
Special Article- Knee Arthroplasty
Austin J Musculoskelet Disord. 2017; 4(1): 1038.
Failure by Immune Reaction to Metal Debris in Total Joint Arthroplasty of Hip and Knee
Boddu C1*, Nett MP2, Cushner FD3 and Scuderi GR4
1Clinical Fellow, NSLIJ Adult Reconstruction/Knee, Lenox hill hospital, USA
2Orthopedic Service Line, Northshore Long Island Jewish Health System, USA
3Chief of Orthopedics-Southside Hospital, USA
4Program Director, Orthopedic Service Line, USA
*Corresponding author: Chandrakanth Boddu, Clinical Fellow, NSLIJ Adult Reconstruction/Knee, Lenox hill hospital, USA
Received: November 03, 2016; Accepted: January 12, 2017; Published: January 13, 2017
Abstract
Background: We propose a clinical classification for Failure by Immune Reaction to Metal debris (FIRM) in Total Joint Arthroplasty (TJA) of hip and knee. We also developed an evidence based diagnostic scoring system and estimated the treatment threshold for FIRM.
Methods: Pub Med and Embase search engines were used to identify original articles. We classified FIRM and identified the individual diagnostic criteria for each type of FIRM. For each individual diagnostic criterion we estimated the pooled Diagnostic Odds Ratio (DOR+) and the individual discriminatory FIRM (id FIRM) scores. From these scores total discriminatory FIRM (TdFIRM) scores were calculated.
Results: We identified a total of 39 original articles for meta-analysis. Based on predominant symptom of clinical presentation, we classified FIRM in to two types i.e., FIRMtype1 and FIRMtype2. We identified 8 individual diagnostic criteria for FIRMtype1 and FIRMtype2 and for each type we estimated the pooled diagnostic odds ratio (DOR+) and the individual discriminatory FIRM (id FIRM) scores. TdFIRM score scores for FIRMtype1 and FIRMtype2 were 4.83 and 4.85 respectively. Treatment threshold for FIRMtype1 and FIRMtype2 were estimated to be 3.38 and 3.39 respectively.
Conclusion: This meta-analysis provides a clinically useful tool for decision making when evaluating a patient suspected to have developed clinical complication by immune reaction to metal debris from arthroplasty. Future studies on FIRM should utilize this scoring system in decision making and critically evaluate its validity.
Keywords: Total joint arthroplasty; Osteolysis; Failure by immune reaction to metal debris; Metal allergy; Metal hypersensitivity
Introduction
The terms metal allergy and metal hypersensitivity are used interchangeably in the current literature to describe a spectrum of complications caused by immune reaction to metal debris generated in total joint arthroplasty. At one end of the spectrum is dermatitis. The dermatitis caused by immune reaction to metal debris may be new in onset or exacerbation of previous lesions and, can be localized or generalized manifestation.
At the other end of the spectrum is a consequence of local immune reaction to metal debris generated by total joint arthroplasty presenting clinically as a variable combination of pain in the joint, cystic lesions around the joint, aseptic loosening of implants and instability of the joint. Various terminologies exist to describe histopathological and imaging findings in patients with pain in the joint suspected to be from local reaction to metal debris after total joint arthroplasty. Willert et al termed the histopathological findings in these patients as Aseptic Lymphocyte-dominated Vasculitis-Associated Lesion (ALVAL) and LymphocYte-Dominated Immunological Answer (LYDIA) [1]. Another histopathological term ‘Metallosis’ was used by Korovessis et al to describe similar findings [2]. Pandit et al used the term ‘Pseudotumour’ to describe cystic and solid masses associated with resurfacing devices [3]. Longton et al described an umbrella term Adverse Reaction to Metal Debris (ARMD) to describe clinical failures of hip joint arthroplasty presenting with pain, a large sterile effusion and/or macroscopic necrosis [4].
For the purpose of this study, we used the acronym ‘FIRM’ (Failure by Immune Reaction to Metal debris) to describe all the clinical failures of Total Joint Arthroplasty (TJA) in hip and also knee joint due to immune reaction to metal debris. The objectives of this study are twofold. One is to develop a classification system for FIRM in TJA of hip and knee that is based on distinctive clinical manifestations. Second is to develop a diagnostic scoring system to facilitate the clinician to make an accurate, evidence based diagnosis.
