
Research Article
Austin J Musculoskelet Disord. 2022; 9(1): 1061.
Correlation between Muscle Mass and Fragility Parameters in the Elderly
de Oliveira JCM1, Gouveia CAM1, Moreira LDP2, Viscardi LGA1 and de Oliveira AS1,2*
¹Physiotherapy Course, Universidade Anhembi Morumbi’,São Paulo, SP, Brazil
²Postgraduate Program in Biomedical Engineering, Anhembi Morumbi University, São Paulo, SP, Brazil
*Corresponding author: Adriana Sarmento de Oliveira, Postgraduate Program in Biomedical Engineering, Anhembi Morumbi University, Rua Doutor Almeida Lima 1134, Mooca, São Paulo-SP, Brazil
Received: December 06, 2021; Accepted: January 20, 2022; Published: January 27, 2022
Abstract
Introduction: The frailty syndrome is an unstable condition related to the elderly’s functional decline and vulnerability to maintain homeostasis in the face of stressful factors. Grip strength is used as a predictor of general strength status, is closely related to mortality and disability and is related to several factors such as muscle mass.
Objective: To evaluate the correlation between muscle mass and frailty parameters in the elderly.
Methodology: An analytical, prospective, cross-sectional observational study was carried out. Elderly people aged 60 years or more, of both sexes, members of one of the elderly groups of the Integrated Health Center at Universidade Anhembi Morumbi were included in the research. The phenotype of Fried et al., [1]. was used to classify frailty, handgrip strength, gait speed (seconds at a distance of 4.6 meters), unintentional weight loss (greater than or equal to 4.5 kg), were evaluated. Exhaustion report (CES-D Depression Scale) and level of physical activity, measured through session 4 of the IPAQ long version. Bioelectrical impedance was performed using a tetrapolar device (Quantum II, RJL System, Clinton Twp, MI, USA).
Results: It was observed that the groups were similar to each other, except for height, as the non-frail ones were taller and had greater maximum handgrip strength. There was a statistical trend (p=0.05) in relation to BMI. In the group without frailty, there was a moderate and positive correlation between skeletal muscle and maximum grip strength (r=0.7; p<0.02). In the frail group there was no significant correlation (r=0.3; p<0.10). In assessing the correlation between skeletal muscle and age, no significant correlation was observed in the nonfrail group (r=0.2; p<0.57) or in the frail group (r=0.3; p<0.11). When evaluated between skeletal muscle and basal metabolism in the group without frailty, there is a strong and positive correlation (r=0.8; p<0.01), which did not occur in the frail group (r=-0, 1; p<0.79). Finally, the non-frail and frail groups did not present a significant correlation between skeletal muscle and gait speed (r=-0.3; p<0.39; r=-0.2; p<0.19, respectively).
Conclusion: The frail elderly have lower maximum handgrip strength compared to the non-frail elderly, but no correlations were observed between skeletal muscle and the variables maximum grip strength, age, basal metabolism and gait speed. In turn, the non-frail elderly showed a moderate and positive correlation between skeletal muscle and the variables maximum grip strength and basal metabolism, but there was no correlation between skeletal muscle and the variables age and gait speed.
Keywords: Aging; Skeletal muscle; Frailty
Introduction
It is notorious to observe that frailty syndrome in the elderly is tangible in the Brazilian and global scenario [2]. In addition, with the increase in the life expectancy of the population, the proportion of elderly aged 80 years or more has increased considerably in recent years and has gained 4.8 million elderly since 2012, surpassing the mark of 30.2 million in 2017, resulting in a phenomenon of great repercussion in Brazil, since longevity is reflected in all dimensions and sectors of society [3].
According to a census by the Ministry of Health it is estimated that 10 to 25% of people over 65 years old and 46% over 85 years old, living in the community, are frail. A recent U.S. Cardiovascular Health Study of 5,317 participants involving the prevalence of frailty showed that 6.7% of the elderly aged 65 years and older were frail. Moreover, 80 to 89 year olds accounted for 30% of the frail elderly [1].
Thus, all these clinical manifestations lead to an increased risk of unfavorable events: such as falls, urinary incontinence, decreased handgrip strength, hospitalization, and death [1]. According to the World Health Organization [4], a fall is determined as an involuntary eventuality that brings the body to the ground or on another surface. Still, it is estimated that one third of the elderly over 65 years of age experience a fall episode every year, and it is the second leading cause of death from unintentional injuries in the world. In Brazil, about 30% of the elderly fall once a year, and the most affected people are precisely the elderly, aged 80 years or more [5].
According to Santos et al., [6], frailty syndrome is considered a clinical syndrome, which can be characterized by progressive weight loss, decreased muscle strength, decreased lean body mass, altered gait resulting in decreased speed, imbalance, and decreased physical activity.
Furthermore, frailty is a clinical condition that has been widely studied in the last decades. Recently frailty has been proposed as a geriatric syndrome through the characterization of a “phenotype” and the description of a pathophysiology with a complex relationship between multisystem declines. However, despite the progress made in the last two decades, and although age is its main risk factor alone, the concept of frailty is far from an objective and consensual definition [7].
Thus, it is noteworthy to analyze that this disease is not exclusively subsequent to the aging process, since most elderly do not become frail compulsorily thus, according to Dias et al. [8] the loss of muscle strength generated by the absence of physical exercise can be assessed through handgrip strength, as a component of physical frailty, becoming an important tool for health professionals and researchers.
