
Review Article
Austin J Musculoskelet Disord. 2014;1(2): 1011.
Current Tendencies of Posterior Lumbar Interbody Fusion with a Pedicle Screw in the Osteoporotic Spine-Advances and Concerns
Okuyama K1*, Miyakoshi N2, Suzuki T3 and ShimadaY2
1Department of Orthopedic Surgery, Akita Rosai Hospital, Japan
2Department of Orthopedic Surgery, Akita University School of Medicine, Japan
3Department of Orthopedic Surgery, Akita Red Cross Hospital, Japan
*Corresponding author: Okuyama K, Department of Orthopedic Surgery, Akita Rosai Hospital, Karuizawa Aza Shimotai 30, Ohdate, ZIP 018-56, Japan
Received: October 29, 2014; Accepted: December 01, 2014; Published: December 02, 2014
Abstract
PLIF with PS has brought a great evolution in spinal instrumentation surgery because it enables us to perform 3-D correction and solid stabilization of the affected segment immediately after surgery. But complications of PLIF with PS, such as screw loosening, non-union and PJF have not been completely solved, especially in elderly patients with osteoporosis. Herein, we review articles focusing on PLIF with PS and its complication in the osteoporotic spine. Moreover, the current tendencies are discussed with our personal experiences in clinical cases.
Keywords: Posterior Lumbar Interbody Fusion; Pedicle Screw; Osteoporosis; Complications; Current Tendencies; Aging Society
Abbreviations
PLIF: Posterior Lumbar Interbody Fusion; PS: Pedicle Screw; 3-D: Three Dimensional; IBD: Interbody Device; BMD: Bone Mineral Density; QCT: Quantitative Computed Tomography; DEXA: Dual Energy X-ray Absorptiometry; PJF(K): Proximal Junctional Fracture(Kyphosis); PEEK: Polyetheretherketone; CFR/PEEK: Carbon Fiber-Reinforced Polyetheretherketone; Ti2448: Ti-24Nb-4Zr-7.9Sn; CPC: Calcium Phosphate Cement; PMMA: Polymethylmethacrylate; EPS: Expandable Pedicle Screw; CBT: Cortical Bone Trajectory; PTH: Parathyroid Hormone; PLF: Posterior Lumbar Fusion; LLIF: Lumbar Lateral Interbody fusion; XLIF®: Extreme Lumbar Lateral Interbody Fusion; DLIF: Direct Lateral Interbody Fusion
Introduction
History and current Status of PLIF and PS
PLIF with PS is very useful and has become a standard procedure of lumbar fusion surgery nowadays. It has provided a great advantage in performing 3-D correction and solid stabilization of affected segments immediately after surgery. To my best knowledge, PLIF was theoretically proposed as one of the procedures for lumbar degenerative diseases by Capener in 1932 [1]. In 1944, Briggs et al, performed the primitive PLIF procedure as an intercorpus fusion with bone chips although the fusion failed [2]. In the 1940’s~50’s, conventional PLIF without instrumentation was pioneered by Cloward to treat painful intervertebral discs, but biomechanical stability of PLIF using the autologous bone without instrumentation was too fragile to allow an early rehabilitation program at that time [3]. As a result of this downside, PLIF without instrumentation had not been accepted as a standard surgical technique until the late 1980’s. Meanwhile, Judet and Roy-Camille proposed the original idea of PS in 1970 [4]. At first, it was described in French as a pedicle screw plate for traumatic disorders in the thoracolumbar and lumbar spine, and was later written in English in 1986 [5].
There is a cornerstone article that revived the PLIF procedure. Steffee and Sitkowski stated that PLIF, in conjunction with PS, was biomechanically ideal, and reported no dislocation, absorption and pseudoarthrosis of interbody grafts in 67 patients with degenerative disorders in 1988 [6]. After this statement, numerous studies demonstrated that PLIF with PS enhanced the osteosynthesis success rate of the spinal arthrodesis in lumbar degenerative diseases. But the problem of grafted bone collapse and/or non-union, especially in the osteoporotic spine, has not been completely resolved. Allograft bone was also proposed as a PLIF material in the same decade, but it did not gain instant stability of the fixed segment. Furthermore, it introduced a risk of blood-borne pathogen transmission and decreased bone healing. Because of the disadvantages and risks, PLIF using allograft bone, has not been accepted among orthopedic surgeons.
In the 1990’s and early 2000’s, variable kinds of IBDs, which provide solid anterior column support of the functional spinal unit, were introduced and used in vivo for lumbar arthrodesis. IBD has extremely improved the biomechanical stability of PLIF with PS. Lots of articles that describe excellent outcomes of PLIF with PS& IBD have also been published [7-9]. After the development of IBD, the tri-cortical or bi-cortical strut bone graft, which had been used in the original PLIF procedure, was considered non-essential. For instance, Kanayama et al. biomechanically proved that interbody fusion constructs with threaded or non-threaded interbody cages had more solid stiffness in comparison to a calf spine in intact condition [10].
In terms of bone harvesting, the morbidity of harvesting a tri-cortical autologous graft bone from the ilium is also very significant, including a persistent donor site pain [11,12]. If PLIF could be carried out without harvesting autograft bone from the iliac crest, it could be a great benefit for both patients and orthopedic surgeons. Several authors reported clinical efficacy of PLIF using local bone with IBD instead of autologous iliac bone graft. Hashimoto et al. performed a clinical study of 25 single-level PLIF cases using carbon cages with a mixture of the local morselized bone and bioactive ceramic granules, and their result demonstrated a 100% bone union rate including 2 cases of collapsed union [13]. In 2003, Miura et al. reported a 100% bone union rate in 32 patients who underwent PLIF using carbon cages filled with only the local bone and PS [14]. In 2007, we also reported an excellent bone union in PLIF for degenerative spondylolisthesis using 2 titanium cages filled with the excised local bone. Our fusion rate was 93.5% at an averaged 2.3 (2.0 - 4.5) years after follow-up [15].