Materials and Methods
In January 2015, a Pub Med and Embase search was performed independently using two search terms ‘Metal Allergy Joint Replacement’ (method 1) and ‘Metal Hypersensitivity Joint Replacement’ (method 2) for record identification. The search term that yielded the maximum number of results among the two was used for records identification from that particular search engine. The duplicates among Pub Med and Embase search were identified and removed. The selected records were screened by reviewing the abstract for clinical data on TJA patients with FIRM and were included for full-text review. If abstract is not available for a record, it was selected for full-text review by default. Of all the records selected for full-text review, final analysis included only the articles with clinical data on TJA patients with FIRM who had a negative work-up for the possibility of infection or neoplasm (primary or metastatic) and could successfully be managed by removal of implants and reinsertion of new implants that generate no metal debris or by pseudoarthrosis (positive control). In addition, we looked carefully to identify such additional records which we may have missed during Pub Med or Embase search but have the data that we are interested in by reviewing the references section of all the full-text reviewed records and if such record is found, was included in the systematic review and meta analysis.
Part I: FIRM clinical classification
By systematic review of the articles selected for final analysis, we identified all the described complications due to FIRM in patients with TJA and grouped them depending on the most commonly observed patterns of clinical presentation.
Part II: FIRM diagnostic scoring system
A meta-analysis was performed to develop FIRM scoring system using the data from the full-text reviewed records. The first step was calculation of pooled diagnostic odds ratio (also called likelihood ratio) of all the individual diagnostic criteria for each defined type of FIRMtype 1 to n using the formula,
DOR+= (Most commonly observed result of a diagnostic criteria+0.5)/(Least commonly observed result of a diagnostic criteria+0.5).
Where, DOR+ is the pooled diagnostic odds ratio of a positive result. Addition of 0.5 to all counts is a validated statistical method to obtain a definable value of DOR+. Individual discriminatory FIRM score of each diagnostic criterion (idFIRMc1 to n) for FIRMtype 1 to n was derived by converting its linear DOR+ value in to logarithmic value.
If all the individual diagnostic criteria identified for FIRMtype 1 to n are perfectly independent of each other, the total discriminatory FIRM score of FIRMtype 1 to n that would determine the treatment threshold for revision surgery can be mathematically be expressed as,
tdFIRMtype 1 to n = idFIRMc1 + idFIRMc2 ……. + idFIRMcn
Where, tdFIRMtype 1 to n is the total discriminatory score of FIRMtype1 to n (in logarithmic scale), idFIRMc1 is the individual discriminatory FIRM score of a diagnostic criteria c1 for FIRMtype 1 to n (in logarithmic scale), and so on. If we found that the individual diagnostic criteria identified for FIRMtype 1 to n were partially independent of each other, we decided to fix the treatment threshold for revision surgery at 70% of the calculated tdFIRMtype1 to n for FIRMtype1 to n.
Results
When Pub Med and Embase were searched as per method 1 using the term ‘Metal Allergy Joint Replacement’ without applying any other filters, 195 and 76 articles were identified respectively. When a similar search was conducted as per method 2 using the term ‘Metal Hypersensitivity Joint Replacement’, 178 and 194 articles were identified in Pub Med and Embase respectively. The term that yielded maximum number of results among the two search terms was selected for record identification and abstract review. After removing the duplicates there were 334 articles available for screening of abstracts (Figure 1). When we included our unpublished record (MPN), we had a total of 335 records available for screening. We excluded 271 records among the final 335 that were screened, as they were not relevant to answer this study objective. We full text reviewed the remaining 68 records among the 335 records that were screened. Out of 68 records that were full text reviewed, 29 records were excluded for various reasons. Hence we identified a total of 39 original articles with data relevant to this study. During the process of full text review of these 39 records, we could not identify any additional original articles that were not previously identified by either Pub Med or Embase search but had the clinical data on FIRM in patients with TJA. Hence, we had a total of 39 articles available for meta-analysis.