Grip strength is used as a predictor of general strength status and is closely related to mortality and disability, and is related to several factors such as muscle mass thus, it is essential to study the association between muscle mass and parameters of the frailty syndrome in the elderly.
General Objective
To evaluate the correlation between muscle mass and frailty parameters in the elderly.
Specific Objective
To evaluate the correlation between muscle mass and frailty parameters in the elderly in the following variables:
• Maximum grip strength;
• Age;
• Basal metabolism;
• Gait speed.
Materials and Methods
This was an analytical observational study, prospective in nature and cross-sectional in design. This research was approved by the Research Ethics Committee of Anhembi Morumbi University, linked to the Rectory of the University under protocol number 3.264.341 on 04/14/2019. All individuals who agreed to participate in the study were informed about its content and objectives, as well as the reliability of the information collected and their anonymity.
The study included elderly individuals aged 60 years or older, of both genders, members of one of the elderly groups of the Integrated Health Center at the Anhembi Morumbi University, who agreed to participate in the study and who signed the free consent form. Elderly people who did not agree to participate in the study, who did not sign the consent form, who were intolerant to the tests, or who dropped out were excluded from the study.
The instruments mentioned below were used to evaluate the elderly based on the five criteria for defining frailty according to Fried et al., [1]. The data were recorded in an individual evaluation form that was prepared based on the criteria of the cited author. The same instruments were used in all the evaluations to be performed.
To evaluate the handgrip strength in the dominant hand, we used a hand dynamometer, which was adjusted according to gender and Body Mass Index (BMI). A dynamometer is a device that is graduated in such a way as to indicate the intensity of the force applied to one of its extremes, where the answer is given in Kilogram (kg) values. Body mass index is recognized as the standard for assessing the degree of obesity and is calculated by dividing weight (in kg) by height (in m) squared. To calculate the BMI, we used a portable anthropometer with a 200 cm extension and a 0.1 cm scale to measure the height of the elderly (m), and a portable digital electronic scale to measure the body weight (kg) of the elderly. Thus, the cut-off for handgrip strength (kg) as a frailty criterion will be obtained through the individual’s Body Mass Index (BMI) according to (Table 1).
Sexo Feminino
Sexo Masculino
IMC (kg/m2)
Força de Preensão Manual (kg)
IMC (kg/m2)
Força de Preensão Manual (kg)
≤ 23
≤ 17
≤ 24
≤ 29
23.1-26
≤ 17.3
24.1-26
≤ 30
26.1-29
≤ 18
26.1-28
≤ 30
> 29
≤ 21
> 28
≤ 32
BMI: Body Mass Index; Source: Prepared by the author, adapted from Fried et al., [1].
Table 1: Parameter for handgrip strength (kg) as a frailty criterion.
For the assessment of walking speed, the evaluator marked a distance of 8.6 meters with the help of a 10-meter steel tape. The elderly were asked to walk this distance in a habitual manner, so that the time (in seconds) that the elderly would use to cover it could be measured. The timing in seconds was done using a digital stopwatch, where the initial two meters of acceleration and the final two meters of deceleration were disregarded to calculate the time to be spent walking. The elderly were given a verbal command to start the test using their normal shoes and, when necessary, their walking aids. Three measurements were performed, presented in seconds, and the mean value of the three measurements was considered.
For the evaluation of the usual walking speed in seconds over a distance of 4.6 meters, there was an adjustment according to gender and height, but the cut for the walking speed over the same distance, which aims to analyze the fragility criterion, was performed through the height of the elderly (m), according to (Table 2).
Sexo Feminino
Sexo Masculino
Estatura (m)
Tempo (s) para caminhar 4.6m
Estatura (m)
Tempo (s) para caminhar 4.6m
≤ 1.59m
≥ 7 seg.
≤ 1.73m
≥ 7 seg.
> 1.59m
≥ 6 seg.
> 1.73m
≥ 6 seg.
Source: Prepared by the author, adapted from Fried et al., [1].
Table 2: Parameter for gait speed as a frailty criterion.
To evaluate the unintentional weight loss, the anamnesis was used where the elderly were asked if they lost weight involuntarily in the last year, and if the answer was yes, they were then asked how many kilos were lost if it was greater than or equal to 4.5 kg or greater than 5% of their weight, it would be a positive criterion of frailty.
To assess the elderly’s exhaustion, we used two selected statements from the Center for Epidemiologic Studies Depression Scale (CES-D), assessed by self-report of fatigue: “I felt I had to make an effort to do usual tasks” (item 2), and “I couldn’t carry on with my things” (item 2). After the two statements were read, the elderly person selected one of the options with which he or she best identified, according to Table 3. The option “often” or “always” in any of the questions are considered as positive criteria for frailty [9,10] (Table 3).
Dias por semana
Frequência
Pontuação
Menos de um dia
Raramente ou nunca
0
1/2 dias
Poucas ou algumas vezes
1
2/3 dias
Frequentemente
2
> 4 dias
Sempre
3
Source: Prepared by the author, adapted from Fried et al., [1].
Table 3: Parameter for the exhaustion criterion.
To measure the level of physical activity and identify active and sedentary participants, we used domain 4 (time spent on physical activities of recreation, sport, exercise and leisure) of the International Physical Activity Questionnaire (IPAQ) long version. The cut score is 150 minutes spent per week in physical activities. The positive criterion for frailty in this item was applied to those who presented an expenditure of less than 150 minutes per week in physical activities. The same questionnaire was used to define active and sedentary, the value ≥150 minutes were considered active and <150 minutes were considered sedentary.