Several disadvantages of PLIF with PS & IBD are the risks of perioperative complications, including surgical invasion and an overall high technical demand. Especially, it has a high risk of neurological complication because the affected lumbar canal is not wide enough to permit safe passage of materials into the interbody space [16,17]. In our institute, to avoid neurological complications, the inferior one-half of the lamina, and the inferior articular process are en-block excised in the cephalad vertebra. The superior articular process is partially excised at the margin of the pedicle in the caudad vertebra in the non-isthmic cases. The spinous processus, supraspinous and interspinous ligaments to the adjacent vertebra are preserved. This surgical step permits safe passage of IBDs and the local resected bone into the interbody space while the caudad and cephlad nerve roots are directly retracted. Two spacers or cages could be easily placed at the lateral portion of the interbody space [15]. In our initial series of this PLIF procedure, transient nerve root palsy and dural damage without neural deficit were recorded in 12 and 6 of 148 patients, respectively [18]. PLIF with PS & IBD can correct and stabilize the three columns of the affected spinal segment, while also allowing direct observation of the neural tissues in the posterior approach, especially when the facet joints are entirely resected. Nowadays, there is no doubt that PLIF with PS& IBD is accepted as a golden standard procedure for treatment of both degenerative and traumatic disorders. In the latest decade, minimally invasive PLIF, and transforaminal interbody fusion with PS & IBD have also been introduced and are gaining popularity as new alternatives to the conventional PLIF with PS & IBD.
Problems of PLIF with PS in the osteoporotic spine
One of the common problems of PS is screw loosening (radiolucency in the bone-screw interface on X-ray) which may lead to a backout of PS, loss of correction and final non-union of PLIF (Figures 1 and 2). PS loosening was mainly caused by cyclic caudo-cephalad toggling at the bone-screw interface when an axial compression load was transmitted through the plate or rod to the screw [19]. Loosening of PS can easily occur if PS is anchored into the osteoporotic vertebral body through the pedicle. In a selected survey of the American Back Society, the rate of screw loosening was observed in 0.81% of 617 cases, and was ranging from 0.6 to 11 % in the literatures reviewed by Esses et al. [20]. It has been analyzed that several factors affecting the stability of PS, such as its length, outer diameter, design, fitness in the pedicle, BMD of the vertebra and elasticity of cancellous bone in 1980’s ~90’s [21,22]. In particular, BMD is supposed to be a very important parameter influencing the stability of PS [23-27]. A very high correlation between BMD and the stability of PS was studied and confirmed. Some thresholds for the implant failure in PS have also been proposed from the view point of BMD. Wittenberg has concluded that early loosening of PS may be expected at BMD less than 90mg/cc measured by QCT [24]. We also confirmed that the vertebrae with an average BMD of 95±33.3 (mean±SD) mg/ml by QCT could not be anatomically stabilized by PS alone in cadaveric specimens [27]. In general, PLIF with PS& IBD is indicative for patients with degenerative spondylolisthesis, kyphoscoliosis, and vertebral fracture and so forth to correct and stabilize the unstable deformities. But most of the patients are ironically associated with osteoporosis more or less. In 2001, we have proposed that BMD value of 0.674 ± 0.104 g/cm2 and 0.720 ± 0.078g/ cm2 by DEXA in the lumbar spine is a specific threshold below which non-union and screw loosening develops, respectively when PLIF with PS & IBD is carried out in patients with lumbar degenerative disorders [28]. Improving clinical results in the current situation is a major hurdle, and unfortunately, loosing of PS is still unsolved, and is the top priority that orthopedic surgeons face.
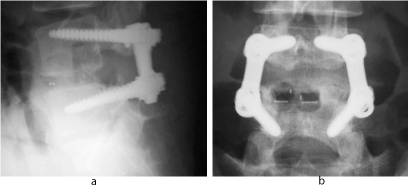
Figure 1: A 26-year-old woman (no-osteoporotic case) with recurrent disc herniation at L4/5. Plain X-rays demonstrating a solid fusion after PLIF with PS & PEEK cages.
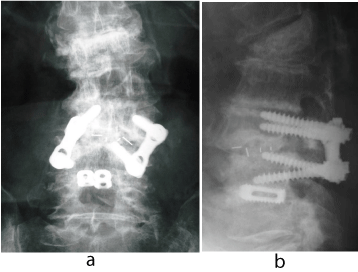
Figure 2: A 76-year-old woman (osteoporotic case) with degenerative spondylolisthesis at L3/4. Plain X-rays demonstrating a definitive non-union after PLIF with PS &PEEK cages.
PJF is included in PJK which is originally described as a pathological kyphotic condition adjacent to the instrumented segments in adult spinal deformity [29]. The same pathological condition often develops in the end and/or adjacent vertebra to the fused segment after multi-segmental PLIF with PS & IBD. This is another serious problem related to multi-segmental PLIF with PS & IBD in the osteoporotic spine. It rarely develops in the caudad, mostly in the cephalad portion [30]. This issue should be immediately resolved. Multiple factors affect the patho-mechanism of PJF(K), but it is mainly caused by stress concentration on the most upper or adjacent vertebra to the fused segment after surgery. Biomechanical research mentioned that PLIF with PS & IBD significantly increased construct stiffness in comparison to the intact specimen. In 1993, Oda et al. demonstrated that the higher construct stiffness resulted in an excessive load on the adjacent segment in the fresh-frozen calf lumbar spine [31]. In addition to this study, Sodo et al. disclosed that excessive loads increased as the number of the segments fused by PLIF with PS & IBD in 2006 [32]. The NIH consensus in 2000 proposed a concept that 70~80% of bone strength was defined by BMD and 20~30% by bone quality. If the patients are suffering from spinal osteoporosis, it is imaginable that their bone strength is too weak to sustain the stress concentration after PLIF with PS & IBD, and a risk of PJF(K) is inevitably increased. Disappointingly, there are no pragmatic predictors or prophylactic measures for development of PJF(K) at the moment (Figure 3).
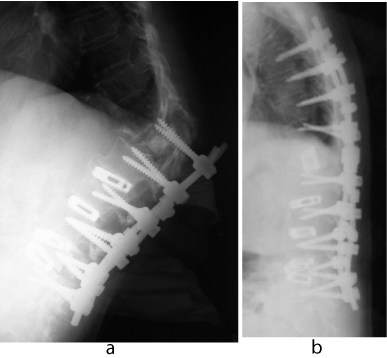
Figure 3: A 74-year-old woman with L4 vertebral fracture and lumbar degenerative kyphoscoliosis. A lateral plain X-ray demonstrating a severe PJF in Th12 after multi-segmental PLIF with PS & titanium cages (a). A salvage surgery, including Th12 corpectomy and augmentation, was done (b).
Numerous clinical studies and research about instrumentation surgery for the osteoporotic spine have been reported on for the last two decades. I have reviewed innovating and challenging articles related to PLIF with PS & IBD and its complication in the osteoporotic spine. All of them are very exciting and promising, and give valuable information for us, orthopedic surgeons, to better guide practice. The following representative articles are very pertinent to this discussion (Table 1).
