
Review Article
Austin J Musculoskelet Disord. 2014;1(2): 1009.
Estrogen: The Current Status and the Future Role in Postconditioning Protection to the Heart
Fawzi A Babiker*
Department of Physiology, Faculty of Medicine, Kuwait University, Kuwait
*Corresponding author: Fawzi A Babiker, Department of Physiology, Faculty of Medicine, Kuwait University, B. O. Box 24923 Safat, 13110 Kuwait
Received: September 15, 2014; Accepted: October 15, 2014; Published: October 16, 2014
Abstract
Ischemic Heart Disease (IHD) is one of the leading causes of worldwide morbidity and mortality. Reperfusion was the first procedure used to rescue the ischemic heart; however, this procedure is associated with subsequent reperfusion injury. Ischemic Preconditioning (IPC) was introduced as an intervention to protect against the potential injury before the insult occurred. Yet, the application of IPC in the clinic was limited because the onset of ischemia is not predictable and neither was the amount of damage caused by the event. Thus, Ischemic Postcondtioning (IPOC) was introduced as an intervention immediately following reperfusion in order to surpass the shortcomings of preconditioning translation in the clinic. Several methods and procedures were used in postconditioning, among them are postconditioning with estrogen (17-β estradiol (E2)) and Selective E2 Receptors Modulators (SERMs). However, the role of E2 is controversial and its research was challenged by the unexpected outcomes of two large clinical trials (heart and Oestrogen/progestin Replacement Study (HERS) and Women’s Health Initiative (WHI). Controversy still exists regarding the results of the experiments using E2; however, many scientists still believe that the potentials of E2 are yet to be unraveled. The aim of this review is to highlight the use and effects of E2 in postconditioning, as well as its possible use in future clinical research.
Keywords: Oestrogen/postconditioning; Ischemic heart disease; Ischemic preconditioning; selective estrogen receptors modulators
Introduction
Ischemic heart disease (IHD) is known to be the leading cause of death in industrialized countries and among the leading causes of morbidity and mortality worldwide [1]. The prognosis of IHD is always running hand in hand with the size of the infarcted area. Indeed, big infarcts are associated with poor prognosis and mortality [2,3]. The first procedure introduced to protect the heart from ischemic injury is reperfusion, which is the reintroduction of blood to the ischemic part of the heart. However, this method of intervention adds to the ischemic damage by causing reperfusion injury [4]. Nevertheless, this method was the only way out for heart protection in the clinic for a long period of time. Various developments in the protective regimen of reperfusion were introduced within the past decades. Several drugs were used as pharmacological treatments to assist reperfusion and to decrease Ischemic Reperfusion (I/R) injury [5].
After the use of reperfusion with its various methods for quite some time, Murry et al. [6] introduced a new procedure of heart protection targeting I/R injury. In this procedure they showed that the heart is likely protected from I/R damage if it is challenged with brief, repetitive, ischemia before the major potentially lethal ischemic event. This technique was later called Ischemic Preconditioning (IPC), which is a repetitive ischemia reperfusion before the major ischemic insult. The protection by the IPC was shown to occur at two different time points, later named windows of protection. One of these is a very short window of 1-3 hours immediately after the IPC intervention, during which any ischemic challenge can be tolerated. The second window starts 12-24 hours IPC and lasts for 2-3 days [7]. Unfortunately, this method of protection cannot be translated in the clinic due to the lack of determination of ischemic attack onset in a healthy population. However, this method could be applied in coronary or cardiac surgery where the ischemia is expected [8,9]. Preconditioning was intensely studied to unravel the underlying mechanisms, which might be used in the protection of the heart against I/R injury. The studies done on its triggers, downstream effectors, and pathways formed the first building blocks for the future methods of heart protection against IHD. About two decades after the discovery of preconditioning, another novel technique of protection was introduced by Vinten Johansen’s group which is repeated episodes of ischemia immediately at the beginning of reperfusion [10]. This procedure resulted in a protection similar to that obtained by IPC and was named Ischemic Postconditioning (IPOC) as it is applied after the ischemic insult [10].
Recently, it has been shown that acute treatment with estrogen (17- β estradiol (E2)) protects the heart against I/R injury [11]. However, in vivo long-term E2 treatment before ischemia did not show any protection against I/R injury [12]. Although the cardioprotective effects of E2 Replacement Therapy (ERT) are still controversial [13,14], E2 pharmacological treatment in preconditioning was found to be feasible in different species [15,16] and in humans [17]. Some researchers, in an attempt to avoid the controversy and the discrepancy shown in the use of E2 started to use Selective Estrogen Receptors Modulators (SERMs). The phytoestrogen genistein was shown to protect the heart against IHD when applied at the beginning of reperfusion [18]. The research on E2 in postconditioning seems to be jeopardized by the decreased popularity of ERT after the negative results obtained in the two large scale epidemiological studies (Heart and E2 Replacement Study (HERS) [19] and Women Health Initiative Study (WHI) [19]. However, several investigations using E2 have shown encouraging results in animal and human studies [15,16,20,21]. Several studies in pre- and postconditioning are expected to be planned by our group and others in the near future. This will require a better understanding of the present E2 research and new areas of interest in the future of pre- and postconditioning E2 research. In this review, we aimed at determining the importance of E2 in pre- and postconditioning by considering gender differences, the effect of presence and withdrawal of E2, and long- and short-term E2 treatment on the outcome of pre- and postconditioning protection. The review of the available literature in the field of E2 in pre- and postconditioning may help illuminate the role of E2 role in these methods of protection and suggest possible translation to clinical research. The aim of the present review, therefore, is to review the recent literature on the use of E2 and its effects in protection of the heart against I/R injury and its future role in postconditioning protection to the heart.
