
Special Article - Knee Arthroplasty
Austin J Musculoskelet Disord. 2017; 4(1): 1040.
Comparison of Local and Systemic Effects of Tranexamic Acid and Transcollation® in Total Knee Arthroplasty
Boddu C1*, Nett MP2, Cushner FD3 and Scuderi GR4
¹Clinical Fellow, NSLIJ Adult Reconstruction/Knee, Lenox hill hospital, USA
²Orthopedic Service Line, Northshore Long Island Jewish Health System, USA
³Chief of Orthopedics-Southside Hospital, USA
4Orthopedic Service Line, Northshore Long Island Jewish Health System
*Corresponding author: Chandrakanth Boddu, Clinical Fellow, NSLIJ Adult Reconstruction/Knee, Lenox hill hospital, USA
Received: November 11, 2016; Accepted: February 02, 2017; Published: February 06, 2017
Abstract
Background: Use of tranexamic acid (TXA) is a pharmacological intervention to achieve hemostasis during total knee arthroplasty (TKA). Transcollation® using Aquamantys® (radiofrequency bipolar hemostatic sealing device) is a physiological hemostatic sealing method to achieve hemostasis during TKA. The local and systemic postoperative effects of these two interventions were never compared in the total knee arthroplasty literature.
Methods: This is a retrospective cohort study. Two groups of patients who underwent unilateral TKAs were identified: the TXA group and the Aquamantys® group. There were 96 patients in the TXA group and 95 patients in the Aquamantys® group. The proportion of cases in the TXA group and Aquamantys® group that developed each individual clinically significant local and systemic effect postoperatively was calculated and the odds ratio of such an outcome among these two groups was analyzed.
Results: There was a clinically meaningful trend towards decreased odds of developing superficial surgical site infection requiring antibiotic therapy in the TXA group compared to Aquamantys® group. We also found that on postoperative day 1, the odds of developing fever >101°F is low in TXA group compared to Aquamantys® group.
Conclusions: This study provides preliminary clinical evidence that is suggestive of beneficiary effect of TXA on wound healing after TKA, specifically, the prevention of superficial surgical site infection.
Level of Evidence: Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
Keywords: Total Knee Arthroplasty; Tranexamic Acid; Transcollation®; Aquamantys®; Surgical Site Infection (SSI); Wound Healing
Introduction
In addition to meticulous surgical technique, at least one pharmacological and one physical intervention is available clinically to reduce perioperative blood loss in patients undergoing a total knee arthroplasty (TKA). The pharmacological intervention to decrease the blood loss during TKA is the use of tranexamic acid (TXA), a synthetic anti-fibrinolytic agent that reversibly blocks the lysine-binding sites of plasminogen and inhibits fibrinolysis [1]. The physical intervention to decrease the blood loss during TKA is the use of Transcollation® using Aquamantys®, a radiofrequency bipolar hemostatic sealing device that heats saline to just less than 100°C and causes coagulation and sealing of superficial tissues [2].
TXA is an effective method to reduce blood loss and transfusions in patients undergoing TKA [3,4]. The beneficial effect of Transcollation® using Aquamantys® or similar devices in reducing the perioperative blood loss has been well studied in orthopedic literature including spine surgery [5,6] and hip arthroplasty [7-10].
There are at least three reasons why this study was conducted. One, we did not come across any published literature on the effect of Transcollation® using Aquamantys® or similar devices in TKA.
Two, the local effects of either TXA or Aquamantys® in TKA was not studied, specifically, in terms of their effect on wound healing. Three, the systemic effects of using TXA and Aquamantys® in TKA were also not studied beyond their effect on blood loss.
Methods
This is a retrospective, therapeutic, cohort study. The time period during which the clinical data was collected is from Feb 2012 to Mar 2013. The study was conducted at two tertiary level care hospitals belonging to the same health care system where the senior author (FDC) had operating privileges. All the surgeries were performed by the senior author (FDC). All of the patients at one tertiary level care hospital received TXA during surgery during the study period were considered for inclusion in the ‘TXA group’. All of the patients at another tertiary level care hospital received Transcollation® using Aquamantys® during the surgery during the same study period were considered for inclusion in the ‘Aquamantys® group’.
We preliminarily identified a total of 97 patients who received TXA while performing TKA (Figure 1). Among these 97 patients, all except one patient had a follow up record for at least one year postoperatively. Excluding that one patient with a follow up for less than one year, the remaining 96 patients were included in the cohort ‘TXA group’.
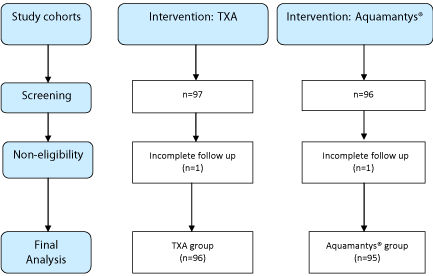
Figure 1: Flow diagram showing the steps of study design analyzing the local
and systemic effects of tranexamic acid (TXA) and Transcollation® in total
knee arthroplasty.
