common ADL COPD patients accomplishes [5]. Therefore, regular physical activity promotes pulmonary and systemic benefits for COPD patients [18,26,27], such as the increase of muscular vessels, oxidative enzymes and mitochondrias [3-19,28,29]. Those changes contribute to increased body composition, peripheral muscle strength, exercise capacity and decreased dyspnea in COPD patients [11].
Nevertheless, it is not fully understood if the aerobic physical activity may influence on COPD patients perceived dyspnea. Therefore, we aimed to analyze the perceived dyspnea within physically active and inactive COPD patients.
Materials and Methods
This was a retrospective study with 166 patients comprised from the Clínica Médica Espaço Vital database. The study was analyzed and approved by the Ethics Committee of the Vale do Itajaí University (n° 393/09). Physical activity practices, age, smoking history, Body Mass Index (BMI), forced expiratory volume in the first second (FEV1) and LCADL score were the measured variables.
The inclusion criteria were COPD diagnosis (FEV1/FVC<0.7 and FEV1<80% predicted), smoking history over 20 packs/year and clinical stability within the past month prior to the initial assessment.
Exclusion criteria were inability to perform any study’s assessments, disease exacerbation during the protocol, having any associated disease that might have limited physical practice, being part of any supervised muscular training program and not have signed a written consent.
The final sample consisted of 38 COPD patients that were eligible according to our study inclusion/exclusion criteria.
Protocol
At first patients were taught they were being submitted to the protocol and were asked to sign an informed consent. Afterwards the Body Mass Index (BMI), physical activity, spirometry and dyspnea were measured.
Body mass index (BMI)
Height and weight were measured on a weighting scale (Filizola®, São Paulo, Brazil). BMI was calculated using the formula: weight/ height [2,30].
Physical activity
Patients were asked if they practiced any physical activity. Patients were considered Physically Active (PA) when they practiced moderate or intense level of physical activity throughout a 30 minutes period every day or for three days per week, respectively. Moderate or intense level of PA was defined according to the amount of load bared by patients during exercise; this amount corresponded to over 60% of the maximum load achieved in a Cardiopulmonary Exercise Test (CPET) previously performed by a trained physical therapist or athletic trainer [10]. Patients who were part of a pulmonary rehabilitation program or any other regular physical activity program were excluded since this is a health care supervised type of exercise.
Spirometry
Spirometry was carried out according to the American Thoracic Society (ATS) guidelines. Bronchodilator challenge was made after fifteen minutes after patients inhaled 400 mcg of albuterol [31,32].
Activities of daily living (ADL) and dyspnea
Dyspnea and ADL limitation was measured by means of the LCADL. This scale in composed of 15 questions within four domains: personal care, domestic activities, physical activity and leisure. Every item of the domains is to score from zero to five. The highest the value the higher is the inability to accomplish any ADL due to excessive dyspnea. LCADL total score may range from 0 to 75 points [5,25].
Statistical analysis
The Kolmogorov-Smirnov test was used to identify sample normality and its distribution was found to be parametric. Therefore data for age, pack/years, BMI, FEV1 and LCADL are described as mean ± standard deviation and percentage value of the LCADL. Differences of these variables between groups PA and PI were obtained by using the non-paired Student`s t test. In order to test the proportional variation between physical activity and LCADL scores the Qui-squared test was used. A p≤0.05 was considered as statistical significant.
Results
Out of 38 recruited patients, 24 (63%) were male and 14 (37%) were female. Overall baseline characteristics of patients are displayed on Table 1.
Variables
Mean± standard deviation
Age (years)
65.76 ± 07.81
Pack/years
48.28 ± 23.29
BMI (Kg/m2)
23.85 ± 04.27
FEV1 % pred. post bd
36.47 ± 11.86
LCADL (total)
28.13 ± 15.47
LCADL (%)
46.18 ± 20.21
Table 1: Baseline characteristics of 38 COPD patients.
According to the American College of Sports Medicine (ACSM) classification, 15 (39.47%) patients were considered physically active (PA) and 23 (60.53%) were considered Physically Inactive (PI). Patients characteristics divided according to physical fitness are shown in Table 2.
Variables
Physically active
Physically inactive
p value
(n=15)
(n=23)
Age (years)
67.0 ± 06.0
65.0 ± 09.0
0.4
Pack/years
49.3 ± 26.4
47.7 ± 21.7
0.8
BMI (Kg/m2)
24.5 ± 04.2
23.5 ± 04.4
0.5
FEV1 % pred. post bd
40.7 ± 11.7
33.7 ± 11.4
0.07
LCADL (total)
21.0 ± 13.6
32.8 ± 15.1
0.01
LCADL (%)
35.5 ± 16.7
53.1 ± 19.5
0.006
Table 2: Baseline characteristics of physically active (PA, n=15) and physically inactive (PI, n=23) COPD patients.
