Editorial
Austin J Nanomed Nanotechnol. 2014;1(1): 1002.
Rapidly Growing Use of Graphene in Immunodiagnostics
Sandeep Kumar Vashist*
HSG-IMIT - Institut für Mikro- und Informationstechnik, Germany
*Corresponding author: : Sandeep Kumar Vashist, HSG-IMIT - Institut für Mikro- und Informationstechnik, Georges-Koehler-Allee 103, 79110 Freiburg, Germany
Received: November 29, 2013; Accepted: December 15, 2013; Published: January 03, 2014
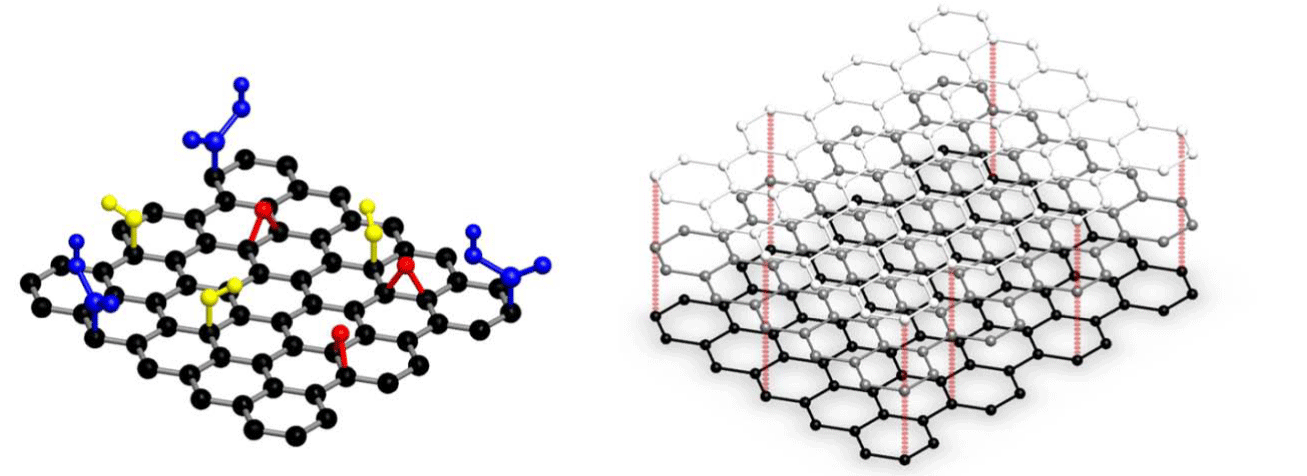
Graphene has been widely employed in immunodiagnostics [1-4] due to its increased surface area, low cost and high electrical conductivity. It is a two-dimensional planar sheet of sp2-bonded carbon atoms packed densely in a honeycomb crystal lattice (Figure 1). Although graphene has been synthesized by numerous procedures, its mass production is done by chemical vapor deposition (CVD) and chemical or thermal reduction of graphene oxide. There have been significant advances in the chemical modification of graphene, preparation of graphene nanocomposites and biomolecular immobilization strategies, where researchers have induced specific functional groups on graphene and bound it to the specific biomolecules [5].
Graphene nano platelets are commercially-available crystalline or flake form of graphite that consists of many graphene sheets stacked together (Figure 1). We have recently developed graphene nano platelets (GNPs)-based sandwich enzyme-linked immunosorbent assay (ELISA) procedure for the highly sensitive detection of human lipocalin-2 (LCN2) [2].GNPs, mixed with 3-aminopropyltriethoxysilane (APTES), were functionalized on 96-well microtiter plate (MTP) and bound covalently to the anti- LCN2 antibodies. The increased surface area provided by the GNPsfunctionalization led to higher antibody immobilization density, which was responsible for significant signal enhancement that led to the highly sensitive detection of LCN2 in plasma, serum and whole blood. The dynamic range of the assay was 0.6-5120pg/ml, while the limit of detection was 0.5pg/ml. The developed assay had 80-fold higher analytical sensitivity and 3-fold lesser immunoassay duration than the commercial ELISA. Moreover, the antibody-bound MTPs were found to be highly stable for 8 weeks, which is appropriate for healthcare and industrial applications.
Figure 1: Single- and multi-layered graphene. The yellow, red and blue groups in single-layered graphene are hydroxyl, ether and carboxyl groups, respectively. Reproduced with permission from Omics Publishing Group.