Category
Authors
Specific Items
Material
Nakahara et al. [40]
Wang et al. [43]
CFR/PEEK with surface hydroxyapatite coating
Ti2448
Design
Yazu et al. [45]
Wu et al. [48]
Novel-concept PS with CPC
Expandable PS
Construct
Ponnappan et al. [41]
Lim et al. [52,53]
PEEK rod system
Ideal position of transverse connectors etc.
Technique
Santoni et al. [57]
Matsukawa et al.[58]
Cortical bone trajectory
Bone strength
Ohtani et al. [63]
Miyakoshi et al. [64]
Intermittent administration of human PTH
Table 1: List of challenging issues in PLIF with PS & IBD in the osteoporotic spine.
Challenges in PLIF with PS&IBD
Implant material
PEEK: Wrought titanium 6Al-4V ELI alloy in IBD is corrosion resistant, strong enough to obtain the immediate stability of the fixed spinal segment, has a biologically intimate affinity to the bone tissue, and enhances bone in growth around the alloy itself [9,33]. But the titanium IBD has a disadvantage of much higher construct stiffness (110GPa) in comparison to the cortical (18GPa) or cancellous bone (4GPa). This higher construct stiffness has a side effect of stress shielding that would develop absorption of the grafted bone within the cage or around the spacer. Therefore, the bone in growth is still controversial [34]. Contrarily, PEEK was introduced to the orthopedic sphere in late 1990s’, and also has been used for a kind of IBD material. PEEK is biocompatible and radiolucent. Moreover, it has a similar construct stiffness (3.6 GPa) to the bone tissue. From the point of biomechanical characteristics, a better bone fusion in PLIF with PEEK is expected than wrought titanium 6Al-4V ELI alloy. This may be a main reason why a PEEK cage is widely preferred instead of a titanium IBD for PLIF. In 2006, Trouillier et al. reported an excellent result of PLIF for lumbar canal stenosis using PEEK and titanium cages filled with the local autologous bone [35]. Rousseau et al. also described a good result of circumferential arthrodesis with PEEK cages in 57 patients with degenerative disorders of the lumbar spine [36]. In addition, Jiya et al. demonstrated a higher fusion rate of a PEEK cage than a Poly-L-Lactide-Co-D,L-lactide cage in 2009 [37]. In spite of these good results, there still remains a concern that PEEK does not induce bone in growth on its surface. This disadvantage of PEEK cages results in a lack of bone in growth area, and might easily lead to non-union [33]. A typical case of non-union was demonstrated in Figures 4. A 79-old-female patient underwent a revision surgery at L4/5 after multiple PLIF with PS using the resected local bone and PEEK cages. The photomicrograph shows very few bone growth in the extracted PEEK cages. Sinclair et al. has pointed out that scar tissue, which is barely seen around the titanium cage, is present around the PEEK cage in the cervical spine of a gout model in 2012 [38]. Further detailed studies are expected to disclose the concern. In the near future, application of CFR/PEEK with or without surface hydroxyapatite coating as an IBD may solve this problem [39,40]. Surprisingly, PEEK rod systems have also been proposed by Ponnappen et al. in 2009. Their study has suggested that the PEEK rod system can greatly withstand the angular displacements of normal physiological range of motion in human cadavers. Is it really possible for a PEEK rod to sustain a physiological load in vivo after multiple segmental fusions? So far, no clinical results have been conclusive [41].
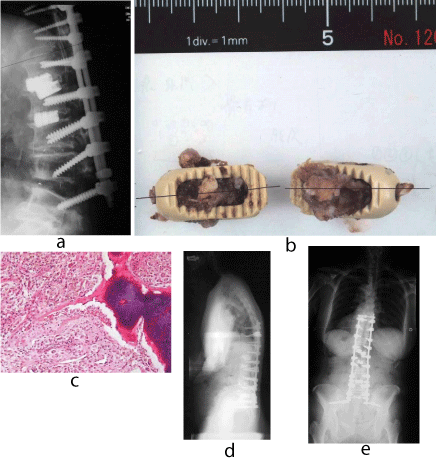
Figure 4: A 79-year-old woman with lumbar degenerative kyphosis. A lateral plain X-ray demonstrating a backout of PEEK cages at L4/5, 6 weeks after the initial surgery (a). A photograph showing the extracted PEEK cages (b). A photomicrograph of cross-section of the extracted PEEK cages demonstrating a very rare boney tissue in the cage (Original magnification X40. H.E.) (c). Plain X--rays after the salvage surgery. PLIF with titanium cages at L4/5 and iliac screw fixation were performed (d,e).
Ti2448: Ti2448, a new low-rigid titanium alloy has been developed. The elastic modulus of Ti2448 is 33GPa, which is very close to bone tissue. In 2009, Sha et al. demonstrated greater callus formation and bone growth in the rodent femur fixed by a Ti2448 nail than by a titanium 6Al-4V ELI nail [42]. Wang et al. demonstrated biomechanics of PLIF with Ti2448 PS & IBD using a finite element analysis. Their results have suggested that the single level PLIF with Ti2448 PS&IBD induces less intra-discal pressure at the adjacent level and lessens stress-shielding effects at implant-bone surface with increased stabilization performance in comparison to the titanium 6Al-4V ELI alloy [43]. Ti2448 PS&IBD has a high potential to provide a better bone fusion rate and lower frequency of PJF(K). Clinical application of Ti2448 PS & IBD is encouraging.