To measure body mass, the elderly were asked to get on the scale without shoes, in an orthostatic position, wearing light clothing and not carrying objects. To measure height, the elderly were asked to stand barefoot, in the standing position, with their heels together and leaning against the anthropometer and with their heads in the horizontal plane. Two measurements were made, taking the average as an estimate of height. The allowed variation between the two measurements was 0.5 cm, and if this value was exceeded, the two measurements should be cancelled and repeated.
Electrical bioimpedance was performed using a tetrapolar device (Quantum II, RJL System, Clinton Twp, MI, USA) that indirectly assesses fat mass and fat-free mass. This method consists in administering an electric current between four points on the individual’s body and measuring the opposition to the current passage, which depends on the tissue composition, providing resistance and reactance values. The quantification through mathematical formulas of bioimpedance allows to evaluate the percentage of body water, fat free mass (lean mass), and fat mass.
The procedure was in the supine position for the administration of the electric current between two parts of the body (arm and leg on the right side) of the participants.
Regarding the statistical analysis, for the evaluation of the descriptive characteristics of the sample, measures of central tendency and dispersion were used, mean and standard deviation, respectively. Student’s t-test was used for unpaired data. Pearson’s correlation coefficient (r) was used to verify the linear correlation between two variables, reflecting the intensity of a linear relationship, followed by one-factor anova. Reference values from 0 to 0.3, represent a negligible correlation, from 0.3 to 0.5 a weak correlation, from 0.5 to 0.7 the correlation is moderate, from 0.7 to 0.9 the correlation is strong, above 0.9, very strong. A significance level of 5 % a was adopted for Student’s t-test and one-way Anova. All statistical analysis was performed in Microsoft Excel version 2010.
Results
A total of 128 elderly individuals were screened, 75 of whom were excluded for not agreeing to participate in the study. Thus, the final sample of the study was composed of 53 elderly people. Of these, 11 elderly subjects did not present frailty syndrome and 24 elderly subjects presented frailty or pre-frailty (Figure 1).
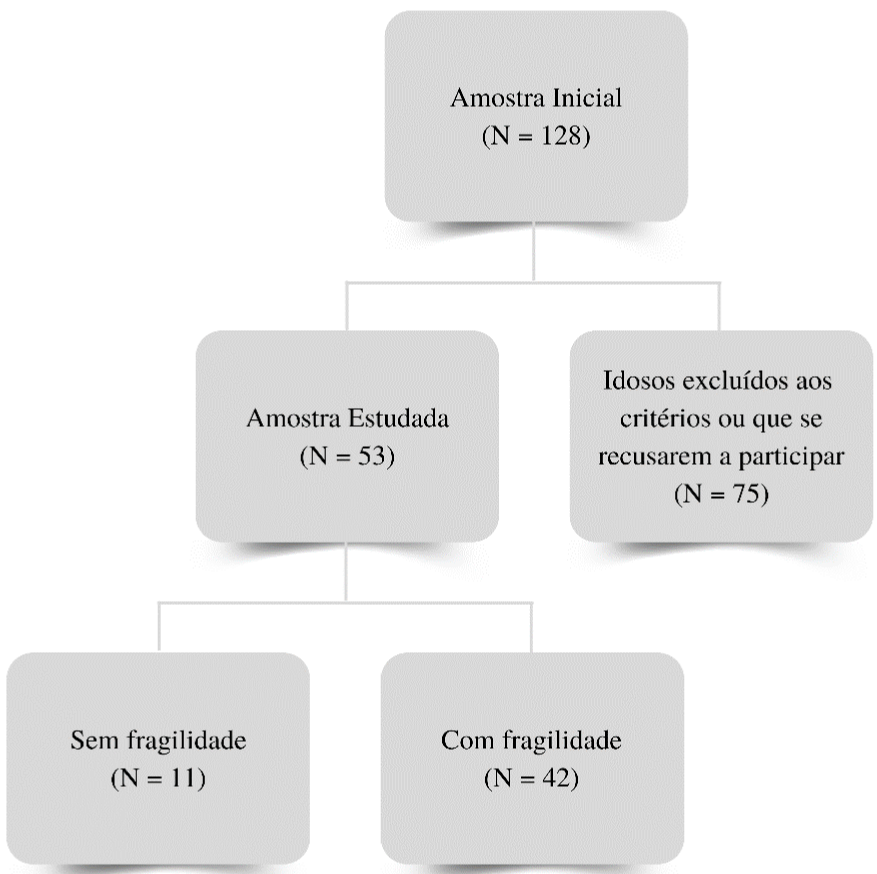
Figure 1: Flow chart of the survey.
Table 4 shows the characterization of the sample and some evaluation criteria of the frailty syndrome in non-frail elderly and frail elderly. It was observed that the groups were similar to each other except for height because the non-fragile were taller and had maximum handgrip strength. There was a statistical trend (p=0.05) regarding BMI( (Table 4)
Sem Fragilidade
(n=11)Com Fragilidade
(n=42)p
Idade (anos)
66±6.1
70±5.2
0.18
Estatura (m)
1.65±6.1
1.58±0.1
0.001*
IMC (kg/m2)
25.3±2.2
29±5.1
0.050
Metabolismo Basal
1434±158
1422±167
0.83
Sexo (n.%)
Feminino
8 (73%)
39 (93%)
0.07
Masculino
3 (27%)
3 (7%)
0.07
Critérios da SF
Velocidade da Marcha
3±0.8
5±1.4
0.09
Força de Preensão Máxima
26±7.7
21±4.3
0.001*
Table 4: Characterization of the sample and evaluation criteria for frailty syndrome.