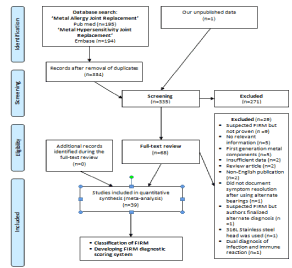
Figure 1: PRISMA Diagram showing the study flow from identification of original articles through meta-analysis.
Part I: FIRM clinical classification
Based on predominant symptom of clinical presentation, we classified FIRM in to two types i.e., FIRMtype1 and FIRMtype2 (Table 1). Further subclassification of cutaneous reaction was attempted based on the location of skin lesions i.e., localized or generalized. Similarly, subclassification of painful joint effusions was attempted based on further clinical qualifiers i.e., evidence of osteolysis or component loosening or dislocation.
FIRMType1
Presenting complication is predominantly dermatitis with minimal or no joint pain
FIRMtype1a
Localized cutaneous reaction (either new or exacerbation of previously existing lesion; located around the replaced joint or elsewhere)
FIRMtype1b
Generalized cutaneous reaction (either new or exacerbation of previously existing lesions)
FIRM Type 2: Presenting complication is predominantly painful joint effusion (with negative work-up of infection or neoplasm) with minimal or no dermatitis
FIRMtype2a
Well fixed implants without evidence of osteolysis/cystic lesions
FIRMtype2b
Well fixed implants with evidence of osteolysis/cystic lesions
FIRMtype2c
Aseptic loosening of component(s)
FIRMtype2d
Instability/Dislocation of the joint
Table 1: The clinical classification of total joint arthroplasty Failure by Immune Reaction to Metal ions (FIRM).
Part II: FIRM diagnostic scoring system
Nine among the 39 articles selected for final analysis had data on FIRMtype1 (Table 2). A total of 12 joints that developed FIRMtype1 were identified among these 9 articles. We identified 8 individual diagnostic criteria that were at least partially independent of each other. When we tested the statistical weight of these 8 individual diagnostic criteria, we identified that all are useful for diagnosis of FIRMtype1, but the contribution of each to the identified FIRMtype1 idFIRMc1 to 8 scores to tdFIRMtype1 was variable. The idFIRMc1 to 8 scores of FIRMtype1 are represented in descending of their statistical weight in (Table 3). The estimated tdFIRMtype1 score of the FIRMtype1 diagnostic criteria was 4.83. As the identified individual diagnostic criteria were partially independent, the treatment threshold for revision surgery was estimated to be 3.38 i.e., 70% of 4.83.
First Author (alphabetical order)
Sex
Joint
Articulation
Patch test
LTT/LAT/LFT
Serum (Co>19µg/L
Cr>17 µg/L)
SB/RLNB
Imaging XR/CT/MRI
♀
♂
THA
TKA
M
N
+
-
+
-
+
-
+
-
+
-
Bizzoto N [5]
0
1
1
0
1
0
1
0
NT
1
0
1
0
1
0
!Dietrich KA [6]
3
0
0
4
0
4
4
0
4
0
NT
NT
0
4
Erall MD [7]
1
0
1
0
1
0
NT
NT
NT
1
0
0
1
Handa S [8]
1
0
0
1
0
1
1
0
NT
1
0
NT
0
1
Gao X [9]
0
1
0
1
0
1
1
0
NT
1
0
1
0
0
1
Post ZD [10]
1
0
0
1
0
1
1
0
0
1
NT
NT
1
0
Thomsen M [11]
1
0
0
1
0
1
NT
0
1
NT
NT
0
1
Von Opstal N [12]
1
0
0
1
0
1
0
1
NT
NT
NT
1
0
Wong CC [13]
1
0
1
0
1
0
1
0
NT
1
0
1
0
0
1
DOR+
3.8
2.7
2.7
6.3
1.8
9
9
2.7
idFIRMtype1 score
0.58
0.43
0.43
0.80
0.26
0.95
0.95
0.43
LTT/LAT/LFT Lymphocyte Transformation Test(s)/Lymphocyte Activation Test(s)/ Lymphocyte Function Test(s); SB/RLNB: Skin Biopsy/ Regional Lymph Node Biopsy; XR/CT/MRI: X-rays/Computerized Tomography/Magnetic Resonance Imaging; THA: Total Hip Arthroplasty; TKA: Total Knee Arthroplasty; M: Hip or knee implant with Metal on Metal bearings or implants with Mores taper between two modular CoCr components
N Hip or knee implant with CoCr component but has no Metal on Metal bearings or Mores taper between two modular CoCr components; NT: Not Tested
! Only 3 patient’s gender was described among the 4 total reported cases.