LCADL score was 32 and 51.1 points for the physically active and for the physically inactive groups, respectively. Therefore, the physically inactive group presented higher LCADL scores than the physically active group (p=0.015) (Table 3). The LCADL (%) confidence interval was 27.0 to 43.9 and 45.2 to 61.1 for the physically active and inactive COPD patients, respectively (Figure 1).
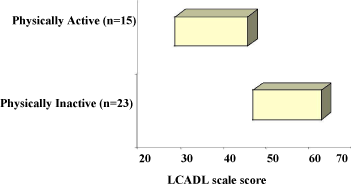
Figure 1: London Chest Activity of Daily Living (LCADL) confidence interval scores in physically active (n=15) and inactive (n=23) COPD patients.
LCADL lower 50%
LCADL above 50%
Total (n)
p value
Physically active (n)
13
2
15
-
Physically inactive (n)
11
12
23
-
TOTAL (n)
24
14
38
0.015
Table 3: Comparison of London Chest Activity of Daily Living (LCADL) scores and physical fitness within 38 COPD patients.
Discussion
This study main goal was to verify an association between the LCADL score and aerobic physical activity in COPD patients. Moderate and severe COPD patients were asked if they practiced any physical activity and, according to Pattè et al. [16], they were classified into physically active if they accomplished 30 daily minutes of mild or moderate intensity whole body physical activity such as cycling or walking.
Most of our sample was classified as physically inactive (60.53%). Therefore, this data supports the idea that COPD patients remain most of their daily time lying or sitting still as oppose to walking [17].
According to Ainsworth’s et al. [33] classification of physical activity, our physically active patients accomplished enough labor and leisure activities within three out of four domains for physical fitness. The domains physically active patients presented were: in commuting, domestic activities and leisure.
Pitta et al. [17] reported that physical inactivity in COPD patients leads to a worsening of the dyspnea perception. The authors found higher scores in the MRC dyspnea scale for physically inactive patients that those who performed any kind of moderate physical activity for at least three times a week. Nevertheless, we choose to assess our patient’s dyspnea by means of the LCADL scale since it brings a higher specificity for activity of daily living accomplishment. Also, it is a reliable and valid tool to assess ADL limitation [25] and presents adequate responsiveness to therapeutic approaches such as pulmonary rehabilitation programs [5].
The reason we calculated the LCADL percentage value was to diminish the lower limit scoring in the domestic activity domain since our sample was mostly composed by male patients. Overall and up to this date, most of the domestic activities are still being accomplished by women on a daily basis. Therefore the LCADL total score did not consider questions which the score was equal to zero. Only the question with a score equal or higher than one was considered to calculate the LCADL total score percentage value [25].
Our study showed that the mean LCADL percentage values were higher in the PI (53.14%) than the PA 35.5% group, showing a deterioration in ADL accomplishment in the PI group.
In the same manner, Miravitlles et al. [34] showed a strong direct relationship between daily walking time and functional status (assessed by the LCADL; p=0.001). They found that patients who walked less than 30 minutes per day presented a LCADL score of 31 % as compared to 20 % from those who walked over 60 minutes per day. They concluded that poor health status is one of the main factors associated with a low walking time in COPD patients.
Simon et al. [20] studied the LCADL associated to the BODE index and found that patients presented a LCADL score lower than 50% (threshold line) is associated to a severe ADL limitation. Our sample showed a difference between PA and PI confidence intervals. The upper limit of the PA group and the lower limit of the PI group divide the sample in distinct LCADL percentage values. Therefore, it is possible that physical activity may interrupt the vicious cycle of dyspnea and ADL limitation. Indeed, several studies have shown that disease severity is directly associated to ADL decrease making patients with severe and very severe COPD perform their daily activities in fewer and shorter bouts than those in mild and moderate stages [35-38].
Simon et al. [20] showed a higher MRC dyspnea perception for a physically inactive compared to physically active patients. The inactive patients presented severe dyspnea perception in different intensity-level activities [7]. Velloso et al. [23] also showed the dyspnea as one of the main factors that limits ADL accomplishment. However, other studies reports that ADL limitation may occur due to other factors than dyspnea, such as: airway obstruction [18-22], dynamic hyperinflation [10] and peripheral muscle weakness [27].