We preliminarily identified a total of 96 patients who underwent Transcollation® using Aquamantys® while performing TKA (Figure 1). Among these 96 patients, all except one patient had a follow up record for at least one year postoperatively. Excluding that one patient with a follow up for less than one year, the remaining 95 patients were included in the cohort ‘Aquamantys® group’.
All of the surgeries were performed with inflation of tourniquet before surgery and deflation after complete wound closure and application of sterile dressing and elastic compression bandage. The Legion/Genesis II posterior stabilized implant system (Smith & Nephew, Andover, MA) was used. All patients received the standard antibiotic prophylaxis as per the clinical practice guidelines developed jointly by the American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), the Surgical Infection Society (SIS), and the Society for Healthcare Epidemiology of America (SHEA) [11].
The surgical technique was the same in both TXA and the Aquamantys® groups. The standard TKA operative procedure was followed. Exposure was achieved by either medial parapatellar or midvastus approach [12]. Initially the operation included tibial preparation (resection of proximal surface), femoral preparation (distal surface resection, sizing of the femoral component, anteroposterior bone cuts and chamfer bone cuts) and removal of medial and lateral menisci and posterior osteophytes on the medial and lateral femoral condyle. Initial balancing was performed with spacer blocks and appropriate soft tissue releases. These steps were followed by the final preparation of the tibia (sizing of tibial component, reaming and broaching of the proximal tibia to accommodate the actual tibial component), femur (box cut for the posterior stabilized design) and patella. Final balancing was performed with trial components and appropriate soft tissue releases. Afterwards the tibial, femoral and patellar components were implanted. Closure was performed in 4 layers. Starting at the center of the medial and lateral quadriceps flaps, the arthrotomy was closed continuously by knotless suturing with PDO size ‘0’ bidirectional QuillTM device (Wyomissing, PA). Similarly, starting at the center of the medial and lateral skin flaps, the deep tissues were closed in one layer continuously by knotless suturing with PDO size ‘2-0’ bidirectional QuillTM device. The subcuticular suturing was done with MonodermTM size ‘3-0’ bidirectional QuillTM. Secure skin closure was obtained using DermabondTM (Ethicon, Somerville, NJ). Sterile compression dressing was then applied and the tourniquet was deflated.
The two cohorts i.e., TXA group and the Aquamantys® group differed in only the intervention. The TXA group received 1000mg loading dose of intravenous TXA after the induction of anesthesia but prior to the skin incision. A second dose of TXA (1000mg) was also given intravenously during immediate postoperative period. The Aquamantys® group did not receive TXA. However, hemostatic sealing was obtained using Aquamantys® diligently at all the appropriate potential areas of bleeding from deep to superficial: the posterior capsule, the medial and lateral meniscal rims, the synovial folds adjacent to the resected femoral condyles and the suprapatellar synovial folds, the medial and lateral flaps of the quadriceps and the subcutaneous tissue.
All of the patients remained in-hospital care for 3 nights postoperatively. Antithrombotic protocol was the same in the two study groups (enoxaparin sodium 30mg subcutaneously every 12 hours). Postoperative pain management protocol was the same in both groups and decided by the pain management team. All patients were infused with acetaminophen 1gm intravenously every 8 hours for the first 24 hours (scheduled). All patients received Pregabalin 25mg orally three times a day (Scheduled). For a moderate pain of 4-6 on a scale of 0-10, Oxycodone 5-10mg immediate release tablet was given orally every 4 hours (PRN). For a severe pain of 7-10 on a scale of 0-10, Morphine 2-4mg was given intravenously every 3 hours (PRN). Patients who were medically indicated received Aspirin. Continuous passive motion devices were part of the standard postoperative nursing protocol.
The variables that were studied in each of the two study groups were broadly based on the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database reporting guidelines [13]. We arranged these variables in to two subgroups: local effects and systemic effects (Table 1). Four individual local effects were identified (Table 1). For each of those 4 individual local effects identified, the odds of developing such an outcome was calculated and statistically compared between the TXA and Aquamantys® groups using Z-test. Similarly, 7 individual systemic effects were identified (Table 1). For each of those 7 individual systemic effects identified, the odds of developing such an outcome was calculated and statistically compared between the TXA and Aquamantys® groups using Z-test.