The classes of medications used by the elderly volunteers are shown in Table 5. It was observed that the classes of antihypertensive, hypoglycemic, statins and fibrates, besides the, T4 hormone repositories and insomnia medication did not present significant differences between the groups. On the other hand, the medication for insomnia, passiflora, representing 9% of the sample in the nonfragility group, showed a significant difference (p=0.05) (Table 5).
Sem Fragilidade
(n=11)Com Fragilidade
(n=42)P
HAS
4 (36%)
19 (45%)
0.60
Angiotensina do receptor da angiotensina
0 (0%)
2 (5%)
0.46
Alfa bloqueador
0 (0%)
1 (2%)
0.61
Inibidores ECA
2 (%)
2 (5%)
0.13
Bloqueadores do receptor AT. da angiotensina II (BRA)
1 (%)
11 (26%)
0.23
Diuréticos
0 (0%)
9 (21%)
0.09
Betabloqueador
2 (18%)
3 (7%)
0.26
DM II
0 (0%)
12 (28%)
0.18
Sulfonilunereias
0 (0%)
1 (2%)
0.61
Biguanidas
1 (9%)
11 (16%)
0.23
Tiazolidínedionas
0 (0%)
1 (2%)
0.61
Dislipidemia
1 (9%)
13 (31%)
0.14
Fibratos
0 (0%)
2 (5%)
0.46
Estatinas
1 (9%)
13 (31%)
0.14
AINE (AAS)
1 (9%)
3 (7%)
0.83
Hipotireoidismo
1 (9%)
8 (19%)
0.43
Levotiroxina
1 (9%)
8 (19%)
0.43
Insônia
1 (9%)
2 (5%)
0.58
Passiflora
1 (9%)
0 (0%)
0.05*
Zolpidem
0 (0%)
1 (2%)
0.61
Pregabalina
0 (0%)
2 (5%)
0.46
Depressão
2 (18%)
9 (21%)
0.81
ISRSs
0 (0%)
7 (17%)
0.51
Tetracíclico
0 (0%)
1 (2%)
0.61
Benzodiazepínicos
1 (9%)
0 (0%)
0.05*
Antipsicótico
0 (0%)
1 (2%)
0.61
Tricíclico
1 (9%)
2 (5%)
0.58
Table 5: Use of medications in both groups.
Figure 2 shows the relationship between skeletal muscle variables and maximum grip strength. In the non-fragile group, a moderate and positive correlation is observed (r=0.7; p<0.02). In the group with frailty, there was no significant correlation (r=0.3; p<0.10). Figure 2 shows the relationship between skeletal muscle variables and maximum grip strength. In the non-fragile group, a moderate and positive correlation is observed (r=0.7; p<0.02). In the group with frailty, there was no significant correlation (r= 0.3; p<0.10) (Figure 2).
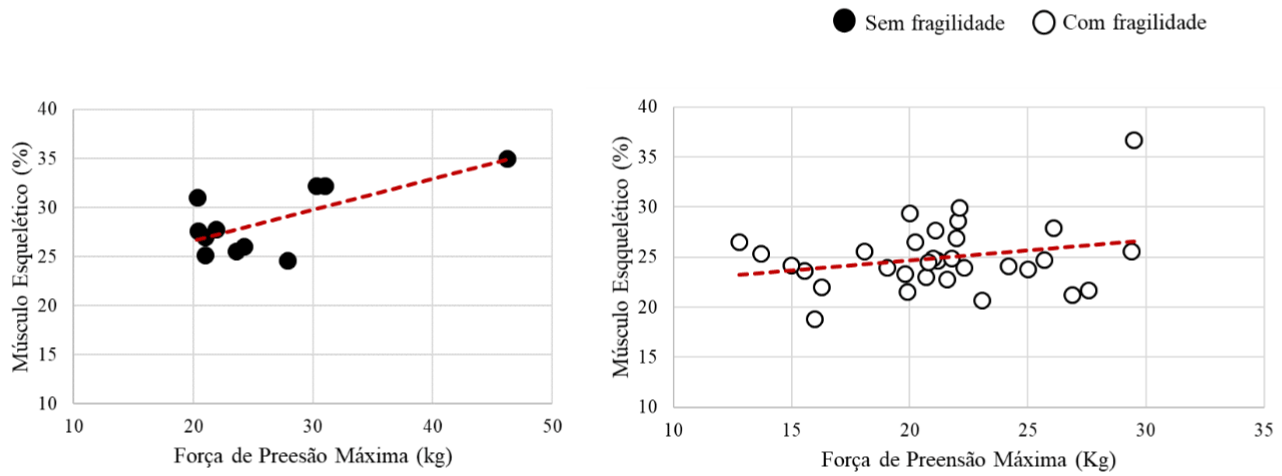
Figure 2: Correlation graph of skeletal muscle and maximum grip strength variables.
The correlation between skeletal muscle and age variables was plotted in Figure 3. No significant correlation was observed in the group without frailty (r=0.2; p<0.57) nor in the group with frailty (r=0.3; p<0.11) (Figure 3).
