Review Article
Austin J Nanomed Nanotechnol. 2014;1(1): 1003.
Graphene based materials and their composites as coatings
Yao Tong1, Siva Bohm2 and Mo Song1*
1Department of Materials, Loughborough University, Loughborough LE11 3TU, United Kingdom
2TATA Steel RD&T, Swinden Technology Centre, Moorgate, Rotherham S60 3AR, United Kingdom
*Corresponding author: : Mo Song, Department of Materials, Loughborough University, Loughborough, LE11 3TU, United Kingdom
Received: December 05, 2013; Accepted: December 27, 2013; Published: December 31, 2013
Abstract
Coating plays a vital role in improving surface quality and providing protection for a substrate. Consistent efforts have been made to produce excellent properties coating. Graphene, a new discovered carbon allotrope, has received worldwide attention and it was studied in almost every area due to its extraordinary properties arisen from its unique structure. It is a very promising new generation material for developing advanced coating. In this article, the recent activities about utilizing pristine graphene based materials and graphene based composites as coatings were reviewed. The synthesis and functionalization of graphene were also described briefly. The future perspectives of graphene based coatings were suggested.
Keywords: Graphene; Graphene based composites coating applications.
Introduction
Coating is usually used to improve the surface properties of a substrate, wettability, corrosion resistance and adhesion, for example. The coating industry has been driven to seek new technologies and materials to improve the efficiency of coatings by economic benefits and growing environmental concerns. There are several factors affect the effectiveness of a coating against all the possible damaging sources: they are the quality of the coating, the substrate characteristics, the properties of the coating⁄substrate interface, and the corrosiveness of the environment [1]. In order to satisfy the industrial requirements nowadays, polymer nanocomposites has been more and more investigated and applied in coating because nanocomposites provide superior properties with a relative low cost. Additionally, the processing procedure can be much less complicated than multi–layer coatings [1,2].
Graphene, a new generation material, is an allotrope of carbon element which was first isolated by simple mechanical exfoliation in 2004 [3]. It is a two dimension honey comb single layer crystal lattice formed by the tightly packed sp2 bonded carbon atoms. Due to the unique structure of graphene, these carbon atoms form an excellent electrons carrier space. Therefore, graphene has extraordinary electrical properties such as high electron mobility at room temperature (250,000 cm2⁄V) [4,5] and ballistic transport and quantum hall effect at room temperature [6]. In addition, graphene also has excellent optical properties [5]. Excellent mechanical properties of graphene (i.e. 1TPa Young’s Modulus and 130GPa tensile strength) were also reported and the mechanical properties relate to the number of graphene layers and the internal defects of the graphene layers [5,7,8]. It is possible that the energy band gap of graphene can be changed by the uniaxial strain on graphene and this indicates that the uniaxial strain applied on graphene is able to affect the electronic properties of graphene [9,10]. In terms of thermal properties, the highest thermal conductivity at room temperature was reported as 5000 Wm−1K−1 [11]. Some potential applications of graphene have been suggested by researchers such as gas detection [12], transistors [13], nanocomposites [14], energy storage devices [6], barrier applications [15] and so on. However, graphene is still agiant gold mine that can be dug deeper.
Due to the excellent properties of graphene, it is believed that it could be used to enhance the performance of coating significantly. Graphene is very ideal to be efficient filler for high quality polymer matrix nanocomposite coating. In addition, it can be used with nanoparticles to form graphene–nanoparticles composites coating and as a high quality coating materials solely. Graphene is identified as a high water and oil repellent material while graphene oxide (GO) is hydrophilic [16]. This property can make graphene suitable for the coating that provides water and oil resistance. A test about frictional properties and wear resistance of multi–layers graphene film has been performed using an AFM by Lin and his co–workers [17]. Superb frictional properties and high wear resistance were reported. These results mean that graphene is able to become a protective coating against scratch or other physical damage toward a substrate. Graphene was also proved to be an effective corrosion barrier material because it was considered inert under the conditions where chemical reactions of other substrates will take place [18]. As a result, it is also promising in improving anti–corrosion property of a coating system.
In coating applications, graphene is believed to be promising but the articles designated for coating applications are limited. In this review, the synthesis methods and functionalization of graphene were described briefly. The articles about utilization of graphene in coating published in these years were reviewed. The conclusions summarized the published researches and suggested the future research perspectives.
Graphene based materials: Synthesis methods and functionalization
Synthesis methods: Different methods have been developed to synthesize graphene. However, not all of them can be used to synthesize good quality graphene efficiently. Three major routes, mechanical exfoliation, reduction from graphene oxide and chemical vapour deposition (CVD), are regarded as the promising routes to synthesize graphene and they are possible to be used for large scale graphene production in the future. Among them, the chemical route, graphene reduced from graphene oxide were frequently used to study the utilization of graphene based materials in different applications.
Mechanical exfoliation: The first graphene sheet was produced by simple mechanical exfoliation [3]. Therefore, mechanical exfoliation became a very attractive method for the researchers to produce graphene initially. The very first mechanical exfoliation method was a simple peeling process where pre–treated graphite was fixed onto a photoresist and graphene layers were peeled off by a scotch tape [3]. Although this simple method can produce graphene with extraordinary properties, it is limited by its low efficiency. Many efforts have been made to improve mechanical exfoliation method. Ultrasonic devices, solvent and surfactants were used to modify this process to produce high quality graphene in larger scale. The solvents and the surfactants can be intercalated into the atomic layers of graphite to form graphite intercalation compounds to prevent agglomeration and assist further separation of graphene single layer [5,19,20]. The influence of ultrasonic power, time and solvent used on the volume of graphite intercalation compounds was also investigated [5]. Although the use of solvent and surfactants can help to produce good quality graphene in larger scale, their major drawbacks are high solvent cost and the difficulties in following graphene deposition caused by high solvent boiling point [5,19]. Graphite oxides produced by chemical methods were also used in mechanical exfoliation. However, the subsequent produced graphene has inevitable structure defects which could disrupt the electronic structure of graphene. These structure defects could not be restored by chemical reduction or thermal annealing [21-23]. Hence, physical exfoliated graphene is preferred when graphene structure is required in an application. However, it is still extremely challenging to scale up mechanical exfoliation process to produce large amount of graphene in a cost effective way with commercial available technologies and devices.