Methods
For this review we used Google scholar search engine and PubMed and Scopus databases for searching literature relevant to our review. We used ischemia, ischemia reperfusion, preconditioning, postconditioning, pharamcological postconditioning remote postconditioning and pacing postconditioning as keywords for our search. The recent findings cited in our review manuscript were presented in the references list given at the end of the manuscript
Ischemic Heart Disease and its Associated Complications
Ischemic heart disease is a complex disorder that is associated with various systemic diseases and conditions [22]. The risk of getting this life-threatening condition increases with age, smoking, hyperlipidemia, hypertension, obesity, insulin resistance, and diabetes [22]. Generally, IHD develops as a consequence of reduction or stop of blood supply to the myocardium [4]. Atherosclerosis is reported to be the major cause of this problem of blood supply [23]. The prognosis of IHD depends mainly on the severity of the disease itself [22]. Overtime, in presence of atherosclerosis, the heart might be weakened the consequences of ischemia ending up eventually in heart failure [4]. One of the potential effects of ischemia is stunning which is a reversible post-ischemic contractile dysfunction that may negatively affect the viability of the myocytes [24]. Calcium overload and ROS were proven to be essential inducers of the myocardial stunning [25,26]. Furthermore, ischemia was documented to be a cause for arrhythmia and sudden cardiac death [27]. Elevated intracellular calcium and generation of Reactive Oxygen Species (ROS), which were induced by ischemia were recognized as crucial causes for arrhythmia. Increased cytosolic calcium has been shown to be responsible for the genesis of early and delayed after depolarization contributing to the arrhythmias [28]. Additionally, ROS were reported to be directly associated with myocardial arrhythmias events [29].
Estrogen as a Hormone and a Therapeutic Drug
However, a lot of controversy exists in E2 studies, significant gender differences were elucidated in basic cardiovascular functions [30]. Many experimental animal studies demonstrated presence of a better papillary muscle shortening in the female rats compared to males [31]. These notions were supported by clinical studies that showed a greater myocardial and left ventricle chamber functions in women compared to men [32]. Furthermore, a higher mass-to-volume ratio was reported in men, showing a higher myocardial mass in men compared to women [33]. These gender differences shown in heart functions are mediated by E2. However, some studies reported a lack of influence of E2 decrease or withdrawal on heart functions [34]. In contrast, a significant fall in aortic peak flow velocity, mean aortic acceleration time, ejection fraction, fractional shortening and ventricular mass was demonstrated after E2 hormone withdrawal [35]. Gender also imposes evident influences on the vascular homeostasis, demonstrated by a higher arterial compliance in women compared to men [36]. Estrogen is also known to reduce arterial collagen and stiffness resulting in a more distensible blood vessel [37]. Interestingly, impairment of endothelium-dependent vasorelaxation induced by atherosclerosis was proved to be gender dependent, with females showing a better relaxation than males [38]. However, some other hormones are involved [39,40], there is a strong evidence that suggests protective effects for E2 against cardiac hypertrophy [41]. Presence of E2 was proven to abrogate and its deficiency potentiates, the development of left ventricular hypertrophy [42]. Indeed, a rapid induction of LV hypertrophy was reported in men compared to women [43]. In a previous study we also demonstrated blockade of LVH. By a pathway involving atrial natriuretic peptide [44].
Ischemic heart disease is one of the key inducers of heart failure, therefore its control is essential in the regression of disease. Estrogen was reported to play a protective role against this serious disease [45]. Observational studies demonstrated an inverse relationship between E2 use and myocardial infarction and death from ischemic heart disease [46,47]. Administration of E2 prevented ischemic [48] and reperfusion [49] arrhythmias and reduced infarct size [50]. Importantly, E2 also increased distal coronary perfusion and other hemodynamic functions of the heart during reperfusion [50]. Use of exogenous E2 by postmenopausal women, significantly decreased myocardial infarction, heart failure and the morbidity and mortality following IHD [51]. In experimental animal models of I/R, E2 was shown to improve coronary artery dilation and spare the myocardium [50].
Mechanisms of E2 Signaling in Pre- and Postconditioning
The controversy that exists in the knowledge of E2 pathways and their protective role in the cardiovascular system dictates the importance of the E2 research. The presence of E2 throughout a long period of time (premenopause) and its absence during a considerable period (postmenopause) in females, necessitates the investigation of the effects of its presence and withdrawal on the outcome of diseases, their control, and treatment. Pre- and postconditioning, as very important methods of protection against IHD, require significant knowledge of E2 influence in order to incorporate their use in the clinic. Estrogen, in its protective role against heart disease, is believed to function by genomic and non-genomic pathways [35]. Genomic transduction pathways of E2 are mediated by the classical E2 receptors (ERs) alpha (ERα) and beta (ERβ) [35]. These pathways are usually of slow effect, requiring time ranging from minutes to hours [52]. However, non-genomic actions of E2 are mediated by membrane ERs through a G protein-coupled receptor and do not require gene transcription [53-55]. These non-genomic pathways of E2 are involved in rapid vasodilatation [56], inhibition of vessel injury [57] and reduction of I/R injury [58]. Recently a G protein-coupled receptor 30 (GPER30) was shown to bind E2 and cause protection via non-genomic signaling [59,60].
Although the mechanisms of protection of preconditioning and postconditioning are not completely understood, both seem to follow the same signaling pathways resulting in protection [20,61]. Preconditioning and postconditioning depend mainly on the instant regulation of specific elements, which activate other downstream elements or the final effectors ending in protection [62,63]. However, their individual methods and times of application are completely different occurring either before or after the insult respectively [20,61]. Up to date the most important components of the protection pathways known today include adenosine (A1) [64,65] and angiotensin II (AT1) receptors [66]; opening of Mitochondrial Potassium mito KATP channel [67,68] and sarcolemmal potassium (sarc KATP) [20] channels; activation of protein kinase C (PKC) [69,70] and PI3K-Akt [69,71,72]; prevention of the opening of the mPTP [71,73,74]; production of ROS [75,76]; and induction of NO [77,78] (Figure 1 and Table 1). However, the arrangement of these components within signaling cascades is not completely understood.