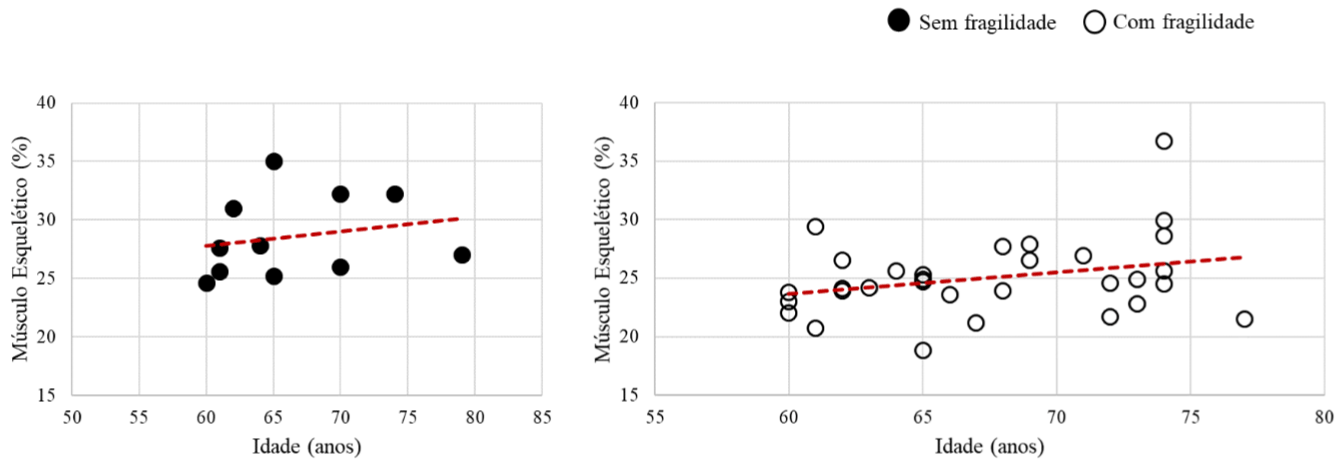
Figure 3: Correlation graph of skeletal muscle and age variables.
Figure 4 shows the relationship between skeletal muscle and basal metabolism variables. In the group without frailty, a strong and positive correlation is observed (r=0.8; p<0.01). On the other hand, in the frailty group there was no statistical correlation (r= -0.1; p<0.79) (Figure 4).
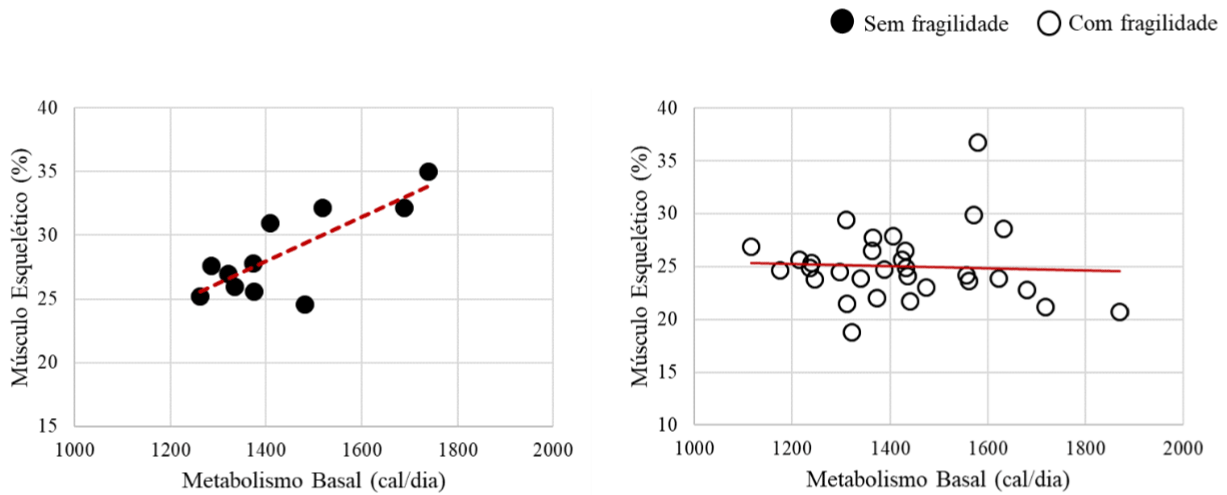
Figure 4: Correlation graph of skeletal muscle and basal metabolism variables.
Finally, the relationship between skeletal muscle variables and gait speed was represented in Figure 5. The groups without frailty and with frailty showed no significant correlation (r=-0.3; p<0.39; r=-0.2; p<0.19, respectively) (Figure 5).