Graphene has been widely used for the detection of a wide range of analytes [6].It has immense potential for electrochemical biosensors as it enables the direct electron transfer between enzyme and electrode surface [7]. Graphene-functionalized electrodes have superior analytical performance with negligible interference from physiological substances and drug metabolites, and excellent antibiofouling [6,7].The intrinsic peroxidase activity of graphene oxide(GO) has been employed to develop a naked-eye colorimetric immunoassay for the detection of prostate specific antigen (PSA) [4]. The capture anti-PSA antibody was immobilized onto the surface of magnetic bead, while the secondary anti-PSA antibody was bound to GO (GO-Ab2). The presence of PSA leads to the formation of sandwich immune complex, which is removed by applying external magnetic field. Thereafter, various amounts of GO-Ab2 were mixed with hydroquinone and H2O2. It displayed different colors corresponding to the various PSA concentrations, which enables naked eye detection that is ideal for point-of-care (POC) applications.This POC immunoassay can easily differentiate between the normal persons (having 1-4 ng/mL PSA) and those having prostate cancer (i.e. 4-10ng/ml PSA) based on the differences in color. Moreover, GO is much cheaper than horse radish peroxidase that is used in conventional immunoassays, which significantly reduces the cost of analysis.
An electrochemical immunosensor has also been developed for the simultaneous detection of multiple cancer biomarkers, i.e. carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP), using the carboxyl graphene nanosheets (CGS) as immunosensing probes [3]. The capture antibodies for these analytes were bound to chitosan-Au nanoparticles by heterobifunctional cross linking, while anti-CEA and anti-AFP detection antibodies were bound to CGS by immobilization of toluidine blue and prussian blue, respectively. It enabled simultaneous detection of CEA and AFP in the linear range of 0.5-60ng/ml with LOD of 0.1 and 0.05ng/ml for CEA and AFP, respectively. The developed immunoassay had good correlation with conventional ELISA. Recently, a potential signal transduction strategy based fluoroimmunoassay was also developed by regulating the interaction between graphene and graphene quantum dots [1]. It was employed for human IgG detection using luminescence resonance energy transfer mechanism.
Taking into account the growing concerns about the safety of nanomaterials, there is a need for the stringent evaluation of toxicity and biocompatibility of graphene, especially for in vivo applications. But the absence of international regulatory guidelines to evaluate the toxicity of nanomaterials [8] has led to highly contradictory results. Therefore, there is an immense need to draft these guidelines. Moreover, the industrial and healthcare requirements [8] also need to be complied with in order to develop commercially-viable graphenebased immunodiagnostics. The production of graphene should be considerably improved and scaled up to reduce its commercial cost and improve its quality. However, the numerous advantages provided by graphene will enable the development and commercialization of analytically-superior graphene-based immunodiagnostic kits in the coming years, which will be extensively used for healthcare, environmental monitoring, and industrial applications.
References
- Zhao H, Chang Y, Liu M, Gao S, Yu H, et al. A universal immunosensing strategy based on regulation of the interaction between graphene and graphene quantum dots. Chem Commun. 2013; 49: 234-236.
- Vashist SK. Graphene-based immunoassay for human lipocalin-2. Anal Biochem. 2013; 446: 96-101.
- Chen X, Jia X, Han J, Ma J, Ma Z. Electrochemical immunosensor for simultaneous detection of multiplex cancer biomarkers based on graphene nanocomposites. Biosens Bioelectron. 2013; 50: 356-361.
- Qu F, Li T, Yang M. Colorimetric platform for visual detection of cancer biomarker based on intrinsic peroxidase activity of graphene oxide. BiosensBioelectron. 2011; 26: 3927-3931.
- Georgakilas V, Otyepka M, Bourlinos AB, Chandra V, Kim N, et al. Functionalization of Graphene: Covalent and non-covalent approaches, derivatives and applications. Chem Rev. 2012; 112: 6156-6214.
- Liu Y, Dong X, Chen P. Biological and chemical sensors based on graphene materials. Chem Soc Rev. 2012; 41: 2283-2307.
- Zheng D, Vashist SK, Al-Rubeaan K, Luong JHT, Sheu F-S. Mediatorless amperometric glucose biosensing using 3-aminopropyltriethoxysilane-functionalized graphene. Talanta. 2012; 99: 22-28.
- Vashist SK, Venkatesh AG, Mitsakakis K, Czilwik G, Roth G, et al. Nanotechnology-based biosensors and diagnostics: technology push versus industrial/healthcare requirements. BioNanoSci. 2012; 2: 115-126.