Abbreviations: ER: Estrogen Receptor; A1: Adenosine Receptor 1; At1: Angiotensin Receptor 1; TK: Tyrosine Kinase; SAC: Stretch Activated Channel.
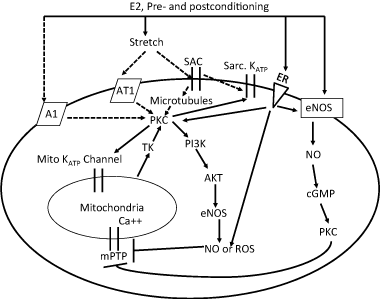
Figure 1: Schematic representation showing the potential pathways for preconditioning, postconditioning and estrogen treatment. Dashed lines showed pathways which were not known in estrogen treatment.
Abbreviations: ER: Estrogen Receptor; A1: Adenosine Receptor 1; At1: Angiotensin Receptor 1; TK: Tyrosine Kinase; SAC: Stretch Activated Channel.
References
Element
Preconditioning
Postconditioning
E2 treatment
PKC
Hausenloy DJ, [61]
Babiker FA, [20]
Haynes MP, et al [54]
Pl3-AKT
Hausenloy DJ� et al [69]
Bopassa JC et al, [71]
Yu HP et al, [83]
MitoKATP
Vinten-Johansen J, et al [67]
Vinten-Johansen J, et al [67]
Raphael J, 2005
Sarc KATP
Gross GJ and Fryer RM 1999
_____
____
eNoS
Xuan YT et al,[78]
Krolikowski JG et al [77]
Fujiwara N et al, 2001
ROS
Forbes RA. [75]
Penna C et al, [76]
Parkash J,[90]
NO
Sasaki N et al [85]
Krolikowski JG et al [77]
Hisamoto K [86]
A1
Solenkova NV et al. [65]
Philipp S et al, [64]
_____
Microtubules
Nakamura Y et al [70]
Babiker FA. [20]
_______
AT1
Sato M et al [66]
_____
_______
ER
___
Vornehm ND et al,[114]
________
mPTP
Bopassa JC et al [74]
Argaud L et al, [73]
Parkash J, [90]
GPER30
_____
_____
Noel SD, et al, [59]
Table 1: Components or elements which were known for a given protection method. Lines indicate that the component is not studied in the given method.
Interestingly, E2 protects the heart via cell signaling events similar to those of the classical pre- and postconditioning (Figure 1 and Table 1). In E2 preconditioning and postconditioning the pathways of protection are likely of non-genomic nature, which happens immediately after acute application of E2. Among these protective non-genomic effects are the antioxidant effect of E2 [79], inhibition of L-type Ca2 channels [80] and opening of mito KATP [17,81]. Estrogen can also bind to a plasma membrane G protein-coupled receptor and activate some of the very important elements known in the classical pre- and postcondtioning such as PI3-kinase, AKt and eNOS pathways [54]. Some other studies showed PI3K and its downstream elements like serine/threonine kinase Akt as E2 downstream elements, which lead finally to the salvage of the myocardium [54,82-84]. One of the elements which seems to be very important in pre and postconditioning protection is nitric oxide (NO), which is proven to be produced non-genomically by E2 [58,85,86]. Another essential element of pre- and postconditioning thought to be involved in potentially protective pathways is PKC [70,87]. This enzyme was also proven to be involved in E2 protection [88]. Recently, E2 was shown to protect the heart by inhibition of mPTP opening by a pathway involving G protein-coupled receptor [71,74,82]. E2 also played a specific role in the protection using a pathway involving reactive oxygen species (ROS); however, conflicting results were obtained regarding the protective role. Some studies have suggested a protective role for E2 by decreasing ROS production [89]. However, other studies suggested an increase of ROS by E2, which ultimately initiates protective cell signaling pathways [90]. These protective, non-genomic pathways of E2 are similar to the potential pathways of pre- and postconditioning. From this, one could speculate that E2, via similar pathways to pre- and postconditioning, will at least not compromise their effects. Acute presence or treatment with E2 therefore, will be protective rather than destructive, which will encourage its use in clinical studies.
E2 in preconditioning
The presence of E2 is essential for the protection of the myocardium against I/R injury due to the premenopausal female myocardium being shown to be more resistant to I/R injury compared to male myocardium [91]. E2 was proven to be very effective in decreasing myocardial infarction (MI) when applied before the ischemic period (preconditioning) [92]. However, controversy exists in regards to the protection of male and female animals [93-95]. Ischemic preconditioning was accompanied by gender-based differences, which can be explained by the differences in sex hormone levels [35]. Some reports have shown a protective effect for the presence of E2 leading to a reduced infarct size, improved cardiac function, and reduced adverse post-MI remodeling [82,96-98]. In contrast, increased infarct size and mortality following E2 supplementation in the studies of ischemia were also reported [21,99]. Yet, other studies did not show any positive effect for E2 in protection against I/R [19,100]. This controversy in the outcome and the negative results of some E2 studies [101-103] has hampered the research of E2 for the past few years. Interestingly, the introduction of the relatively new methods of protection against I/R like IPC led to an upsurge in E2 research. Many studies were designed to investigate E2’s role in preconditioning. Acute treatment with E2 was proven to be crucial for IPC protection [81,104]. Chronic withdrawal of E2 blocked IPC protection and abrogated its beneficial effects [105] while short term E2 replacement protected the heart against I/R in ovariectomized rats [11]. From these conclusions one could deduce that the presence of E2 and short periods of E2 treatments are protective. On the other hand, one study showed that long term treatments with E2 did not affect ischemia or IPC [12]. But, a different study noted that E2 treatment hindered IPC when applied for long periods [106]. This was confirmed in a study where premenopausal women showed higher mortality rates compared to postmenopausal women [107]. Understanding the effects of the presence or absence of E2 is critical for protection with IPC. The conflicting results in IPC studies seem to be mainly because of the E2 level and duration of availability. Fluctuating, normal E2 levels did not show negative impact on the protective effects of IPC of the female heart. However, the non-fluctuating E2 levels (chronic treatment) showed a negative influence on IPC.