Table 2: The results of meta-analysis of FIRMtype1 diagnostic criteria.
Diagnostic criteria(C1-8) of FIRMtype1
idFIRM scores
C1. Histopathology of skin/regional lymph node(s)
Positive
0.95
Negative
0
C2. Serum (Co>19µg/L or Cr>17µg/L)
Positive
0.95
Negative
0
C3. Skin patch test (Ni or Co or Cr)
Positive
0.80
Negative
0
C4. Sex
Female
058
Male
0
C5. Joint
Hip
0.43
Knee
0
C6. Articulation
M type!
0.43
N type@
0
C7. Imaging (XR/CT/MRI)
Positive
0.43
Negative
0
C8. LTT/LAT/LFT
Positive
0.26
Negative
0
tdFIRMtype1 score# =
!M type Hip or knee implant with Metal on Metal bearings or implants with Mores taper between two modular CoCr components; @N type: Hip or knee implant with CoCr component but has no Metal on Metal bearings or Mores taper between two modular CoCr components; LTT/LAT/LFT: Lymphocyte Transformation Test(s)/Lymphocyte Activation Test(s)/Lymphocyte Function Test(s); #: The recommended cutoff value for the treatment threshold is 3.38.
Table 3: The diagnostic scoring system for FIRMtype1 is represented below.
Thirty among the 39 articles selected for final analysis had data on FIRMtype2 (Table 4). A total of 177 joints that developed FIRMtype2 were identified among these 30 articles. We identified 8 individual diagnostic criteria that were partially independent of each other. When we tested the statistical weight of these 8 individual diagnostic criteria, with the exception of lymphocyte function testing, the rest were found to be useful for diagnostic purpose. The contribution of each to the identified FIRMtype2 idFIRMc1 to 7 scores to tdFIRMtype2 was also variable. Interestingly, the contribution of each to the identified FIRMtype1 idFIRMc1 to 8 scores to tdFIRMtype1 score was different from the contribution of FIRMtype2 idFIRMc1 to 7 scores to the tdFIRMtype2 score. The idFIRMc1 to 7 scores of FIRMtype2 are represented in descending of their statistical weight in (Table 5). The estimated tdFIRMtype2 score of the FIRMtype2 diagnostic criteria was 4.85. As the identified individual diagnostic criteria were partially independent, the treatment threshold for revision surgery was estimated to be 3.39 i.e., 70% of 4.85.
First Author (alphabetical order)
Sex
Joint
Articulation
Patch test (Co,Cr,Ni)
LTT/LAT
Serum (Co>19µg/L
Cr>17 µg/L)
HP
Imaging (XR/CT/MRI)
♀
♂
THA
TKA
M
N
+
-
+
-
+
-
+
-
+
-
Anand A [14]
0
1
0
1
0
1
1
0
NT
NT
1
0
1
0
Bergschmidt P [15]
1
0
0
1
0
1
1
0
NT
NT
1
0
0
1
Bernasek TL [16]
ND
2
0
2
0
NT
NT
1
1
1
1
0
2
Blumenfeld TJ [17]
1
0
1
0
1
0
NT
NT
NT
1
0
0
1
Campbell P [18]
4
1
5
0
5
0
NT
NT
NT
5
0
4
1
Carr AM [19]
2
1
3
0
3
0
NT
NT
NT
3
0
3
0
Engh CA Jr [20]
ND
1
0
1
0
NT
NT
NT
1
0
NT
Jensen P [21]
1
0
1
0
1
0
1
0
NT
NT
1
0
1
0
Kemp MA [22]
3
0
3
0
3
0
NT
NT
NT
3
0
3
0
!Kiran M [23]