Simon et al. [20] showed a correlation between lower FEV1 and higher LCADL scores. In our study, the severe airway obstruction shown in our patients may have contributed to exercise limitation. The severe patients in the PI group presented a 7% mean difference for FEV1 as compared to the PA group. This knowledge supports the hypothesis that the higher the airway obstruction the higher the dyspnea scores perceived; a limiting factor to exercise accomplishment, higher dyspnea and LCADL scores in these patients [23]. Watz et al. [24] showed a correlation between FEV1 and physical activity level. They stated that the lower the FEV1, the lower was the physical activity accomplished by these patients. Other studies have shown an association of physical activity with health related quality of life [39,40], disease severity [41-43], mortality [44,45] and dynamic hyperinflation [46].
Our study showed that the physical activities associated to a lower dyspnea-ADL limitation [33]. Rodrigues et al. [18] showed that COPD patients increased the overall functional physical capacity and upper limbs incremental and endurance capacity and decreased dyspnea during daily activities after a pulmonary rehabilitation program.
It is already known that comprehensive physical training can decrease dyspnea in COPD patients [14,47]. Nevertheless, as we know that COPD patients are ADL limited due to disease severity and dyspnea, we must educate our patients to practice an oriented-type of exercise in order to decrease dyspnea-related ADL limitation.
Our study presents two limitations: (1) the small sample size within the PI and PA groups, however, our sample size presented enough power to detect our primary outcome; (2) we did not perform the CPET to determine the amount of PA (moderate or intense) and only collected the data from another centre.
Conclusion
We conclude that the higher scores in the LCADL scale in physically inactive COPD patients is related to higher dyspnea perception which leads to a lower ability to perform activities of daily living.
References
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2013.
- Takubo Y, Guerassimov A, Ghezzo H, Triantafillopoulos A, Bates JH, Hoidal JR, et al. Alpha1-antitrypsin determines the pattern of emphysema and function in tobacco smoke-exposed mice: parallels with human disease. Am J Respir Crit Care Med. 2002; 166: 1596-1603.
- Eid AA, Ionescu AA, Nixon LS, Lewis-Jenkins V, Matthews SB, Griffiths TL, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 164: 1414-1418.
- Garrod R, Paul EA, Wedzicha JA. Supplemental oxygen during pulmonary rehabilitation in patients with COPD with exercise hypoxaemia. Thorax. 2000; 55: 539-543.
- Garrod R, Paul EA, Wedzicha JA. An evaluation of the reliability and sensitivity of the London Chest Activity of Daily Living Scale (LCADL). Respir Med. 2002; 96: 725-730.
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996; 153: 976-980.
- Hajiro T, Nishimura K, Tsukino M, Ikeda A, Oga T, Izumi T. A comparison of the level of dyspnea vs disease severity in indicating the health-related quality of life of patients with COPD. Chest. 1999; 116: 1632-1637.
- Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999; 160: 1856-1861.
- Man WD, Soliman MG, Gearing J, Radford SG, Rafferty GF, Gray BJ, et al. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003; 168: 562-567.
- Marin J, Carrizo SJ, Gascon M, Sanchez A, Gallego B, Celli BR. Inspiratory capacity, dynamic hyperinflation, breathlessness, and Exercise performance during the six minute walk test in Chronic Obstructive Pulmonary disease. Am J Respir Crit Care Med. 2001; 163: 1395-1399.
- Marquis K, Debigaré R, Lacasse Y, Léblanc P, Jobin J, Carrier G, et al. Midthigh muscle cross sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002; 166: 809-13.
- Matsudo VKR, Matsudo SMM, Araujo TL, Ribeiro MA. Dislipidemias e a promoção da atividade física: uma revisão na perspectiva de mensagens de inclusão. R. bras. Ci e Mov. 2005; 13: 161-170.
- McArdle W, Katch F, Katch V. Fisiologia do exercício: Energia, Nutrição e Desempenho humano. 4th edn. Guanabara Koogan, 1998.
- Neder JA, Nery LE. Fisiologia Clínica do Exercício: Teoria e prática. Artes Médicas. 1st edn. 2003.
- Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med. 2003; 167: 544-549.
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995; 273: 402-407.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005; 171: 972-977.
- Rodrigues SL, Viegas CAA, Lima T. Efetividade da reabilitação pulmonar como tratamento coadjuvante da doença pulmonar obstrutiva crônica. Am. J. Pneumol. 2002; 28: 65 – 70.
- Ramirez-Sarmiento A, Orozco-Levi M, Guell R, Barreiro E, Hernandez N, Mota S, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med. 2002; 166: 1491-1497.