E2 in postconditioning
If postconditioning is to be considered for clinical use, several aspects of E2 must be investigated such as the availability based on gender, short and long-term treatments with E2, and the effects of E2 withdrawal. Up to date the use of E2 in postconditioning of the myocardium is rarely considered. Only a single study considered E2 in postconditioning of the heart [108]. However, postconditioning with E2 treatment was used frequently in the protection of other organs. Estrogen postconditioning was shown to be protective in gastric epithelial cells [109,110], in neurons [111] and the mesentery [112]. Some studies manipulating individual receptors such as ERβ [113], ERα [114] and GPR30 [74] showed a protective postconditioning and simultaneously suggested a protective role for E2 in postconditioning. The fast development within postconditioning research suggests an important role in the protection against IHD. Therefore, its translation to the clinic might gain significant interest in the near future. Thus, the knowledge of the effects of the presence of E2 and the consequences of its direct use will be of vital importance for intervention in both male and female patients. Understanding gender differences in postconditioing will lead to the successful introduction of suitable methods and techniques in the clinic. Furthermore, the difference in the nature of the short and long-term E2 treatments is to be dissected for selection of the protective and less harmful treatments.
Use of SERMs in pre- and postconditioning
The risk of E2 use and its possible exacerbation of the risks of breast and endometrial cancer, venous thromboembolism [115,116] along with the discouraging results of HERS and WHI [101-103], sparked a remarkable interest in the use of Selective Estrogen Receptors Modulators (SERMs) for heart protection. This was strengthened by the observation of a decreased prevalence of IHD in populations who consume phytoestrogen [117]. Indeed this indicates that not only E2, but other related SERMs can give similar protective effects. SERMs gained popularity over E2 because of their reduced risk on the body. SERMs possess tissue specific agonist/antagonist effects [102] compared to E2, which could be beneficial for some tissues while detrimental to others [13,99,118]. They mainly act as E2 agonists on the heart and antagonists in the breast and uterus [119]. Furthermore, they can be administered safely to both sexes without any deleterious effects [102,120]. In fact, there are many known SERMs which are cardioprotective and lack the adverse effects of E2 [101-103]. However, every SERM has its own mechanism of action patients must be evaluated individually for its effects [103]. Among and patients the potential postconditioning agents only genistein was investigated and proved to be protective in preconditioning producing protection similar to that attained by E2 [121]. Similar effects were also shown with its use as a postconditioning drug [18]. Genistein, was also found to be effective in improving the hemodynamic and vascular function in animals [122] as well as in female patients [123]. The future research on SERMs will promote them to have significant consideration in postconditioning research for the treatment and prevention of IHD.
Postconditioning the human heart and the potential role of E2 and SERMs
Preconditioning has been proved to be effective in the human heart [124]. Trials of IPC on human atrial sections [125,126] and human skeletal muscles [127] showed significant protection against apoptotic and necrotic cell death. Although, many studies were done to validate the protective effects of postconditioning and to unravel its protective elements, the application of this technique in the clinic is rather limited. Few studies were done in small groups of patients. In these studies it was reported that postconditioning the heart with IPOC immediately after stentin the infarct-related artery, sustainably protected the heart from the subsequent I/R injury [128-131]. Promising results were also demonstrated in the clinical pilot studies using pharmacological postconditioning [132]. Indeed, postconditioning is a novel method of treatment which can save many lives. Its application in the clinic should be enhanced and large scale trials must be designed to determine whether this method of treatment could be introduced as an official method of treatment in the clinic [133]. Although, few studies were done on the effects of E2 in postconditioning, acute treatment with E2 was proved to protect the heart from I/R injury [16,134]. For safe application of postconditioning in the clinic the effects of presence or withdrawal of E2 as well as gender difference are to be considered. Acute pretreatment with E2 protected both male [16,17] and female [81,135] hearts in vivo and its acute presence during angioplasty made the heart more resistant to ischemia [17]. These results can encourage the use of acute E2 treatment in the clinic, which might be protective. Also it may carry the advantage of short term use which might not have the negative effects of long term E2 use on cancer. Acute E2 treatment could also be used as treatment for male patients since the natural absence of E2 will not affect the outcome of the treatment and the short time use might not have negative health threats. SERMs with their selective nature and low affinity for ERα and high affinity for ERβ [136] can be applied in acute or long-term treatments.
Conclusion
Estrogen seems to be one of the essential remedies in protecting the heart against most of the cardiovascular diseases like cardiac hypertrophy, IHD and heart failure. These diseases are known to be a serious clinical problem in the whole world. The control of these diseases and protection of the heart against them is the aim of today’s medicine. Thus, it is very important to study the role of estrogen as a drug in targeting these diseases. Furthermore identification the role of E2 and its effects on postconditioning protection to the heart might open a new avenue in the treatment of the heart against IHD.
References
- Finegold JA, Asaria P, Francis DP. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol. 2013; 168: 934-945.
- Lopes RD, Lokhnygina Y, Hasselblad V, Newby KL, Yow E, Granger CB, et al. Methods of creatine kinase-MB analysis to predict mortality in patients with myocardial infarction treated with reperfusion therapy. Trials. 2013; 14: 123.
- Pride YB, Giuseffi JL, Mohanavelu S, Harrigan CJ, Manning WJ, Gibson CM, et al. Relation between infarct size in ST-segment elevation myocardial infarction treated successfully by percutaneous coronary intervention and left ventricular ejection fraction three months after the infarct. Am J Cardiol. 2010; 106: 635-640.
- Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005; 14: 170-175.
- Yellon DM, Baxter GF. Reperfusion injury revisited: is there a role for growth factor signaling in limiting lethal reperfusion injury? Trends Cardiovasc Med. 1999; 9: 245-249.
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986; 74: 1124-1136.