Local effects
- Superficial surgical site infections (SSIs) requiring antibiotic treatment
- Deep SSIs requiring incision and drainage
- Organ/space SSIs requiring arthrotomy for debridement (with or without component revision)
- Postoperative stiffness requiring manipulation under anaesthesia
Systemic effects
- Postoperative fever > 101°F
- Deep venous thrombosis (DVT)
- Pulmonary vascular
- Pulmonary parenchymal
- Cardiac
- Neurologic (stroke)
- Sepsis
Table 1: Shows the list of local and systemic effect studied postoperatively in the tranexamic acid (TXA) group and Aquamantys® group after total knee arthroplasty.
Several possible biases that could have affected the results were carefully considered while constructing this study.
One, the clinical comparability of patients in the TXA and Aquamantys® groups with each other was tested for all the possible confounding variables such as age, body mass index (BMI) and the baseline medical issues. There were no statistically significant differences in the tested clinical parameters between the TXA and Aquamantys® groups (Table 2).
Patient variables
TXA
group (n=96)
Aquamantys® group(n=95)
Statistical
test
p value
Age
63
68
t-test
>0.05
Sex (M/F)
33/63
36/59
Z-test
>0.05
BMI
34
36
t-test
>0.05
Medical baseline
- Diabetes mellitus
- COPD
- CAD/CHF
19
3
8
23
4
9
t-test
t-test
t-test
>0.05
>0.05
>0.05
Table 2: Shows the clinical comparability of patients in TXA and Aquamantys® groups.
Two, the difference in the instrumentation system was also carefully considered while evaluating the results. In the TXA group, the VisionaireTM instrumentation (Smith & Nephew, Andover, MA) was used in 35 patients and the standard instrumentation was used in 61 patients. In the Aquamantys® group, the VisionaireTM instrumentation system was used in 36 patients and the standard instrumentation system was used in 59 patients. Because the ratio of patients in each group using either of the two instrumentation systems was nearly identical, we did not consider it a significant bias that could affect the results of the study.
Three, operating time can affect the clinical results after TKA. Although we did not calculate the mean duration of surgery in the TXA and the Aquamantys® groups, it was unlikely that the duration of surgery would differ significantly between the TXA and the Aquamantys® groups, because the surgeries were performed by the same surgeon (FDC), with a similar assisting team comprising of an adult reconstruction fellow, physician assistant and orthopedic trained surgical technologist.
Results
The analysis of local effects of the TXA and Aquamantys® is represented in Table 3. We found no statistically significant difference in any of the 4 local effects compared between the TXA and Aquamantys® groups. However, we noted a clinically significant trend towards decreased odds of superficial wound infections requiring antibiotic therapy in the TXA group compared to Aquamantys® group (1/96 vs. 6/95). Though this difference has not reached a conventional statistically significant level of p < 0.05, it was close (0.07), and we considered it a clinically meaningful difference.
TXA group vs.
Aquamantys® group
Risk ratio (odds ratio)
95% CI
Z statistic
Level of significance (p value)
Superficial SSIs requiring oral antibiotics
1/96 vs.
6/95
0.16
0.01 to 1.39
1.65
0.09
Deep SSIs requiring surgery
0/96 vs.
0/95
-
-
-
-
Organ or space SSIs requiring surgery
3/96 vs.
2/95
1.48
0.24 to 9.08
0.42
0.66
Postoperative stiffness treated with manipulation
7/96 vs.
10/95
0.69
0.25 to 1.89
0.71
0.47
Table 3: Shows analysis of local effects noted postoperatively in the tranexamic acid (TXA) group and Aquamantys® group after total knee arthroplasty.
The analysis of systemic effects of the TXA and Aquamantys® is represented in Table 4. On postoperative day (POD) 1, the odds of developing postoperative fever >101°F is statistically low in TXA group compared to Aquamantys® group. Otherwise there was no difference in the odds of developing postoperative fever >101°F between the TXA and the Aquamantys® groups on POD2 or 3. Similarly, we did not find any statistically significant difference in the incidence of other studied systemic effects between the TXA and the Aquamantys® groups.
Discussion
In addition to the desired effect on hemostasis, it is important to understand the local and systemic effects of using TXA and Aquamantys® during TKA. This is the first study to show that TXA decreases the odds of developing superficial wound infections requiring antibiotic therapy in patients undergoing TKA. This study finding on the effect of TXA on wound healing corroborates with the other available clinical research [14-16].
One important limitation of this study is the sample size. Though we could show a clinically meaningful trend towards decreased odds of developing superficial SSI requiring antibiotic therapy in the TXA group compared to the Aquamantys® group (1/96 vs. 6/95), we failed to prove that it is also statistically significant because of the limited sample size (type II error).
We systematically reviewed the basic/animal research to postulate the possible mechanism of action of TXA by which it facilitates wound healing. The effect of TXA on wound healing does not appear to be based on its antifibrinolytic effect, because any other antifibrinolytic agent does not have the same facilitative effect on wound healing [17].