Screw design, augmentation and novel technique
Screw design and augmentation: It is well known that several factors affect the stability of PS in vivo, such as its length, outer diameter, design, fitness in the pedicle, BMD of the vertebra, elasticity of the cancellous bone. In 2010, Cho et al. annotated the biomechanics of PS-based instrumentation [44]. It is very impressive, and should be recommended reading material for clinical orthopedic surgeons because of its simple explanation of PS biomechanics. A PS with unique design has been proposed to increase the bone-screw interface stability in the osteoporotic spine. Yazu et al. has studied pullout strength of a novel-concept PS augmented with CPC in the 15 embalmed human vertebrae (average BMD, 0.866±0.370g/cm2). The PS has 20 small holes leading to a hollow part on the bottom of the thread to be filled with CPC. According to their findings, the maximum pullout strength of the novel-concept screw augmented with CPC was increased by nearly 250% in comparison to that of the conventional PS [45]. To date, clinical results of the novel-concept screw augmented with CPC have not yet been confirmed. In a clinical series, even though PMMA has a risk of exothermic reaction to the neural elements, Piñera et al. reported an efficacy of a cannulated pedicle screw augmented with PMMA in osteoporotic patients over 70 years with lumbar degenerative instability [46]. In 2005, EPS was developed to increase the PS stability in the osteoporotic spine in vitro. Lei and Wu have demonstrated a significant increase of the pull-out force and turning-back torque of EPS compared with the conventional PS in calf vertebrae [47]. They also have comparatively studied screw loosening in the osteoporotic spine between EPS and conventional PS in vivo, and concluded that EPS could decrease the risk of screw loosening and achieve better fixation strength and exhibited clinical results with very low EPS breakage in osteoporotic lumbar spine fusion in 2012 [48]. This is a very hopeful result without PMMA technique, which has a risk of an exothermic reaction to the neural elements [49]. In the clinical application, orthopedic surgeons can feel that the dural thread PS facilitates faster insertion and the PS with double lead and dual threads near the pedicle provides higher insertion torque. However, their higher pullout strength has not yet been reported [44,50]. The transverse connector also increases the pullout strength. In 2001, our colleagues, Suzuki et al. demonstrated that both single and double coupling of PS provided significantly greater pullout strength than that of PS without coupling using 33 cadaveric lumbar vertebrae [51]. Two transverse connectors give us the greatest axial rotatory stability. Lim et al. has advised their ideal position was one in the middle and the other at the proximal 1/8 position of the longitudinal rods, and demonstrated that diagonal trans-fixation could provide more rigidity in flexion and extension but less in lateral bending and axial rotation in comparison with horizontal trans-fixation in vitro [52,53].
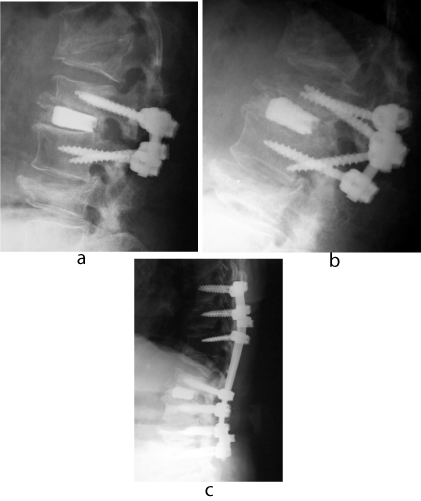
Figure 5: A 80-year-old woman with lumbar canal stenosis at L2/3. A lateral plain X-ray immediately after PLIF with PS &titanium cages at L2/3 (a).New vertebral fracture in L2 and a PS backout developed 6 weeks after the initial surgery (b). A lateral plain X-ray 9 months after the salvage surgery, with intermittent administration of teriparatide, showing a definitive bone fusion at L2/3 (c)
Insertional torque and new trajectories: Measurement of insertional torque of PS both in vitro and in vivo is very simple and reliable to evaluate PS stability. In 2000, we investigated intraoperative insertional torque of PS in 62 consecutive patients, whose mean age at the surgery was 58 (34-74), with lumbar degenerative disorders. In the study, a high correlation between the insertional torque of PS and lumbar BMD of the patients was found (P<0.01), but it could not objectively predict loosening of PS in our clinical setting [54]. In 2010, Deckelmann et al. studied “cut-out” failure of PS in cadavers, and suggested that intraoperative transpedicle measurement of peak breakaway torque was technically feasible and predicted reliable local bone strength and implant failure for dorsal spinal instrumentations [55]. Moreover, Lee et al. have also confirmed that the insertional torque of a PS had a positive correlation with BMD in 181 patients with degenerative lumbar diseases in 2012. In their finding, the predictive torque (Nm) generated during PS insertion was [-0.127 + 1.62 X (BMD at the corresponding lumbar vertebrae)] in vivo [56]. Nonetheless, it is still controversial whether the insertional torque of PS is an excellent and objective predictor of PS loosening and related failure at the moment. It mainly attributes to PS design differences among the studies. In 2009, Santoni et al. proposed CBT of PS as an alternative procedure to the conventional PS. They performed a cadaveric study, based on the insertional torque and pullout test, and have concluded a 30% increase in failure load of CBT screw in uniaxial pullout. Its juxtaposition to higher quality bone justified its use in patients with poor trabecular bone quality [57]. For sure, CBT is a novel PS technique in the lumbar spine. Matsukawa et al. have analyzed insertional torque of CBT during operation in vivo. According to their results, the insertional torque of a screw in CBT was 1.7 times higher than the conventional PS, and they have concluded that CBT of PS has a possibility that its stability is better than the conventional PS, especially in the osteoporotic spine [58]. But the mechanical stability of CBT screws in a constructed unit, such as in conjunction with PLIF, has not yet been disclosed. A further detailed study should be done.
Improvement of vertebral bone strength: To achieve solid bone fusion and avoid implant failure of PLIF with PS&IBD, it is very significant to increase bone strength of the vertebra as well as the implant materials and design adjustment in the osteoporotic spine. For treatment of osteoporosis, bisphosphonate is widely used to increase BMD and prevent vertebral fracture in clinical cases. Bisphosphonate mainly inhibits osteoclast-mediated bone absorption. An efficacy of bisphosphonate on the spinal fusion was also studied in some animal models. In 2005, Huang et al. reported rats that underwent alendronate treatment had radiologically larger and denser fusion masses than those of the controlled rats in spite of lower fusion rates [59]. In 2007, Bransford et al. also investigated an effect of zoledronic acid in an L6/7 rabbit spine fusion model [60]. Contrary to bisphosphonate, hormones PTH increase the formation of bone by stimulating osteoblastic activity. In 2012, Nakamura et al. announced that human PTH administered once per week reduced new vertebral fractures in subjects with primary osteoporosis and fracture risks [61]. Ten years before this announcement, Skripitz and Aspenberg had already reported that intermittent administration of PTH (1-34) significantly increased the mean pull-out strength of stainless-steel screws from 66 to 145N and the removal torque of it from 1.1 to 3.5 Ncm in the proximal tibia of rats. They concluded that intermittent treatment with PTH enhanced the early fixation of orthopedic implants [62]. In particular, PTH has a potential to selectively stimulate bone formation at the operated or fractured site. Hence, intermittent PTH administration has another possibility to increase excellent bone fusion and, as a result, avoid implant failure of PLIF with PS&IBD in the osteoporotic spine. In 2013, Ohtani et al, compared teriparatide and bisphosphonate treatment to reduce PS loosening in postmenopausal women with degenerative spondylolisthesis, and concluded that the incidence of PS loosening in the teriparatide group was significantly lower than that of the risedronate or the control group (p<0.05), even though their fusion method was PLF, without trying PLIF [63]. Currently, we have also been studying the effectiveness of teriparatide and bisphosphonate treatment after multi-segmental PLIF with PS &IBD. In our data, PS loosening has developed in 8.4% (17/204 screws) of the periparatide group, and in 24.3% (49/202 screws) of the bisphosphonate group. The difference was significant (P<0.01). In addition, sclerotic change around PS loosening on the X-rays was observed in 29.4% (5/17screws) of the teriparatide group, and in 91.8% (45/49 screws) of the bisphosphonate group. The difference was also significant (P<0.01) [64]. These studies suggest that intermittent administration of human PTH has a higher possibility to improve bone quality of the vertebrate, and enhance bone fusion in PLIF with PS&IBD (Figure 5). Further detailed investigations in numerous clinical cases are anticipated.