- Pagliaro P, Gattullo D, Rastaldo R, Losano G. Ischemic preconditioning: from the first to the second window of protection. Life Sci. 2001; 69: 1-15.
- Lango R, Mrozinski P. Clinical importance of anaesthetic preconditioning. Anestezjol Intens Ter. 2010; 42: 206-212.
- Succi JE, Gerola LR, Succi GM, Almeida RA, Novais LS, Rocha B. Ischemic preconditioning influence ventricular function in off-pump revascularization surgery. Arq Bras Cardiol. 2010; 94: 319-324, 339-44.
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003; 285: H579-588.
- Fraser H, Davidge ST, Clanachan AS. Activation of Ca(2+)-independent nitric oxide synthase by 17beta-estradiol in post-ischemic rat heart. Cardiovasc Res. 2000; 46: 111-118.
- Peng WJ, Yu J, Deng S, Jiang JL, Deng HW, Li YJ. Effect of estrogen replacement treatment on ischemic preconditioning in isolated rat hearts. Can J Physiol Pharmacol. 2004; 82: 339-344.
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004; 291: 1701-1712.
- Ouyang P, Michos ED, Karas RH. Hormone replacement therapy and the cardiovascular system lessons learned and unanswered questions. J Am Coll Cardiol. 2006; 47: 1741-1753.
- Booth EA, Obeid NR, Lucchesi BR. Activation of estrogen receptor-alpha protects the in vivo rabbit heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2005; 289: H2039-2047.
- Lee TM, Lin MS, Chou TF, Tsai CH, Chang NC. Adjunctive 17beta-estradiol administration reduces infarct size by altered expression of canine myocardial connexin43 protein. Cardiovasc Res. 2004; 63: 109-117.
- Lee TM, Su SF, Chou TF, Tsai CH. Pharmacologic preconditioning of estrogen by activation of the myocardial adenosine triphosphate-sensitive potassium channel in patients undergoing coronary angioplasty. J Am Coll Cardiol. 2002; 39: 871-877.
- Tissier R, Waintraub X, Couvreur N, Gervais M, Bruneval P, Mandet C, et al. Pharmacological postconditioning with the phytoestrogen genistein. J Mol Cell Cardiol. 2007; 42: 79-87.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002; 288: 321-333.
- Babiker FA, Lorenzen-Schmidt I, Mokelke E, Vanagt WY, Delhaas T, Waltenberger J, et al. Long-term protection and mechanism of pacing-induced postconditioning in the heart. Basic Res Cardiol. 2010; 105: 523-533.
- van Eickels M, Patten RD, Aronovitz MJ, Alsheikh-Ali A, Gostyla K, Celestin F, et al. 17-beta-estradiol increases cardiac remodeling and mortality in mice with myocardial infarction. J Am Coll Cardiol. 2003; 41: 2084-2092.
- Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007; 59: 418-458.
- Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009; 204: 334-341.
- Sunderdiek U, Schmitz-Spanke S, Korbmacher B, Gams E, Schipke JD. Left ventricular dysfunction and disturbed O(2)-utilization in stunned myocardium: influence of ischemic preconditioning. Eur J Cardiothorac Surg. 2001; 20: 770-776.
- Kim SJ, Depre C, Vatner SF. Novel mechanisms mediating stunned myocardium. Heart Fail Rev. 2003; 8: 143-153.
- Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation. 2001; 104: 3158-3167.
- De Groot JR, Coronel R. Acute ischemia-induced gap junctional uncoupling and arrhythmogenesis. Cardiovasc Res. 2004; 62: 323-334.
- Jeong EM, Liu M, Sturdy M, Gao G, Varghese ST, Sovari AA, et al. Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol. 2012; 52: 454-463.
- Barth AS, Tomaselli GF. Cardiac metabolism and arrhythmias. Circ Arrhythm Electrophysiol. 2009; 2: 327-335.
- Colom B, Oliver J, Roca P, Garcia-Palmer FJ. Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res. 2007; 74: 456-465.
- Curl CL, Delbridge LM, Wendt IR. Sex differences in cardiac muscle responsiveness to Ca2+ and L-type Ca2+ channel modulation. Eur J Pharmacol. 2008; 586: 288-292.
- Bella JN, Palmieri V, Roman MJ, Paranicas MF, Welty TK, Lee ET, et al. Gender differences in left ventricular systolic function in American Indians (from the Strong Heart Study). Am J Cardiol. 2006; 98: 834-837.
- Nikitin NP, Loh PH, de Silva R, Witte KK, Lukaschuk EI, Parker A, et al. Left ventricular morphology, global and longitudinal function in normal older individuals: a cardiac magnetic resonance study. Int J Cardiol. 2006; 108: 76-83.
- Mihmanli I, Mihmanli V, Kantarci F, Albayram MS, Atakir K, Cebi D, et al. The effect of an acute decrease in serum estrogen concentration on vessel walls: determination with color and pulsed Doppler ultrasound. Arch Gynecol Obstet. 2003; 267: 134-138.
- Babiker FA, De Windt LJ, van Eickels M, Grohe C, Meyer R, Doevendans PA. Estrogenic hormone action in the heart: regulatory network and function. Cardiovasc Res. 2002; 53: 709-719.
- Zhou L, Chen Y, Sun N, Liu X. Family history of hypertension and arterial elasticity characteristics in healthy young people. Hypertens Res. 2008; 31: 833-839.
- Lydrup ML, Fernö M. Correlation between estrogen receptor alpha expression, collagen content and stiffness in human uterine arteries. Acta Obstet Gynecol Scand. 2003; 82: 610-615.
- Kojda G, Hüsgen B, Hacker A, Perings D, Schnaith EM, Kottenberg E, et al. Impairment of endothelium-dependent vasorelaxation in experimental atherosclerosis is dependent on gender. Cardiovasc Res. 1998; 37: 738-747.