1
1
2
0
2
0
2
0
NT
2
0
1
0
2
0
Korovessis P [2]
ND
9
0
9
0
NT
NT
NT
9
0
9
0
Kosukegawa [24]
1
0
1
0
1
0
NT
NT
NT
1
0
1
0
Kumar [25]
1
1
2
0
2
0
2
0
NT
1
0
1
0
2
0
Lohmann CH [26]
24
3
27
0
27
0
NT
NT
17
10
15
13
20
7
Mahendra G [27]
ND
30
0
30
0
NT
NT
NT
30
0
25
5
Melosev I [28]
ND
25
0
25
0
NT
NT
NT
13
4
16
9
Mikhael MM [29]
0
2
2
0
2
0
NT
1
0
0
1
1
0
0
2
Mc Master WC [30]
0
1
0
1
1
0
NT
0
1
1
0
1
0
0
1
@Nett MP
0
2
2
0
2
0
NT
NT
0
2
2
0
2
0
Pandit H
13
0
13
0
13
0
NT
NT
NT
13
0
10
3
Perumal V [31]
1
0
1
0
1
0
1
0
NT
NT
1
0
1
0
Rajpura A [32]
5
8
13
0
13
0
NT
NT
NT
13
0
5
8
Roessler PP [33]
0
1
1
0
1
0
1
0
NT
0
1
1
0
1
0
Singh C [34]
1
0
1
0
1
0
NT
NT
NT
1
0
1
0
Thakur RR [35]
4
1
0
5
0
5
2
2
NT
NT
5
0
2
3
Theruvil B [36]
3
0
3
0
3
0
NT
NT
NT
3
0
3
0
Thomas P [37]
8
8
16
0
16
0
10
6
8
8
NT
16
0
8
8
Toms AP [38]
0
1
1
0
1
0
NT
NT
NT
1
0
1
0
Viveganathan B [39]
1
0
0
1
0
1
1
0
NT
NT
NT
1
0
#Willert HG
9
10
19
0
19
0
NT
NT
NT
19
0
12
5
DOR+
2.3
21
21
2.3
1.0
1.5
8.2
2.6
idFIRMtype2 score
0.36
1.32
1.32
0.36
0
0.17
0.91
0.41
LTT/LAT/LFT: Lymphocyte Transformation Test(s)/Lymphocyte Activation Test(s)/Lymphocyte Function Test(s); HP: Histopathology of joint tissue; XR/CT/MRI: X-rays/Computerized Tomography/ Magnetic Resonance Imaging; THA: Total Hip Arthroplasty TKA Total Knee Arthroplasty; M: Hip or knee implant with Metal on Metal bearings or implants with Mores taper between two modular CoCr components; N: Hip or knee implant with CoCr component but has no Metal on Metal bearings or Mores taper between two modular CoCr components, NT: Not Tested; ND: No Data; !Of the three reported cases, one case not included because of dual diagnosis of infection and immune reaction to metal ions; @: Unpublished data of author (MPN); #: Histopathology finding were not reported in 2 among the total of 19 patients.
Table 4: The results of meta-analysis of FIRMtype2 diagnostic criteria.
Diagnostic criteria(C1-7) of FIRMtype2
idFIRM score
C1. Joint
Hip
1.32
Knee
0
C2. Articulation
M type!
1.32
N type@
0
C3. Histopathology from the joint tissue
Positive
0.91
Negative
0
C4. Imaging (XR/CT/MRI)
Positive
0.41
Negative
0
C5. Sex
Female
0.36
Male
0
C6. Skin patch test (Ni or Co or Cr)
Positive
0.36
Negative
0
C7 Serum (Co>19µg/L or Cr>17 µg/L)
Positive
0.17
Negative
0
tdFIRMtype2 score# =
!: M type Hip or knee implant with Metal on Metal bearings or implants with Mores taper between two modular CoCr components; @ N type: Hip or knee implant with CoCr component but has no Metal on Metal bearings or Mores taper between two modular CoCr components; LTT/LAT/LFT: Lymphocyte Transformation Test(s) /Lymphocyte Activation Test(s)/Lymphocyte Function Test(s); #: The recommended cutoff value for the treatment threshold is 3.39.