To minimize PJF(K)
PJF(K) is a pathological kyphosis adjacent to the instrumentated segments [29]. Schairer et al. retrospectively reviewed 836 adult cases who had fusion surgery for spinal deformity, and reported that 51.9 % (14/27) of the patients were re-admitted due to PJK within 90 days from surgery [65]. This rate seems to be a very high percentage compared to other surgical complications. As I mentioned before in the current review, it is biomechanically proven that the stress concentration on the most upper and adjacent vertebra to the fused segment is a critical factor that develops PJF(K) after instrumentation surgery. Further risk factors of PJF(K) have been pointed out. Kim et al., Helgeson et al. and Yagi et al. documented rigidity of the implant, all PS fixation, inclusion of the sacrum and extreme corrective force while surgery as extrinsic risk factors [66-68]. Meanwhile, Kim et al., Dewald et al. and Watanabe et al. also mentioned patients aged more than 55 years with poor bone quality and obesity as intrinsic factors [69-71]. Based on these studies, a detailed numerous analysis article about PJF(K) was proposed by the Department of Mechanical Engineering, Quebec in 2014. Cammarata et al. have performed biomechanical analysis of PJK through computer simulations and sensitivity tests. They have developed and validated spine models of 6 patients with adult scoliosis, and performed 576 simulations. In their conclusions, they have recommended preserving more posterior intervertebral elements above the upper instrumented vertebra, using the transverse process hook at the upper instrumented vertebra instead of PS, using tapered transition rods (if manufacturing were possible), and decreasing the sagittal preoperative rod curvature to reduce patho-mechanisms of PJK [72]. Their conclusions are very suggestive for spinal surgeons to minimize the risks of PJF(K) in osteoporotic patients who are supposed to be treated by multi-segmental PLIF with PS&IBD.
In the future
An intriguing article was published by the American Board of Orthopedic Surgery database in 2014. The study disclosed that the rate of cases with degenerative spondylolisthesis treated by posterior-approaching interbody fusion techniques increased significantly throughout the study period (2009-2011) from 13.6% to 32% (p<0.001). Why is this? The authors do not exactly explain the reasons of the tendency, and the article is unfortunately rated evidence level 4 [73]. From my perspective, the greatest advantage of PLIF with PS&IBD is that it could safely provide orthopedic surgeons complete neural decompression, 3-D correction of the deformity, and immediate, excellent stability of the fixed segments through a posterior approach alone. This merit is probably the main reason for a preference of surgeons utilizing PLIF with PS&IBD. Surgical fusion is an essential method to stabilize the lumbar spine. The procedure includes anterior lumbar interbody fusion, PLF, PLIF and PLIF plus PF. Controversy exists about what the optimum fusion procedure is. Scientific evidence to support the preference of PLIF is still lacking. In meta-analyses from seventeen comparative studies by Liu et al., it was found that moderate-quality evidence showed PLIF was more effective than PLF for the improvement of clinical satisfaction, postoperative back pain, fusion rate and for the reduced complication rate on the basis of four relevant randomized controlled trials and six observational studies. They have also stated that PLIF may be better than PLF and PLIF plus PLF methods in the treatment of lumbar spondylolisthesis, although the conclusions need to be treated with caution due to a lack of high quality evidence [74]. In the latest decade, LLIF, XLIF® or DLIF have been invited as new surgical maneuvers for degenerative disorders, but the comparative effectiveness and safety of LLIF/XLIF®/DLIF versus PLIF has not been proven, including cost-effectiveness and indications [75]. In addition to these issues, prolonged exposure to “low-level” radiation as an occupational risk in LLIF/XLIF®/DLIF remains a concern for medical personnel as much as minimally invasive PLIF [76]. Unfortunately, other surgical techniques superior to PLIF with PS&IBD could not be discovered at the present time.
The proportion of elderly people has been increasing in our globe. Out of all the advanced countries, Japan, in terms of the aging society phenomenon, is at the center of this problem. As of 2014,the rate of people over 65 years and 75 years is 25.7 % and 12.5%, respectively. Moreover, the rate of people over 65 years is rapidly increasing and estimated to rise to 33.4% by 2034, even though the total population of Japan is going to naturally decrease. This is unsurprising data, and one we must confront. Undoubtedly, the current situation of Japan is bound to develop in other countries in the near future. In 2009, based on the date of BMD in the lumbar spine, Yoshimura et al, have calculated that 970,000 (160.000 males, 810.000 females) people from 40 to 79 years old would annually develop osteoporosis in Japan [77]. Even though some extent of racial differences is present, similar incidences of osteoporosis can be expected in other countries. Chin et al, also have reported that the incidence of osteoporosis among patients older than 50 years, who required spine surgery, was 14.5% in men and 51.3% in women [78]. The elderly population does not uncommonly have spinal degenerative and traumatic disorders with symptomatic 3-D deformity and instability. Thereby, orthopedic surgeons have to indicate PLIF with PS&IBD as a surgical intervention to treat these disorders more frequently in the aging societies of the future. As the previously reviewed excerpts have shown, numerous orthopedic surgeons and researchers are endeavoring to improve PLIF with PS&IBD in the osteoporotic spine. In my view, PLIF with PS&IBD will continue to be a mainstay of the lumbar fusion surgery if the proportion of elderly people continues to grow. Finally, I would like to pay great respect to the authors and co-workers whose research and findings I have cited in this article.