- Bell JR, Mellor KM, Wollermann AC, Delbridge LM. Cardiac ischaemic stress: cardiomyocyte Ca²⁺, sex and sex steroids. Clin Exp Pharmacol Physiol. 2011; 38: 717-723.
- Jochmann N, Stangl K, Garbe E, Baumann G, Stangl V. Female-specific aspects in the pharmacotherapy of chronic cardiovascular diseases. Eur Heart J. 2005; 26: 1585-1595.
- Murphy E. Estrogen signaling and cardiovascular disease. Circ Res. 2011; 109: 687-696.
- Xu Y, Arenas IA, Armstrong SJ, Davidge ST. Estrogen modulation of left ventricular remodeling in the aged heart. Cardiovasc Res. 2003; 57: 388-394.
- Luchner A, Bröckel U, Muscholl M, Hense HW, Döring A, Riegger GA, et al. Gender-specific differences of cardiac remodeling in subjects with left ventricular dysfunction: a population-based study. Cardiovasc Res. 2002; 53: 720-727.
- van Eickels M, Grohé C, Cleutjens JP, Janssen BJ, Wellens HJ, Doevendans PA. 17beta-estradiol attenuates the development of pressure-overload hypertrophy. Circulation. 2001; 104: 1419-1423.
- Reis SE, Holubkov R, Young JB, White BG, Cohn JN, Feldman AM. Estrogen is associated with improved survival in aging women with congestive heart failure: analysis of the vesnarinone studies. J Am Coll Cardiol. 2000; 36: 529-533.
- de Vries CS, Bromley SE, Farmer RD. Myocardial infarction risk and hormone replacement: differences between products. Maturitas. 2006; 53: 343-350.
- Reiner AP, Heckbert SR, Vos HL, Ariëns RA, Lemaitre RN, Smith NL, et al. Genetic variants of coagulation factor XIII, postmenopausal estrogen therapy, and risk of nonfatal myocardial infarction. Blood. 2003; 102: 25-30.
- Chen CC, Lin CC, Lee TM. 17beta-Estradiol decreases vulnerability to ventricular arrhythmias by preserving connexin43 protein in infarcted rats. Eur J Pharmacol. 2010; 629: 73-81.
- Wang Y, Wang Q, Zhao Y, Gong D, Wang D, Li C, et al. Protective effects of estrogen against reperfusion arrhythmias following severe myocardial ischemia in rats. Circ J. 2010; 74: 634-643.
- Babiker FA, Joseph S, Juggi J. The protective effects of 17beta-estradiol against ischemia-reperfusion injury and its effect on pacing postconditioning protection to the heart. J Physiol Biochem. 2014; 70: 151-162.
- Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, et al. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012; 345: e6409.
- Nuedling S, Kahlert S, Loebbert K, Doevendans PA, Meyer R, Vetter H, et al. 17 Beta-estradiol stimulates expression of endothelial and inducible NO synthase in rat myocardium in-vitro and in-vivo. Cardiovasc Res. 1999; 43: 666-674.
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, et al. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007; 67: 1859-1866.
- Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, et al. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000; 87: 677-682.
- Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004; 101: 12354-12357.
- Han G, Ma H, Chintala R, Miyake K, Fulton DJ, Barman SA, et al. Nongenomic, endothelium-independent effects of estrogen on human coronary smooth muscle are mediated by type I (neuronal) NOS and PI3-kinase-Akt signaling. Am J Physiol Heart Circ Physiol. 2007; 293: H314-321.
- Bourghardt J, Bergström G, Krettek A, Sjöberg S, Borén J, Tivesten A. The endogenous estradiol metabolite 2-methoxyestradiol reduces atherosclerotic lesion formation in female apolipoprotein E-deficient mice. Endocrinology. 2007; 148: 4128-4132.
- Node K, Kitakaze M, Kosaka H, Minamino T, Funaya H, Hori M. Amelioration of ischemia- and reperfusion-induced myocardial injury by 17beta-estradiol: role of nitric oxide and calcium-activated potassium channels. Circulation. 1997; 96: 1953-1963.
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2009; 23: 349-359.
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005; 307: 1625-1630.
- Hausenloy DJ, Tsang A, Yellon DM. The reperfusion injury salvage kinase pathway: a common target for both ischemic preconditioning and postconditioning. Trends Cardiovasc Med. 2005; 15: 69-75.
- Piper HM, Abdallah Y, Schäfer C. The first minutes of reperfusion: a window of opportunity for cardioprotection. Cardiovasc Res. 2004; 61: 365-371.
- Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004; 61: 481-497.
- Philipp S, Yang XM, Cui L, Davis AM, Downey JM, Cohen MV. Postconditioning protects rabbit hearts through a protein kinase C-adenosine A2b receptor cascade. Cardiovasc Res. 2006; 70: 308-314.
- Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol Heart Circ Physiol. 2006; 290: H441-449.
- Sato M, Engelman RM, Otani H, Maulik N, Rousou JA, Flack JE 3rd, et al. Myocardial protection by preconditioning of heart with losartan, an angiotensin II type 1-receptor blocker: implication of bradykinin-dependent and bradykinin-independent mechanisms. Circulation. 2000; 102: III346-351.
- Vinten-Johansen J, Zhao ZQ, Jiang R, Zatta AJ, Dobson GP. Preconditioning and postconditioning: innate cardioprotection from ischemia-reperfusion injury. J Appl Physiol (1985). 2007; 103: 1441-1448.
- Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003; 83: 1113-1151.
- Hausenloy DJ, Mocanu MM, Yellon DM. Cross-talk between the survival kinases during early reperfusion: its contribution to ischemic preconditioning. Cardiovasc Res. 2004; 63: 305-312.
- Nakamura Y, Miura T, Nakano A, Ichikawa Y, Yano T, Kobayashi H, et al. Role of microtubules in ischemic preconditioning against myocardial infarction. Cardiovasc Res. 2004; 64: 322-330.