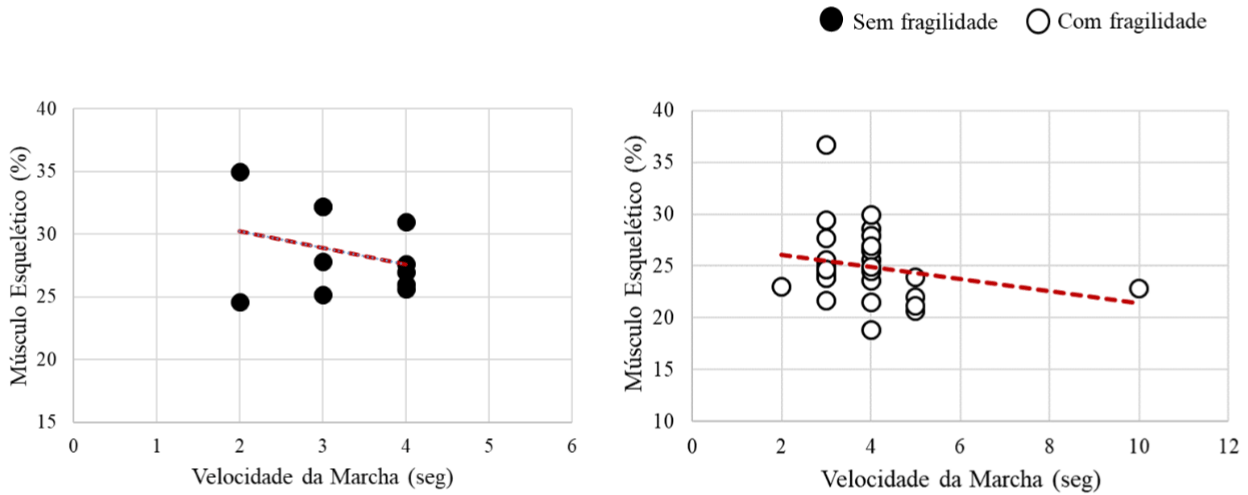
Figure 5: Correlation graph of skeletal muscle and gait speed variables.
Discussion
The main finding of this study was a reduction in height and maximum grip strength, and an increase in BMI in the frail group. On the other hand, in the non-fragile group, a strong positive correlation was demonstrated between skeletal muscle with maximal grip strength, and skeletal muscle with basal metabolism. The frail elderly showed lower handgrip strength compared to the non-frail elderly.
According to Lana and Schneider [11]. Frailty is identified as an age-associated syndrome characterized by manifestations such as weight and muscle mass loss, decreased grip strength, fatigue, postural instability, and reduced food intake, increasing the risk for an unfavorable evolution in the face of external aggressions and acute illnesses. Gottlieb et al., [12] show that after the age of 75 the prevalence of frailty syndrome is higher in men (58%) than in women (45%). On the other hand, Roubenoft et al., [9] evidenced that with aging, men have greater muscle loss due to the decline in Growth Hormone (GH), Insulin-Related Growth Factor (IGF-1), and testosterone. However, although muscle loss is greater in men, it is important to note the importance of sarcopenia in older women, which in this study was predominantly in women.
Lenardt et al., [13] state that reduced muscle mass and handgrip strength have been explored as an early manifestation of frailty and may be present even before the onset of other functional disabilities. Furthermore, report that low handgrip strength is directly related to the occurrence of chronic morbidities and musculoskeletal disorder. According to Neri and Pinto [14] the physiological changes of aging cause an increase in the likelihood of the elderly to present low handgrip strength. Taking into consideration the findings of this study, it is noticeable that due to the decrease in muscle mass, the individual tends to have a lower grip strength in the frail group, as shown in Figure 2.
Correspondingly, Macedo, Freitas, and Scheicher [15], state in the course of the frailty syndrome, there is a decrease in muscle mass and muscle quality, however, this decline varies according to muscle type and gender. Moreover, there is a tendency to decrease maximum grip strength with advancing age, and the decrease in grip strength occurs more rapidly than the lower limb strength. According to Santos et al., [2] it is reported that in some research What is found are that women present the fragility component for the variable grip strength. Similar to what was found in this study where women were the ones who presented the greatest fragility.
In a study conducted by NERI and Bez [10], the elderly who showed reduced handgrip strength also had body mass deficits, which is in line with the findings of this research. The results reveal that the number of elderly with decreased handgrip strength in this study is significant. In a pioneering, study conducted in Brazil on frailty in Brazilian elderly it was observed that 20.5% of the elderly had reduced handgrip strength as a result of unintentional weight loss, low calorie consumption, and decreased muscle mass. On the other hand, longitudinal studies have shown that age-related decline in muscle strength is not proportionally related to loss of muscle mass [16]. This may, at least in part, explain our observation that muscle strength is independently and significantly associated with outcomes, regardless of muscle mass. Otherwise, Shintakuya et al., [17] state that the loss of muscle mass with aging may not be more useful than muscle strength. However, maintaining muscle mass with advancing age is critical because it serves as a metabolic reservoir that is needed to effectively resist disease.
Our study also indicated that seniors with frailty tend to decrease in height, as presented in Table 4. Dorneles, Vielmo, and Ely [18] consider that due to changes in the spine, such as flattening of the vertebrae, reduction of the discs, arching of the lower limbs, and flattening of the plantar arch, there is a reduction in stature, which is usually one centimeter per decade after the age of 40, and may be accentuated after the age of 70. Similarly, Schneider and Irigara [19] point out that there is evidence that after 40 years of age, the stature of individuals may decrease by one to two and a half centimeters per decade. Furthermore, the reduction in height observed in the elderly is one of the consequences of aging. Thoracic kyphosis, reduction of the intervertebral discs, and plantar flattening contribute to this process. The reduction reaches 1 cm in men and 1.5 cm in women per decade, from 40-50 years of age [20].
Regarding the high BMI index in the elderly, Civinski, Montibeller and Braz [21], consider that the lack of regular physical activity, healthy eating, daily tasks and physical skills can result in increased body mass and sedentary lifestyle. Furthermore, authors point out that BMI above 27 kg/m2 (overweight) negatively impacts the functional capacity of the elderly, by influencing activities that involve balance and squats [22]. According to the aging process is related to an increase in BMI and, mainly, to a reduction in the functional capacity of the elderly, due to the decline in motor performance and mobility.