Conclusion
Numerous spinal surgeons and researchers are endeavoring to minimize the complications of PLIF with PS&IBD related to osteoporosis. The current review article demonstrates that material and design change of implants, augmentation and novel techniques of PLIF with PS&IBD, and an increase of vertebral bone strength have high potentialities to improve the clinical results, and to reduce its complications in the osteoporotic spine. In the near future, orthopedic surgeons will have more opportunities to indicate PLIF with PS&IBD for treatment of lumbar degenerative and traumatic disorders associated with osteoporosis. In my view, PLIF with PS&IBD will continue to be a mainstay of lumbar fusion surgery even in increasingly aging societies.
References
- Capener N. Spondylolisthesis. Br J Surg. 1932; 19: 374-386.
- Briggs H, Milligan PR, Orange E, Jersey N. Chip fusion of the low back following exploration of the spinal canal. J Bone Joint Surg [Am]. 1944; 26: 125-130.
- Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953; 10: 154-168.
- Roy-Camille R, Roy-Camille M, Demeulenaere C. [Osteosynthesis of dorsal, lumbar, and lumbosacral spine with metallic plates screwed into vertebral pedicles and articular apophyses]. Presse Med. 1970; 78: 1447-1448.
- Roy-Camille R, Saillant G, Mazel C. Plating of thoracic, thoracolumbar, and lumbar injuries with pedicle screw plates. Orthop Clin North Am. 1986; 17: 147-159.
- Steffee AD, Sitkowski DJ. Posterior lumbar interbody fusion and plates. Clin Orthop Relat Res. 1988; 227: 99-102.
- Shimizu K, Iwasaki R, Matsushita M, Yamamuro T. Posterior lumbar interbody fusion using AW-GC vertebral spacer. Yamamuro T, Kokubo T, Nakamura T, editors. In: Bioceramics 5. Kobunshi Kankokai. 1992; 435-441.
- Ido K, Asada Y, Sakamoto T, Hayashi R, Kuriyama S. Use of an autologous cortical bone graft sandwiched between two intervertebral spacers in posterior lumbar interbody fusion. Neurosurg Rev. 2000; 24: 119-122.
- Kroppenstedt S, Gulde M, Schönmayr R. Radiological comparison of instrumented posterior lumbar interbody fusion with one or two closed-box plasmapore coated titanium cages: follow-up study over more than seven years. Spine (Phila Pa 1976). 2008; 33: 2083-2088.
- Kanayama M, Cunningham BW, Haggerty CJ, Abumi K, Kaneda K, McAfee PC. In vitro biomechanical investigation of the stability and stress-shielding effect of lumbar interbody fusion devices. J Neurosurg. 2000; 93: 259-265.
- Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989; 3: 192-195.
- Fernyhough JC, Schimandle JJ, Weigel MC, Edwards CC, Levine AM. Chronic donor site pain complicating bone graft harvesting from the posterior iliac crest for spinal fusion. Spine (Phila Pa 1976). 1992; 17: 1474-1480.
- Hashimoto T, Shigenobu K, Kanayama M, Harada M, Oha F, Ohkoshi Y, et al. Clinical results of single-level posterior lumbar interbody fusion using the Branigan I/F carbon cage filled with a mixture of local morselized bone and bioactive ceramic granules. Spine. 2002; 27: 258-262.
- Miura Y, Imagama S, Yoda M, Mitsuguchi H, Kachi H. Is local bone viable as a source of bone graft in posterior lumbar interbody fusion? Spine (Phila Pa 1976). 2003; 28: 2386-2389.
- Okuyama K, Kido T, Unoki E, Chiba M. PLIF with a titanium cage and excised facet joint bone for degenerative spondylolisthesis--in augmentation with a pedicle screw. J Spinal Disord Tech. 2007; 20: 53-59.
- Fraser RD. Interbody, posterior, and combined lumbar fusions. Spine (Phila Pa 1976). 1995; 20: 167S-177S.
- Hosono N, Nametaka M, Makino T, Miwa T, Kaito T, Kaneko N, et al. Perioperative complications of primary posterior lumbar interbody fusion for nonisthmic spondylolisthesis: analysis of risk factors. J Neurosurg Spine. 2008; 9: 403-407.
- Okuyama K, Abe E, Suzuki T, Tamura Y, Chiba M, Sato K. Posterior lumbar interbody fusion: a retrospective study of complications after facet joint excision and pedicle screw fixation in 148 cases. Acta Orthop Scand. 1999; 70: 329-334.
- Law M, Tencer AF, Anderson PA. Caudo-cephalad loading of pedicle screws: mechanisms of loosening and methods of augmentation. Spine (Phila Pa 1976). 1993; 18: 2438-2443.
- Esses SI, Sachs BL, Dreyzin V. Complications associated with the technique of pedicle screw fixation. A selected survey of ABS members. Spine (Phila Pa 1976). 1993; 18: 2231-2238.
- Krag MH, Beynnon BD, Pope MH, Frymoyer JW, Haugh LD, Weaver DL. An internal fixator for posterior application to short segments of the thoracic, lumbar, or lumbosacral spine. Design and testing. Clin Orthop Relat Res. 1986; : 75-98.
- Krag MH. Biomechanics of thoracolumbar spinal fixation. A review. Spine (Phila Pa 1976). 1991; 16: S84-99.
- Coe JD, Warden KE, Engr MB, Herzig MA, McAfee PC. Influence of bone mineral density on the fixation of thoracolumbar implants: A comparative study of transpedicular screws, laminar hooks, and spinous process wires. Spine. 1990; 15: 902-907.
- Wittenberg RH, Shea M, Swartz DE, Lee KS, White AA 3rd, Hayes WC. Importance of bone mineral density in instrumented spine fusions. Spine (Phila Pa 1976). 1991; 16: 647-652.
- Carlson GD, Abitbol JJ, Anderson DR, Krag MH, Kostuik JP, Woo SL, et al. Screw fixation in the human sacrum. An in vitro study of the biomechanics of fixation. Spine (Phila Pa 1976). 1992; 17: S196-203.
- Yamagata M, Kitahara H, Minami S, Takahashi K, Isobe K, Moriya H, et al. Mechanical stability of the pedicle screw fixation systems for the lumbar spine. Spine (Phila Pa 1976). 1992; 17: S51-54.
- Okuyama K, Sato K, Abe E, Inaba H, Shimada Y, Murai H. Stability of transpedicle screwing for the osteoporotic spine. An in vitro study of the mechanical stability. Spine (Phila Pa 1976). 1993; 18: 2240-2245.