- Bopassa JC, Ferrera R, Gateau-Roesch O, Couture-Lepetit E, Ovize M. PI 3-kinase regulates the mitochondrial transition pore in controlled reperfusion and postconditioning. Cardiovasc Res. 2006; 69: 178-185.
- Yang XM, Philipp S, Downey JM, Cohen MV. Postconditioning's protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol. 2005; 100: 57-63.
- Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005; 111: 194-197.
- Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010; 298: H16-23.
- Forbes RA, Steenbergen C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ Res. 2001; 88: 802-809.
- Penna C, Rastaldo R, Mancardi D, Raimondo S, Cappello S, Gattullo D, et al. Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res Cardiol. 2006; 101: 180-189.
- Krolikowski JG, Weihrauch D, Bienengraeber M, Kersten JR, Warltier DC, Pagel PS. Role of Erk1/2, p70s6K, and eNOS in isoflurane-induced cardioprotection during early reperfusion in vivo. Can J Anaesth. 2006; 53: 174-182.
- Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Bolli R. Endothelial nitric oxide synthase plays an obligatory role in the late phase of ischemic preconditioning by activating the protein kinase C epsilon p44/42 mitogen-activated protein kinase pSer-signal transducers and activators of transcription1/3 pathway. Circulation. 2007; 116: 535-544.
- Prokai L, Simpkins JW. Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol Ther. 2007; 114: 1-12.
- Cairrão E, Alvarez E, Carvas JM, Santos-Silva AJ, Verde I. Non-genomic vasorelaxant effects of 17β-estradiol and progesterone in rat aorta are mediated by L-type Ca2+ current inhibition. Acta Pharmacol Sin. 2012; 33: 615-624.
- Lee TM, Su SF, Tsai CC, Lee YT, Tsai CH. Cardioprotective effects of 17 beta-estradiol produced by activation ofmitochondrial ATP-sensitive K(+)Channels in canine hearts. J Mol Cell Cardiol. 2000; 32: 1147-1158.
- Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol. 2009; 297: H1806-1813.
- Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, et al. The PI3K/Akt pathway mediates the nongenomic cardioprotective effects of estrogen following trauma-hemorrhage. Ann Surg. 2007; 245: 971-977.
- Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, et al. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001; 104: 330-335.
- Sasaki N, Sato T, Ohler A, O'Rourke B, Marbán E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation. 2000; 101: 439-445.
- Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, et al. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2001; 276: 3459-3467.
- Vinten-Johansen J, Zhao ZQ, Zatta AJ, Kin H, Halkos ME, Kerendi F. Postconditioning--A new link in nature's armor against myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2005; 100: 295-310.
- Sylvia VL, Walton J, Lopez D, Dean DD, Boyan BD, Schwartz Z. 17 beta-estradiol-BSA conjugates and 17 beta-estradiol regulate growth plate chondrocytes by common membrane associated mechanisms involving PKC dependent and independent signal transduction. J Cell Biochem. 2001; 81: 413-429.
- Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol. 2005; 68: 959-965.
- Parkash J, Felty Q, Roy D. Estrogen exerts a spatial and temporal influence on reactive oxygen species generation that precedes calcium uptake in high-capacity mitochondria: implications for rapid nongenomic signaling of cell growth. Biochemistry. 2006; 45: 2872-2881.
- Johnson MS, Moore RL, Brown DA. Sex differences in myocardial infarct size are abolished by sarcolemmal KATP channel blockade in rat. Am J Physiol Heart Circ Physiol. 2006; 290: H2644-2647.
- Das B, Sarkar C. Similarities between ischemic preconditioning and 17beta-estradiol mediated cardiomyocyte KATP channel activation leading to cardioprotective and antiarrhythmic effects during ischemia/reperfusion in the intact rabbit heart. J Cardiovasc Pharmacol. 2006; 47: 277-286.
- Penna C, Tullio F, Merlino A, Moro F, Raimondo S, Rastaldo R, et al. Postconditioning cardioprotection against infarct size and post-ischemic systolic dysfunction is influenced by gender. Basic Res Cardiol. 2009; 104: 390-402.
- Pitcher JM, Nagy RD, Tsai BM, Wang M, Kher A, Meldrum DR. Is the preconditioning threshold different in females? J Surg Res. 2005; 125: 168-172.
- Song X, Li G, Vaage J, Valen G. Effects of sex, gonadectomy, and oestrogen substitution on ischaemic preconditioning and ischaemia-reperfusion injury in mice. Acta Physiol Scand. 2003; 177: 459-466.
- Babiker FA, De Windt LJ, van Eickels M, Thijssen V, Bronsaer RJ, Grohé C, et al. 17beta-estradiol antagonizes cardiomyocyte hypertrophy by autocrine/paracrine stimulation of a guanylyl cyclase A receptor-cyclic guanosine monophosphate-dependent protein kinase pathway. Circulation. 2004; 109: 269-276.
- Gabel SA, Walker VR, London RE, Steenbergen C, Korach KS, Murphy E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005; 38: 289-297.
- Wang M, Baker L, Tsai BM, Meldrum KK, Meldrum DR. Sex differences in the myocardial inflammatory response to ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2005; 288: E321-326.
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998; 280: 605-613.
- Mendelsohn ME, Karas RH. The time has come to stop letting the HERS tale wag the dogma. Circulation. 2001; 104: 2256-2259.
- Palacios S. The future of the new selective estrogen receptor modulators. Menopause Int. 2007; 13: 27-34.
- Palacios S. Selective estrogen receptor modulators: the future in menopausal treatment. Minerva Ginecol. 2011; 63: 275-286.
- Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB. Selective estrogen receptor modulators: an update on recent clinical findings. Obstet Gynecol Surv. 2008; 63: 163-181.
- Sovershaev MA, Egorina EM, Andreasen TV, Jonassen AK, Ytrehus K. Preconditioning by 17beta-estradiol in isolated rat heart depends on PI3-K/PKB pathway, PKC, and ROS. Am J Physiol Heart Circ Physiol. 2006; 291: H1554-1562.