- Simon KM, Hass AP, Carpes MF, Zimmerman JL. Índice prognóstico de mortalidade BODE e atividade física em doentes pulmonares obstrutivos crônicos. Rev Bras Med Esporte. 2009; 15: 16-20.
- Schönhofer B, Ardes P, Geibel M, Köhler D, Jones PW. Evaluation of a movement detector to measure daily activity in patients with chronic lung disease. Eur Respir J. 1997; 10: 2814-2819.
- Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005; 172: 19-38.
- Velloso M, Stella SG, Cendon S, Silva AC, Jardim JR. Metabolic and ventilatory parameters of four activities of daily living accomplished with arms in COPD patients. Chest. 2003; 123: 1047-1053.
- Watz H, Waschki B, Boehme C, Claussen M, Meyer T, Magnussen H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: a cross-sectional study. Am J Respir Crit Care Med. 2008; 177: 743-751.
- Carpes MF, Simon KM, Garrod R, Jardim J, Mayer A. Versão brasileira da escala London Chest activity daily living para uso em pacientes com doença pulmonar obstrutiva crônica. J. Bras. de Pneumol. 2008; 34.
- Carpes MF, Guerra J, Pereira KJ. Influence of peripheral muscle training on the BODE index of mortality in individuals with chronic obstructive pulmonary disease. Rev. Bras. Fisioter. 2008; 1 –54.
- Casaburi R, Porszasz J, Burns MR, Carithers ER, Chang RS, Cooper CB. Physiologic benefits of exercise training in rehabilitation of patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997; 155: 1541-1551.
- Casaburi R. Skeletal muscle function in COPD. Chest. 2000; 117: 267S-71S.
- Donaldson G, Seemungal T, Patel I, Browmik A, Wilkinson TMA, Hurst JR, et al. Airway and systemic inflammation and decline in lung Function in patients with COPD. Chest. 2005; 128: 1995-2004.
- Lerario MC, Sachs A, Lazaretti-Castro M, Saraiva LG, Jardim JR. Body composition in patients with chronic obstructive pulmonary disease: which method to use in clinical practice? Br J Nutr. 2006; 96: 86-92.
- Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004; 23: 932-946.
- Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999; 159: 179-187.
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000; 32: S498-504.
- Miravitlles M, Cantoni J, Naberan K. Factors associated with a low level of physical activity in patients with chronic obstructive pulmonary disease. Lung. 2014; 192: 259-265.
- Donaire-Gonzalez D, Gimeno-Santos E, Balcells E, Rodríguez DA, Farrero E, de Batlle J, et al. Physical activity in COPD patients: patterns and bouts. Eur Respir J. 2013; 42: 993-1002.
- Esteban C, Quintana JM, Aburto M, Moraza J, Egurrola M, Pérez-Izquierdo J, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010; 36: 292-300.
- Eliason G, Zakrisson AB, Piehl-Aulin K, Hurtig-Wennlöf A. Physical activity patterns in patients in different stages of chronic obstructive pulmonary disease. COPD. 2011; 8: 369-374.
- Jehn M, Schmidt-Trucksäss A, Meyer A, Schindler C, Tamm M, Stolz D. Association of daily physical activity volume and intensity with COPD severity. Respir Med. 2011; 105: 1846-1852.
- Esteban C, Quintana JM, Aburto M, Moraza J, Egurrola M, Pérez-Izquierdo J, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010; 36: 292-300.
- Marín Royo M, Pellicer Císcar C, González Villaescusa C, Bueso Fabra MJ, Aguar Benito C, Andreu Rodríguez AL, et al. [Physical activity and its relationship with the state of health of stable COPD patients]. Arch Bronconeumol. 2011; 47: 335-342.
- Eliason G, Zakrisson AB, Piehl-Aulin K, Hurtig-Wennlöf A. Physical activity patterns in patients in different stages of chronic obstructive pulmonary disease. COPD. 2011; 8: 369-374.
- Jehn M, Schmidt-Trucksäss A, Meyer A, Schindler C, Tamm M, Stolz D. Association of daily physical activity volume and intensity with COPD severity. Respir Med. 2011; 105: 1846-1852.
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007; 175: 458–463.
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006; 61: 772-778.
- Waschki B, Kirsten A, Holz O, Müller KC, Meyer T, Watz H, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011; 140: 331-342.
- Garcia-Rio F, Lores V, Mediano O, Rojo B, Hernanz A, Lopez-Collazo E, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009; 180: 506–512.
- Belman MJ. Exercise in patients with chronic obstructive pulmonary disease. Thorax. 1993; 48: 936-946.