Table 5: The diagnostic scoring system for FIRMtype2 is represented below.
Type1 FIRM
Type2 FIRM
Principle stimulus
Ni
Co,Cr
Particle size
Sub micrometer
Sub nanometer
Sub micrometer
Joint tissue metal content
Not known


Joint metal ion level
Not known


Serum metal ion level



Interaction with APC
Extra cellular
Intracellular
Extra cellular
Pathway
Th17 inflammation
Th1 inflammation
Th17 inflammation
Helper T cells
Th17cell
Th1 cell
Th17 cell
Cytokine mediator
IL-17
INF-γ
IL-17
Effector cells
Activated CTLs
Activated Macrophage
Activated CTLs
Histological appearance
PVLI, DLI
Coagulative necrosis
PVLI, DLI
Clinical effect
Synovitis (=pain/effusion)
Dermatitis
Osteolysis (= pseudo tumors, aseptic loosening)
Synovitis (=pain/effusion)
Table 6: Etiology and pathogenesis of FIRM in patients with total joint arthroplasty.
Discussion
Significance of the study
This is the first proposed comprehensive classification system of FIRM in patients with either THA or TKA that identified and statistical weighed the strength of individual diagnostic criteria, developed of a clinically applicable diagnostic scoring system and estimated a threshold level for the revision surgery.
Concerns
There are three specific concerns that we should address in detail regarding the methodology used in this meta-analysis. First, is the use of DOR+ (also called Likelihood ratio) as a single indicator of a diagnostic criterion’s performance compared to the traditional paired indices of test validity such as predictive values. The attractive features of likelihood ratios that are not shared by the predictive values are applicability to specific patient rather than relate test results to populations, applicability across the disease frequencies and ability to combine the tests in order to refine the clinical judgment [40].
Second issue concerning the methodology is to provide an explanation for use of logarithmic scale than a more familiar linear scale. The best way to estimate the strength of a differentially used diagnostic criteria across the studies is to convert linear scores in to logarithmic scores so that, the plotted score-treatment threshold curve will be changed from hyperbolic to sigmoid curve where usually, between 25% to 75% of the maximum response, the relation between the diagnostic scoring system and the treatment threshold will be linear, so that a better understanding and interpretation is possible. In other words, if all the included studies for meta-analysis did used exactly the same set of diagnostic criteria for decision making in all their cases, there would have been no need to convert the linear scores into logarithmic scale.
Third and the most important issue concerning this scoring system is the selection of 70% of tdFIRM score as the treatment threshold. Nomogram created for the Bayes theorem indicates that an odds ratio of 10 to 1, 100 to 1 and 1000 to 1 are equivalent to a post-test probability of 50%, 75% and >95% respectively [41]. As the minimum required tdFIRMtype1 and tdFIRMtype2 score for treating threshold is considered 3.38 and 3.39 respectively, the odds of being correct is more than 2000 to 1 or a post-test probability of approximately 99%.
Clinical application
It is important to understand that though 7 out of 8 tested diagnostic criteria for FIRMtype1 and FIRMtype2 are the same, their contribution to their respective tdFIRM score is different based on the strength of the odds ratio of each individual diagnostic criteria. The gender of the patient, the joint under evaluation and availability of previous surgical records provides pre-test tdFIRM score for a patient under evaluation for FIRM. Depending on the pre-test tdFIRM score, this scoring system provides clinicians with flexibility to decide which among the 5 tests (Histopathology/Imaging/Path test/Serum ion level/Lymphocyte based tests) to perform to either rule in or rule out a possibility of reaching the treatment threshold value of tdFIRM. For example, a male patient with total knee arthroplasty with an N type implant under evaluation for suspected FIRMtype1 has a pre-test score of zero. To make a diagnosis of FIRMtype1 in him all the 5 tests need to be positive to cross the treatment threshold of 3.38. On the contrary, a female patient with total hip arthroplasty with an M type implant under evaluation for suspected FIRMtype2 has a pre-test score of 3. Hence, she would reach a treatment threshold of 3.39 if either histopathology or imaging is positive. If the imaging is negative for her and the clinician would like to reserve histopathology as the last resort for diagnosis as it is an operative procedure, treatment threshold can be reached with a positive patch test and positive serum metal ion levels.