In a research Silva et al., [23] observed statistically significant differences between frailty and grip strength, body mass, and stature in men, with the non-frail ones exhibiting higher mean body mass and greater stature. From another perspective, for females, the only statistically significant difference was for height, with the non-fragile elderly showing higher mean. Thus, it is revealed that BMI is directly linked to grip strength [23].
Our study also includes the BMI variable being compatible with normal weight, which in turn showed a significant association in frail elderly, as shown in Table 4. A result proportional to this, was observed in a cross-sectional study conducted in Campina Grande, with 420 elderly, whose objective was to verify the correlation between handgrip strength and flexibility to the variables age and anthropometric measurements. The results revealed that there was a significant correlation between BMI and reduced grip strength, however, only for females [23]. Furthermore, point out that the progressive reduction of muscle mass in the elderly is related to physical disability, mobility, and mortality. Their authors proposed that healthcare professionals identify functional and muscle changes in the elderly, particularly in the elderly, as these may predict those individuals with frailty and without frailty. However, recent studies have suggested that high BMI may be closely associated with low muscle mass, thus may be derived from frailty syndrome and obesity.
Muscle mass and frailty syndrome criteria should be investigated with priority in the evaluation and physiotherapeutic approach and multidisciplinary professional work. In a study by Goodpaster et al., [24] demonstrated that there was a much faster reduction in muscle strength than in muscle mass in the elderly, reporting that in the frailty syndrome, there is a compromise of muscle quality, and that gaining muscle mass alone may not prevent the decline in muscle strength. Taking muscle quality into consideration, Rebelatto and Morelli [25] state that sarcopenia occurs mainly due to the decrease in crosssectional area. As a result, the elderly will have lower quality in their muscle contraction, less strength, less coordination of movements, and probably a greater probability of suffering accidents (for example, falls, low physical activity, and altered grip strength). Furthermore, according to Deschenes [26], the decrease in the number of muscle fibers is the dominant cause of frailty syndrome, although fiber atrophy, particularly type II, is also involved. However, Silva et al., [23] said that with the passing of years the fast contraction fibers or type II decrease in number and volume and the slow contraction fibers or type I also decrease, but in a smaller proportion than the former. In this way, this fact may explain the lower speed observed in the movements of the elderly. According to Clark and Manini [27] the force generated by a muscle is not directly proportional to the amount of muscle fiber present in it. Based on this fact, recently the term dinapenia (dyna = strength; penia = loss) was proposed by Clark and Manini to define the specific loss of muscle strength related to the aging process, and to dissociate the loss of strength from the loss of muscle mass.
Okuma [28] states that the increase in muscle capacity to generate force is explained from the overload principle; the muscle group is subjected to work with higher loads than it is used to withstanding, generating an increase in size and strength. A study by Fiatarone et al., [29] demonstrated that very elderly individuals (mean age 87 years) who underwent resistance training associated with nutritional supplementation for ten weeks had increased muscle strength, as well as objective improvements in gait, speed, and spontaneous physical activity. The potential long-term benefits are fewer falls, increased mobility, and independence. In addition, Hasten et al., [30] showed increased muscle protein synthesis in response to resistance training. Furthermore, changes in innervation and muscle activation pattern also occur with training, improving motor performance.
It should be remembered that resistance exercise is still the most effective intervention to increase muscle mass and strength in the elderly. Stephen and Borst [31] reinforce that some elderly people may have reduced food intake and increased protein needs, making it difficult to obtain the effects of strength training if the nutrition is not appropriate for the energy intake of each individual. In one study Sousa et al., [32] found a 32 to 48% increase in muscle strength through progressive strength training, furthermore, the study findings were statistically significant (p<0.05), indicating that strength training may be directly associated with the prevention of frailty syndrome. Finally, Sousa et al., [32] states that increased functional abilities with strength training have only been verified for frail individuals. Similar to this, our study demonstrated that the elderly with frailty presented a lack of muscle mass and strength, caused by the frailty syndrome and not prevention through resistance training.
Conclusion
The frail elderly showed lower handgrip strength compared to the non-frail elderly, but no correlations were observed between skeletal muscle and the variables handgrip strength, age, basal metabolism, and gait speed. In turn, the non-fragile elderly showed a moderate and positive correlation between skeletal muscle and the variables maximum grip strength and basal metabolism, but there was no correlation between skeletal muscle and the variables age and gait speed.
References
- Fried LP, et al. Frailty in Older Adults: Evidence for a Phenotype. The Journals of Gerontology: Series A, New Jersey. 2001; 56: 146-156.
- Santos A, et al. Sono, Fragilidade E Cognição: Estudo Multicêntrico Com Idosos Brasileiros. Revista Brasileira De Enfermagem, Brasília. 2013; 66: 355-356.
- Pereira LF, et al. Retrato Do Perfil De Saúde-Doença De Idosos Longevos Usuários Da Atenção Básica De Saúde. Revista Enfermagem UERJ, Rio De Janeiro. 2015; 23: 649-655.
- Organização Mundial Da Saúde. Caídas. WHO. 2021.
- Ansai JH, et al. Revisão De Dois Instrumentos Clínicos De Avaliação Para Predizer Risco De Quedas Em Idosos. Revista Brasileira De Geriatria E Gerontologia, Rio De Janeiro. 2004; 17: 177-189.
- Santos I, et al. Perfıl De Fragilidade E Fatores Associados Em Idosos Cadastrados Em Uma Unidade De Saúde Da Família. Ciências & Saúde Coletiva, Rio De Janeiro. 2015; 20: 1917-1994.