The principle effect by which TXA facilitates wound healing is probably prevention of leakage of albumin from the exposed capillaries at the healing edges of the wound thus preventing interstitial tissue edema. There are at least two described mechanisms by which TXA prevents albumin leakage from the exposed capillaries: up regulation of occlude in expression [18] and down regulation of kinin expression [19]. Tensile forces caused due to edema are known to affect the behavior of cells within the healing epithelia by altering the material properties of extracellular matrices, such as substrate stiffness leading to altered morphology, proliferation, differentiation and migration of many different cell types [20].
Conclusion
This study provides preliminary clinical evidence that is suggestive of beneficiary effect of TXA on wound healing after TKA, specifically, the prevention of superficial SSI. Future studies are needed to confirm and substantiate the findings of this study that TXA has beneficial effect on wound healing.
References
- Benoni G., Lethagen S., and Fredin H.: The effect of tranexamic acid on local and plasma fibrinolysis during total knee arthroplasty. Thromb Res 1997; 85: 195-206
- Demmy TL. Transcollation: lost in translation? Eur J Cardiothorac Surg. 2015; doi:10.1093/ejcts/ezv145.
- Kelley TC, Tucker KK, Adams MJ, Dalury DF. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion. 2014; 54: 26-30.
- Oremus K, Sostaric S, Trkulja V, Haspl M. Influence of tranexamic acid on postoperative autologous blood retransfusion in primary total hip and knee arthroplasty: a randomized controlled trial. Transfusion. 2014; 54: 31-41.
- Frank SM, Wasey JO, Dwyer IM, Gokaslan ZL, Ness PM, Kebaish KM. Radiofrequency bipolar hemostatic sealer reduces blood loss, transfusion requirements, and cost for patients undergoing multilevel spinal fusion surgery: a case control study. Orthop Surg Res. 2014; doi: 10.1186/s13018-014-0050-2.
- Mankin KP, Moore CA, Miller LE, Block JE. Hemostasis with a bipolar sealer during surgical correction of adolescent idiopathic scoliosis. J Spinal Disord Tech. 2012; 25: 259-263.
- Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011; 93: 513-518.
- Falez F, Meo A, Panegrossi G, Favetti F, La Cava F, Casella F. Blood loss reduction in cement less total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013; 37: 1213-1217.
- Morris MJ, Barrett M, Lombardi AV Jr, Tucker TL, Berend KR. Randomized blinded study comparing a bipolar sealer and standard electrocautery in reducing transfusion requirements in anterior supine intermuscular total hip arthroplasty. J Arthroplasty. 2013; 28: 1614-1617.
- Zeh A, Messer J, Davis J, Vasarhelyi A, Wohlrab D. The Aquamantys system--an alternative to reduce blood loss in primary total hip arthroplasty? J Arthroplasty. 2010; 25: 1072-1077.
- Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health-Syst Pharm. 2013; 70: 195-283.
- Nutton RW, Wade FA, Coutts FJ, van der Linden ML. Short Term Recovery of Function following Total Knee Arthroplasty: A Randomised Study of the Medial Parapatellar and Midvastus Approaches. Arthritis. 2014; doi: 10.1155/2014/173857.
- ACS NSQIP Classic Variables & Definitions. 2013.
- Choy A, McCulloch PG. Perioperative and postoperative tranexamic acid reduces the local wound complication rate after surgery for breast cancer. Br J Surg. 1995; 82: 135.
- Bonnema J, van Geel AN, Wiggers T, Ligtenstein DA. Perioperative and postoperative tranexamic acid reduces the local wound complication rate after surgery for breast cancer. Br J Surg. 1994; 81: 1693.
- Oertli D, Laffer U, Haberthuer F, Kreuter U, Harder F. Perioperative and postoperative tranexamic acid reduces the local wound complication rate after surgery for breast cancer. Br J Surg. 1994; 81: 856-859.
- Björlin G, Nilsson IM. The effect of antifibrinolytic agents on wound healing. Int J Oral Maxillofac Surg. 1988; 17: 275-276.
- Nakaigawa N, Kamata K, Komatsu R, Ozaki M. The effect of antifibrinolytic agents on wound healing. Tranexamic acid accelerates skin barrier recovery and upregulates occlude in damaged skin. Int J Oral Maxillofac Surg. 1988; 17: 275-276.
- Yuan C, Wang XM, Yang LJ, Wu PL. Tranexamic acid accelerates skin barrier recovery and upregulates occludin in damaged skin. Int J Dermatol. 2014; 53: 959-965.
- Evans ND, Oreffo RO, Healy E, Thurner PJ, Man YH. Epithelial mechano biology, skin wound healing, and the stem cell niche. J Mech Behav Biomed Mater. 2013; 28: 397-409.