Conclusion
This meta-analysis provides a clinically useful tool for decision making when evaluating a patient suspected to have developed clinical complication by immune reaction to metal debris from arthroplasty. Future studies on FIRM should utilize this scoring system in decision making and critically evaluate its validity.
References
- Willert HG, Buchhorn GH, Fayyazi A. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005; 87: 28.
- Korovessis P, Petsinis G, Repanti M. Metallosis after contemporary Metal-on-Metal total hip arthroplasty: Five to seven year follow up. J Bone Joint Surg Am. 2006; 88: 1183.
- Pandit H, Glyn-Jones S, McLardy-Smith P. Pseudotumour associated with metal-on-metal hip resurfacings. J Bone Joint Surg [Br]. 2008; 90: 847.
- Langton DJ, Jameson SS, Joyce TJ. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg (Br). 2010; 92: 38.
- Bizzotto N, Sandri A, Trivellin G. Chromium-Induced Diffuse Dermatitis and Lymph Node Involvement by Langerhans Cell Histiocytosis After Metal-on-Metal Hip Resurfacing. Br J Dermatol. 2014.
- Dietrich KA, Mazoochian F, Summer B. Intolerance reactions to knee arthroplasty in patients with nickel/cobalt allergy and disappearance of symptoms after revision surgery with titanium-based endoprostheses. J Dtsch Dermatol Ges. 2009; 7: 410.
- Earll MD, Earll PG, Rougeux RS. Wound drainage after metal-on-metal hip arthroplasty secondary to presumed delayed hypersensitivity reaction. J Arthroplasty. 2011; 26: 338.e5.
- Handa S, Dogra S, Prasad R. Metal sensitivity in a patient with a total knee replacement. Contact Dermatitis. 2003; 49: 259.
- Gao X, He RX, Yan SG. Dermatitis associated with chromium following total knee arthroplasty. J Arthroplasty. 2011; 26: 665.e13.
- Post ZD, Orozco FR, Ong AC. Metal sensitivity after TKA presenting with systemic dermatitis and hair loss. Orthopedics. 2013; 36: e525.
- Thomsen M, Rozak M, Thomas P. Pain in a chromium-allergic patient with total knee arthroplasty: disappearance of symptoms after revision with a special surface-coated TKA--a case report. Acta Orthop. 2011; 82: 386.
- Van Opstal N, Verheyden F. Revision of a tibial baseplate using a customized oxinium component in a case of suspected metal allergy. A case report. Acta Orthop Belg. 2011; 77: 691.
- Wong CC, Nixon RL. Systemic allergic dermatitis caused by cobalt and cobalt toxicity from a metal on a metal hip replacement. Contact Dermatitis. 2014; 71: 113.
- Anand A, McGlynn F, Jiranek W. Metal hypersensitivity: can it mimic infection? J Arthroplasty. 2009; 24: 826.e25.
- Bergschmidt P, Bader R, Mittelmeier W. Metal hypersensitivity in total knee arthroplasty: revision surgery using a ceramic femoral component - a case report. Knee. 2012; 19: 144.
- Bernasek TL, Polikandriotis JA, Levering MF, et al. Five- to ten-year outcomes for modular metal-on-metal total hip arthroplasty. J Arthroplasty. 2013; 28: 1231.
- Blumenfeld TJ, Bargar WL, Campbell PA. A painful metal-on-metal total hip arthroplasty: a diagnostic dilemma. J Arthroplasty. 2010; 25: 1168. e1.
- Campbell P, Shimmin A, Walter L, et al. Metal sensitivity as a cause of groin pain in metal-on-metal hip resurfacing. J Arthroplasty. 2008; 23: 1080.
- Carr AM, DeSteiger R. Osteolysis in patients with a metal-on-metal hip arthroplasty. ANZ J Surg. 2008; 78: 144.
- Engh CA Jr, Ho H, Engh CA. Metal-on-metal hip arthroplasty: does early clinical outcome justify the chance of an adverse local tissue reaction? Clin Orthop Relat Res. 2010; 468: 406.