- Ensrud, KE. Frailty and Risk of fall, Fracture, and Mortality in Older Woman: The Study of Osteoporotic Fractures. The Journals of Gerontology: Series A, Baltimore. 2007; 62: 744-751.
- Dias JA, et al. Força De Preensão Palmar: Métodos De Avaliação E Fatores Que Influenciam A Medida. Revista Brasileira Cineantropometria Desempenho Humano, Santa Catarina. 2010; 12: 209-216.
- Roubenoff R, Hughes A. Sarcopenia: Current Concepts. The Journals of Gerontology: Series A, Boston. 2000; 55: 716-724.
- Bez O, Neri L. Velocidade Da Marcha, Força De Preensão E Saúde Percebida Em Idosos: Dados Da Rede FIBRA. Ciência Saúde Coletiva, São Paulo. 2014; 19: 3345-3351.
- Lana D, Schneider H. Síndrome De Fragilidade No Idoso: Uma Revisão Narrativa. Revista Brasileira De Geriatria E Gerontologia, Rio De Janeiro. 2014; 17: 673-680.
- Gottlieb V, et al. Envelhecimento, Estresse Oxidativo E Sarcopenia: Uma Abordagem Sistêmica. Revista Brasileira De Geriatria E Gerontologia, Rio De Janeiro. 2011; 15: 365-380.
- Lenardt H, et al. Fatores Associados À Força De Preensão Manual Diminuída Em Idosos. Escola Anna Nery Revista De Enfermagem, Rio De Janeiro. 2016; 20: 1441-1145.
- Pinto M, Neri L. Doenças Crônicas, Capacidade Funcional, Envolvimento Social E Satisfação Em Idosos Comunitários: Estudo Fibra. Ciências & Saúde Coletiva, Rio De Janeiro, 2013; 18: 3454-3455.
- Macedo D, Freitas L, Scheicher M. Handgrip and Functional Mobility in Elderly with Different Levels of Physical Activity. Original Research, São Paulo. 2014; 21: 151-155.
- Manini T, Clark C. Dynapenia and Aging: An Update. The Journals of Gerontology: Series A, Gainesvillev. 2012; 67: 28-40.
- Shintakuya R, et al. Sarcopenia Is Closely Associated With Pancreatic Exocrine Insufficiency In Patients With Pancreatic Disease. Elsevier, Amsterdam. 2017; 17: 70-75.
- Dorneles G, Vilemo G, Ely B. Envelhecimento E Arquitetura: As Necessidades Espaciais Dos Idosos Em Espaços Abertos. Revista De Arquitetura, Cidade E Contemporaneidade, Pelotas. 2020; 4: 158-159.
- Schneider H, Irigaray Q. O envelhecimento Na Atualidade: Aspectos Cronológicos, Biológicos, Psicológicos E Sociais. Estudo De Psicologia, Campinas. 2008; 25: 586-593.
- Santos O, Machado O, Leite M. Envelhecimento E Alterações Do Estado Nutricional. Sociedade Brasileira De Geriatria E Gerontologia, Recife. 2010; 14: 169-175.
- Civinsk C, Montibeller A, Braz O. An Importância Do Exercício Físico No Envelhecimento. Revista Da Unifebe, Brusque. 2011; 9: 163-175.
- Oliveira T, Duarte P, Reis A. Relação Entre Índice De Massa Corporal E Desempenho Motor De Idosos Pertencentes A Grupos De Convivência. Texto & Contexto Enfermagem, Santa Catarina. 2016; 25: 2-9.
- Silva A, et al. Sarcopenia Associada Ao Envelhecimento: Aspectos Etiológicos E Opções Terapêuticas. Revista Brasileira De Reumatologia, São Paulo. 2006; 46: 391-397.
- Goodpaster B, et al. The Loss of Skeletal Muscle Strength, Mass, and Quality in Older Adults: The Health, Aging and Body Composition Study. The Journals Of Gerontology: Series A, Pennsylvania. 2006; 61: 1059-1064.
- Rebelatto JR, Morelli JGS. Fisioterapia Geriátrica: A Prática Da Assistência Ao Idoso. 2ª Edição. São Paulo, Manole. 2007; 540.
- Deschenes M. Effects of Aging on Muscle Fibre Type and Size. Sports Medicine, Virginia. 2004; 34: 809-824.
- Clark C, Manini T. Sarcopenia Dynapenia. The Journals of Gerontology, Gainesville. 2008; 63: 829-834.
- Okuma SS. O Idoso E A Atividade Física: Fundamentos E Pesquisa. 6ª Edição. Papirus. 1998; 224.
- Fiatarone M, et al. High-Intensity Strength Training in Nonagenarians. Effects on Skeletal Muscle. JAMA The Journal Of The American Medical Association, Chicago. 1990; 263: 3029-3034.
- Hasten D, et al. Resistance Exercise Acutely Increases MHC and Mixed Muscle Protein Synthesis Rates In 78-84 and 23-32 Yr Olds. American Journal Physiology Endocrinology Metabolism, Missouri. 2000; 278: 620-626.
- Borst E. Interventions for Sarcopenia and Muscle Weakness in Older People. Age and Ageing, Gainesville. 2004; 33: 548-555.
- Sousa N, Marques U. Prevenção Da Queda Do Idoso: As Alterações Induzidas Pelo Treino Da Força No Desempenho Do Timed Get-Up & Go Test E Do Functional Reach Test. Revista Digital - Buenos Aires, V. 2002; 53: 1-3.