- Okuyama K, Abe E, Suzuki T, Tamura Y, Chiba M, Sato K. Influence of bone mineral density on pedicle screw fixation: A study of pedicle screw fixation augmenting posterior lumbar interbody fusion in elderly patients. Spine J. 2001; 1: 402-407.
- Glattes RC, Bridwell KH, Lenke LG, Kim YJ, Rinella A, Edward C. Proximal junctional kyphosis in adult spinal deformity following long instrumented posterior spinal fusion. Incidence, outcomes, and risk factor analysis. Spine. 2005; 30: 1643-1649.
- Miyakoshi N, Abe E, Shimada Y, Okuyama K, Suzuki T, Sato K. Outcome of one-level posterior lumbar interbody fusion for spondylolisthesis and postoperative intervertebral disc degeneration adjacent to the fusion. Spine (Phila Pa 1976). 2000; 25: 1837-1842.
- Oda I, Abumi K, Yu BS, Sudo H, Minami A. Types of spinal instability that require interbody support in posterior lumbar reconstruction: an in vitro biomechanical investigation. Spine (Phila Pa 1976). 2003; 28: 1573-1580.
- Sudo H, Oda I, Abumi K, ItoM, Kotani Y, Mimami A. Biomechanical study on the effect of five different Lumbar reconstruction techniques on adjacent-level intradiscal pressure and lamina strain. J Neurosurg Spine. 2006; 5: 150-155.
- Wu SH, Li Y, Zhang YQ, Li XK, Yuan CF, Hao YL, et al. Porous titanium -6Aluminuim-4Vanadium cage has better osseointergration and less micromotion than a poly-ether-ether-ketone cage in sheep vertebral fusion. Artif Organs. 2013; 37: E191-E201.
- Lee JH, Jeon DW, Lee SJ, Chang BS, Lee CK. Fusion rates and subsidence of morselized local bone grafted in titanium cages in posterior lumbar interbody fusion using quantitative three-dimensional computed tomography
- Trouillier H, Birkenmaier C, Rauch A, Weiler C, Kauschke T, Refior HJ. Posterior lumbar interbody fusion (PLIF) with cages and local bone graft in the treatment of spinal stenosis. Acta Orthop Belg. 2006; 72: 460-466.
- Rousseau MA, Lazennec JY, Saillant G. Circumferential arthrodesis using PEEK cages at the lumbar spine. J Spinal Disord Tech. 2007; 20: 278-281.
- Jiya T, Smit T, Deddens J, Mullender M. Posterior lumbar interbodyfusion using nonresorbablepoly-ether-ether-ketone versus resorbablepoly-L-lactide-Co-D, L-lactidefusion devices. A prospective, randomized study to assess fusion and clinical outcome. Spine. 2009; 34: 233-237.
- Sinclair SK, Konz GJ, Dawson JM, Epperson RT, Bloebaum RD. Host bone response to polyetheretherketone versus porous tantalum implants for cervical spinal fusion in a goat model. Spine (Phila Pa 1976). 2012; 37: E571-580.
- Rohner B, Wieling R, Magerl F, Schneider E, Steiner A. Performance of a composite flow moulded carbon fibre reinforced osteosynthesis plate. Vet Comp Orthop Traumatol. 2005; 18: 175-182.
- Nakahara I, Takao M, Goto T, Ohtsuki C, Hibino S, Sugano N. Interfacial shear strength of bioactive-coated carbon fiber reinforced polyetheretherketone after in vivo implantation. J Orthop Res. 2012; 30: 1618-1625.
- Ponnappan RK, Serhan H, Zarda B, Patel R, Albert T, Vaccaro AR. Biomechanical evaluation and comparison of polyetheretherketone rod system to traditional titanium rod fixation. Spine J. 2009; 9: 263-267.
- Sha M, Guo Z, Fu J, Li J, Yuan CF, Shi L, et al. The effects of nail rigidity on fracture healing in rats with osteoporosis. Acta Orthop. 2009; 80: 135-138.
- Wang Z, Fu S, Wu ZX, Zhang Y, Lei W. Ti2448 pedicle screw system augmentation for posterior lumbar interbody fusion. Spine (Phila Pa 1976). 2013; 38: 2008-2015.
- Cho W, Cho SK, Wu C. The biomechanics of pedicle screw-based instrumentation. J Bone Joint Surg Br. 2010; 92: 1061-1065.
- Yazu M, Kin A, Kosaka R, Kinoshita M, Abe M. Efficacy of novel-concept pedicle screw fixation augmented with calcium phosphate cement in the osteoporotic spine. J Orthop Sci. 2005; 10: 56-61.
- Piñera AR, Duran C, Lopez B, Saez I, CorreiaE, Alvarez L. Instrumented lumbar arthrodesis in elderly patients: Prospective study using cannulated cemented pedicle screw instrumentation. Eur Spine J. 2011; 20: S408-S414.
- Lei W, Wu Z. Biomechanical evaluation of an expansive pedicle screw in calf vertebrae. Eur Spine J. 2006; 15: 321-326.
- Wu ZX, Gong FT, Liu L, Ma ZS, Zhang Y, Zhao X, et al. A comparative study on screw loosening in osteoporotic lumbar spine fusion between expandable and conventional pedicle screws. Arch Orthop Trauma Surg. 2012; 132: 471-476.
- Cho W, Wu C, Zheng X, Erkan S, Suratwala SJ, Mehbod AA, et al. Is it safe to back out pedicle screws after augmentation with polymethyl methacrylate or calcium phosphate cement? A biomechanical study. J Spinal Disord Tech. 2011; 24: 276-279.
- Jacob AT, Ingalhalikar AV, Morgan JH, Channon S, Lim TH, Torner JC, et al. Biomechanical comparison of single- and dual-lead pedicle screws in cadaveric spine. J Neurosurg Spine. 2008; 8: 52-57.
- Suzuki T, Abe E, Okuyama K, Sato K. Improving the pullout strength of pedicle screws by screw coupling. J Spinal Disord. 2001; 14: 399-403.
- Lim TH, Eck JC, An HS, Hong JH, Ahn JY, You JW. Biomechanics of transfixation in pedicle screw instrumentation. Spine (Phila Pa 1976). 1996; 21: 2224-2229.
- Lim TH, Kim JG, Fujiwara A, Yoon TT, Lee SC, Ha JW, et al. Biomechanical evaluation of diagonal fixation in pedicle screw instrumentation. Spine (Phila Pa 1976). 2001; 26: 2498-2503.