- Jensen P, Thyssen JP, Retpen JB. Cobalt allergy and suspected aseptic lymphocyte-dominated vascular-associated lesion following total hip arthroplasty. Contact Dermatitis. 2009; 61: 238.
- Kemp MA, Mitra A, da Costa TM. Bearing exchange in the management of Pseudotumour. Ann R Coll Surg Engl. 2013; 95: 266.
- Kiran M, Boscainos PJ. Adverse reactions to metal debris in metal-on-polyethylene total hip arthroplasty using a titanium-molybdenum-zirconium-iron alloy stem. J Arthroplasty. 2015; 30: 277.
- Kosukegawa I, Nagoya S, Kaya M, et al. Revision total hip arthroplasty due to pain from hypersensitivity to cobalt-chromium in total hip arthroplasty. J Arthroplasty. 2011; 26: 978. e1.
- Kumar P, Bryan C, Bowler J. Fialed total knee prosthesis secondary to metal hypersensitivity. Orthopaedics. 1983; 6: 1459.
- Lohmann CH, Meyer H, Nuechtern JV. Periprosthetic tissue metal content but not serum metal content predicts the type of tissue response in failed small-diameter metal-on-metal total hip arthroplasties. J Bone Joint Surg Am. 2013; 95: 1561.
- Mahendra G, Pandit H, Kliskey K. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009; 80: 653.
- Milosev I, Trebse R, Kovac S, et al. Survivorship and retrieval analysis of Sikomet metal-on-metal total hip replacements at a mean of seven years. J Bone Joint Surg Am. 2006; 88: 1173.
- Mikhael MM, Hanssen AD, Sierra RJ. Failure of metal-on-metal total hip arthroplasty mimicking hip infection. A report of two cases. J Bone Joint Surg Am. 2009; 91: 443.
- McMaster WC, Patel J. Adverse local tissue response lesion of the knee associated with Morse taper corrosion. J Arthroplasty. 2013; 28: 375.e5.
- Perumal V, Alkire M, Swank ML. Unusual presentation of cobalt hypersensitivity in a patient with a metal-on-metal bearing in total hip arthroplasty. Am J Orthop (Belle Mead NJ). 2010; 39: E39.
- Rajpura A, Porter ML, Gambhir AK. Clinical experience of revision of metal on metal hip arthroplasty for aseptic lymphocyte dominated vasculitis associated lesions (ALVAL). Hip Int 2011; 21: 43.
- Roessler PP, Witt F, Efe T. Arthroprosthetic cobaltism and pseudotumour also occur in patients with small diameter femoral ball head metal-on-metal total hip arthroplasties. BMJ Case Rep. 2014.
- Singh C, Kaplan A, Pambuccian SE. Necrotic granulomatous pseudotumor following metal-on-metal hip arthroplasty: a potential mimic of sarcoma on fine needle aspiration cytology. Diagn Cytopathol. 2012; 40: E104.
- Thakur RR, Ast MP, McGraw M. Severe persistent synovitis after cobalt-chromium total knee arthroplasty requiring revision. Orthopedics. 2013; 36: e520.
- Theruvil B, Vasukutty N, Hancock N. Dislocation of large diameter metal-on-metal bearings an indicator of metal reaction? J Arthroplasty. 2011; 26: 832.
- Thomas P, Braathen LR, Dörig M. Increased metal allergy in patients with failed metal-on-metal hip arthroplasty and peri-implant T-lymphocytic inflammation. Allergy. 2009; 64: 1157.
- Toms AP, Nolan J, Barker T. Early failure of a Birmingham resurfacing hip replacement with lymphoreticular spread of metal debris: pre-operative diagnosis with MR. Br J Radiol. 2009; 82: 977.e87.
- Vivegananthan B, Shah R, Karuppiah AS. Metallosis in a total knee arthroplasty. BMJ case rep. 2014.
- Grimes DA, Schulz KF. Uses and abuses of screening tests. Lancet. 2002; 359: 881.
- Fagan TJ. Letter: nomogram for Bayes theorem. N Engl J Med. 1975; 293: 257.