- Shinmura K, Nagai M, Tamaki K, Bolli R. Loss of ischaemic preconditioning in ovariectomized rat hearts: possible involvement of impaired protein kinase C epsilon phosphorylation. Cardiovasc Res. 2008; 79: 387-394.
- Grist M, Wambolt RB, Bondy GP, English DR, Allard MF. Estrogen replacement stimulates fatty acid oxidation and impairs post-ischemic recovery of hearts from ovariectomized female rats. Can J Physiol Pharmacol. 2002; 80: 1001-1007.
- Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann Intern Med. 2001; 134: 173-181.
- Crisostomo PR, Wang M, Wairiuko GM, Terrell AM, Meldrum DR. Postconditioning in females depends on injury severity. J Surg Res. 2006; 134: 342-347.
- Du D, Ma X, Zhang J, Zhang Y, Zhou X, Li Y. Cellular and molecular mechanisms of 17beta-estradiol postconditioning protection against gastric mucosal injury induced by ischemia/reperfusion in rats. Life Sci. 2010; 86: 30-38.
- Liu MJ, Fei SJ, Qiao WL, Du DS, Zhang YM, Li Y, et al. The protective effect of 17beta-estradiol postconditioning against hypoxia/reoxygenation injury in human gastric epithelial cells. Eur J Pharmacol. 2010; 645: 151-157.
- Gulinello M, Lebesgue D, Jover-Mengual T, Zukin RS, Etgen AM. Acute and chronic estradiol treatments reduce memory deficits induced by transient global ischemia in female rats. Horm Behav. 2006; 49: 246-260.
- Shih HC, Huang MS, Lee CH. Estrogen augments the protection of hypertonic saline treatment from mesenteric ischemia-reperfusion injury. Shock. 2011; 35: 302-307.
- Wang M, Wang Y, Weil B, Abarbanell A, Herrmann J, Tan J, et al. Estrogen receptor beta mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol Regul Integr Comp Physiol. 2009; 296: R972-978.
- Vornehm ND, Wang M, Abarbanell A, Herrmann J, Weil B, Tan J, et al. Acute postischemic treatment with estrogen receptor-alpha agonist or estrogen receptor-beta agonist improves myocardial recovery. Surgery. 2009; 146: 145-154.
- Boyle P, Maisonneuve P, Autier P. Update on cancer control in women. Int J Gynaecol Obstet. 2000; 70: 263-303.
- Douketis JD, Gordon M, Johnston M, Julian JA, Adachi JR, Ginsberg JS. The effects of hormone replacement therapy on thrombin generation, fibrinolysis inhibition, and resistance to activated protein C: prospective cohort study and review of literature. Thromb Res. 2000; 99: 25-34.
- Barnes S. Evolution of the health benefits of soy isoflavones. Proc Soc Exp Biol Med. 1998; 217: 386-392.
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003; 349: 523-534.
- Lewis JS, Jordan VC. Selective estrogen receptor modulators (SERMs): mechanisms of anticarcinogenesis and drug resistance. Mutat Res. 2005; 591: 247-263.
- Lello S. [Selective estrogen receptor modulators: focus on bazedoxifene]. Minerva Ginecol. 2011; 63: 305-314.
- Deodato B, Altavilla D, Squadrito G, Campo GM, Arlotta M, Minutoli L, et al. Cardioprotection by the phytoestrogen genistein in experimental myocardial ischaemia-reperfusion injury. Br J Pharmacol. 1999; 128: 1683-1690.
- Squadrito F, Altavilla D, Squadrito G, Saitta A, Cucinotta D, Minutoli L, et al. Genistein supplementation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovasc Res. 2000; 45: 454-462.
- Squadrito F, Altavilla D, Morabito N, Crisafulli A, D'Anna R, Corrado F, et al. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis. 2002; 163: 339-347.
- Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006; 47: 2277-2282.
- Vohra HA, Galiñanes M. Myocardial preconditioning against ischemia-induced apoptosis and necrosis in man. J Surg Res. 2006; 134: 138-144.
- Zhang JG, Ghosh S, Ockleford CD, Galiñanes M. Characterization of an in vitro model for the study of the short and prolonged effects of myocardial ischaemia and reperfusion in man. Clin Sci (Lond). 2000; 99: 443-453.
- Martou G, O'Blenes CA, Huang N, McAllister SE, Neligan PC, Ashrafpour H, et al. Development of an in vitro model for study of the efficacy of ischemic preconditioning in human skeletal muscle against ischemia-reperfusion injury. J Appl Physiol (1985). 2006; 101: 1335-1342.
- Laskey WK. Brief repetitive balloon occlusions enhance reperfusion during percutaneous coronary intervention for acute myocardial infarction: a pilot study. Catheter Cardiovasc Interv. 2005; 65: 361-367.
- Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, et al. Postconditioning the human heart. Circulation. 2005; 112: 2143-2148.
- Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G, et al. Long-term benefit of postconditioning. Circulation. 2008; 117: 1037-1044.
- Darling CE, Solari PB, Smith CS, Furman MI, Przyklenk K. 'Postconditioning' the human heart: multiple balloon inflations during primary angioplasty may confer cardioprotection. Basic Res Cardiol. 2007; 102: 274-278.
- Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008; 359: 473-481.
- Yetgin T, Manintveld OC, Duncker DJ, van der Giessen WJ. Postconditioning against ischaemia-reperfusion injury: ready for wide application in patients? Neth Heart J. 2010; 18: 389-392.
- Delyani JA, Murohara T, Nossuli TO, Lefer AM. Protection from myocardial reperfusion injury by acute administration of 17 beta-estradiol. J Mol Cell Cardiol. 1996; 28: 1001-1008.
- Booth EA, Marchesi M, Kilbourne EJ, Lucchesi BR. 17Beta-estradiol as a receptor-mediated cardioprotective agent. J Pharmacol Exp Ther. 2003; 307: 395-401.
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997; 138: 863-870.