- Okuyama K, Abe E, Suzuki T, Tamura Y, Chiba M, Sato K. Can insertional torque predict screw loosening and related failures? An in vivo study of pedicle screw fixation augmenting posteriorlumbar interbodyfusion. Spine. 2001; 25: 858-864.
- Deckelmann S, Schwyn R, Van der Pol B, Windolf M, Heini PF, Benneker LM. DensiProbe Spine: a novel instrument for intraoperative measurement of bone density in transpedicular screw fixation. Spine (Phila Pa 1976). 2010; 35: 607-612.
- Lee JH, Lee JH, Park JW, Shin YH. The insertional torque of a pedicle screw has a positive correlation with bone mineral density in posterior lumbar pedicle screw fixation. J Bone Joint Surg Br. 2012; 94: 93-97.
- Santoni BG, Hynes RA, McGilvray KC, Rodriguez-Canessa G, Lyons AS, Henson MA, et al. Cortical bone trajectory for lumbar pedicle screws. Spine J. 2009; 9: 366-373.
- Matsukawa K, Yato Y, Kato T, Imabayashi H, Asazuma T, Nemoto K. In vivo analysis of insertional torque during pedicle screwing using cortical bone trajectory technique. Spine (Phila Pa 1976). 2014; 39: E240-245.
- Huang RC, Khan SN, Sandhu HS, Metzl JA, Cammisa FP Jr, Zheng F, et al. Alendronate inhibits spine fusion in a rat model. Spine (Phila Pa 1976). 2005; 30: 2516-2522.
- Bransford R, Goergens E, Briody J, Amanat N, Cree A, Little D. Effect of zoledronic acid in an L6-L7 rabbit spine fusion model. Eur Spine J. 2007; 16: 557-562.
- Nakamura T, Sugimoto T, Nakano T, KishimotoH, Ito M, Fukunaga M, et al. Randomized teriparatide [human parathyroid hormone(PTH)1-34] once-weekly efficacy research(TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracturerisk. J Clin Endocrinol Metab. 2012; 97: 3097-3106.
- Skripitz R, Aspenberg P. Implant fixation enhanced by intermittent treatment with parathyroid hormone. J Bone Joint Surg Br. 2001; 83: 437-440.
- Ohtori S, Inoue G, Orita S, Yamauchi K, Eguchi Y, Ochiai N, et al. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine. 2013; 38: E487-E 492.
- Miyakoshi N, Okuyama K, Kudo D, Tsuchie, H, Hongo M, Kasukawa Y, et al. Novel clinical applications of anti-osteoporotic agents for fracture healing and orthopedic surgery. Osteoporosis Jpn. 2014 ; 22 : 14-18 ( in Japanese).
- Schairer WW, Carrer A, Deviren V, Hu SS, Takemoto S, Mummaneni P, et al. Hospital readmission after spine fusion for adult spinal deformity. Spine (Phila Pa 1976). 2013; 38: 1681-1689.
- Kim YJ, Bridwell KH, Lenke LG, Kim J, Cho SK. Proximal junctional kyphosis in adolescent idiopathic scoliosis following segmental posterior spinal instrumentation and fusion: minimum 5-year follow-up. Spine (Phila Pa 1976). 2005; 30: 2045-2050.
- Helgeson MD, Shah SA, Newton PO, Clements DH 3rd, Betz RR, Marks MC, et al; Harms Study Group. Evaluation of proximal junctional kyphosis in adolescent idiopathic scoliosis following pedicle screw, hook, or hybrid instrumentation. Spine (Phila Pa 1976). 2010; 35: 177-181.
- Yagi M, King AB, Boachie-Adjei O. Incidence, risk factors, and natural course of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Minimum 5 years of follow-up. Spine (Phila Pa 1976). 2012; 37: 1479-1489.
- Kim YJ, Bridwell KH, Lenke LG, Glattes CR, Rhim S, Cheh G. Proximal junctional kyphosis in adult spinal deformity after segmental posterior spinal instrumentation and fusion: minimum five-year follow-up. Spine (Phila Pa 1976). 2008; 33: 2179-2184.
- DeWald CJ, Stanley T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65: surgical considerations and treatment options in patients with poor bone quality. Spine (Phila Pa 1976). 2006; 31: S144-151.
- Watanabe K, Lenke LG, Bridwell KH, Kim YJ, Koester L, Hensley M. Proximal junctional vertebral fracture in adults after spinal deformity surgery using pedicle screw constructs: analysis of morphological features. Spine (Phila Pa 1976). 2010; 35: 138-145.
- Cammarata M, Aubin CÉ, Wang X, Mac-Thiong JM. Biomechanical risk factors for proximal junctional kyphosis: a detailed numerical analysis of surgical instrumentation variables. Spine (Phila Pa 1976). 2014; 39: E500-507.
- Kepler CK, Vaccaro AR, Hilibrand AS, Anderson DG, Rihn JA, Albert TJ, et al. National trends in the use of fusion techniques to treat degenerative spondylolisthesis. Spine (Phila Pa 1976). 2014; 39: 1584-1589.
- Liu XY, Qui GX, Weng XS, Yu B, Wang YP. What is optimum fusion technique for adult spondylolisthesis-PLIF or PLF or PLIF plus PLF? A meta-analysis from 17 comparative studies. Spine. 2014; 39: 1887-1898.
- Barbagallo GM, Albanese V, Raich AL, Dettori JR, Sherry N, Balsano M. Lumbar Lateral Interbody Fusion (LLIF): Comparative Effectiveness and Safety versus PLIF/TLIF and Predictive Factors Affecting LLIF Outcome. Evid Based Spine Care J. 2014; 5: 28-37.
- Taher F, Hughes AP, Sama AA, Zeldin R, Schneider R, Holodny EI, et al. 2013 Young Investigator Award winner: how safe is lateral lumbar interbody fusion for the surgeon? A prospective in vivo radiation exposure study. Spine (Phila Pa 1976). 2013; 38: 1386-1392.
- Yoshimura N, Muraki S, Oka H, Mabuchi A, Kinoshita H, Yosihda M, et al. Epidemiology of lumbar osteoporosis and osteoarthritis and their causal relationship--is osteoarthritis a predictor for osteoporosis or vice versa?: the Miyama study. Osteoporos Int. 2009; 20: 999-1008.
- Chin DK, Park JY, Yoon YS, Kuh SU, Jin BH, Kim KS, et al. Prevalence of osteoporosis in patients requiring spine surgery: incidence and significance of osteoporosis in spine disease. Osteoporos Int. 2007; 18: 1219-1224.