Chemical vapour deposition (CVD): Chemical vapour deposition was first reported in 2006. Ni foil was used as a substrate and camphor was used as precursor [24]. Since then, chemical vapour deposition method was received more and more attention for being regarded as a new promising route to produce graphene in large scale [5]. In addition, this method was able to control the number of graphene layers and minimize the folding of graphene, and this meant controlled thickness graphene film could be synthesized [5,23,25,26]. Medium–high carbon solubility (>0.1 atomic%) substrate like Ni and low carbon solubility (<0.1 atomic%) substrate like Cu have different graphene growth mechanisms [5]. For high carbon solubility substrate, graphene layer is grown from the precipitation of carbon on the substrate, which is dissolved into the substrate earlier, after cooling. A typical CVD process generally has three steps [26,27].
1. The substrate is put into a chemical vapour deposition chamber at a setting vacuum and temperature with a diluted hydrocarbon gas.
2. The dissolve of carbon atoms into the substrate starts at a relatively low temperature.
3. Graphene layers are formed from the out–diffused dissolved carbon atoms in the followed rapid quenching.
The type and concentration of the hydrocarbon gas and the thickness of the substrate determine the concentration of dissolved carbon atoms. Both cooling rate and the concentration of dissolved carbon atoms control the thickness and the crystal structure of the graphene layers [26,27]. For the low carbon solubility substrate, the growth of graphene does not company with a diffusion process. The graphene layers are grown on the surface of the substrate and this process is a four–steps process [28]:
1. Methane is deposited on the substrate to form CxHy with exposing the substrate to hydrogen.
2. Nuclei stars to form from the local super–saturation of CxHy on the substrate.
3. Graphene islands are grown from the nuclei on the substrate surface.
4. Graphene covers the substrate surface.
Whether graphene can cover the whole substrate surface depends on the amount of CxHy on the substrate. Some modifications of chemical vapour deposition have been carried out in recent years. For example, plasma can be used to enhance chemical vapour deposition process that provides a route to synthesize graphene with lower temperature and shorter deposition time [29]. Although CVD is believed to be an ideal route to synthesize large area graphene sheet, the graphene produced from this technique still has intrinsic defects which allow the transportation of some molecules. Therefore, detrimental effect on the barrier properties of graphene sheet is resulted [30]. In addition, the costs of equipment and the time required for synthesis of large amount of graphene are the key limitations.
Reduction and synthesis of graphene oxide: Although mechanical exfoliation and CVD can produce high quality graphene, it is still extremely challenging to enlarge the synthesis scale cost effectively with commercial available technologies and devices. Researchers more focus on the synthesis and reduction of graphene oxide (GO) because graphene oxide and reduced graphene oxide can be synthesized easily. Graphene oxide is usually synthesized from the oxidation of graphite by strong oxidants based on Brodie [31], Staudenmaier [32], Hummers’ method [33] or some other modification of these methods. Hummers’ method was more widely used and many modifications had been made to synthesize graphene oxide for designated applications [5,13,34]. GO can be easily dispersed in many solvents and especially well in water which facilitate any subsequent processing [35]. The reduction of GO to is basically a chemical route to produce graphene with compromised properties induced by the chemical process. GO can be reduced in either chemical routes or thermal routes. Various chemicals had been reported to reduce GO such as hydrazine [23], hydroquinone [36], sodium borohydride (NaBH4) [37] and ascorbic acid [38]. Hydrazine hydrate reductant was found to be the best one to produce thin and fine reduced graphene oxide (RGO). However, NaBH4 exhibited the best efficient to reduce graphene oxide although it can slowly react with water [5]. Thermal reduction of GO utilizes heat treatment to remove oxide functional group on GO to produce RGO [5,13]. A simple and low temperature one–step solvothermal method was reported by Dubin et al.[39]. The reduction of GO was resulted from thermal reduction at 200 and the reaction with N–methyl–2– pyrrolidinone (NMP) molecules. The detailed chemistry of reduction and the study toward synthesizing better properties RGO will grow rapidly as the research in graphene moving forward.
Functionalization of graphene
The functionalization of graphene is the major route to stabilise graphene suspension in a complex environment without agglomeration takes place. For composites based coating, functionalization of graphene plays a very important role to achieve good interfacial bonding between matrixes and graphene sheets. Graphene functionalization can be achieved via physical or chemical approaches and there are three major categories of functionalization: functionalization via organic species, functionalization via macromolecules and functionalization via nanoparticles [40].
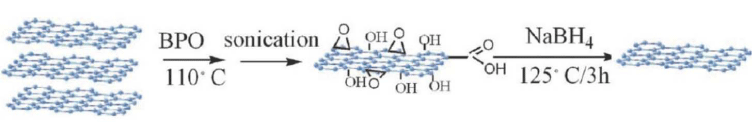
Many organic substances can react with the p bonds of graphene and, therefore, different functional groups can be introduced for different purposes. The oxidation of graphite can generate oxidised functional group on graphene layers which give the opportunity to produce stable graphene suspension in water or some organic solvents. GO is easy to disperse in water because of its hydrophilic nature. However, it is not soluble in every organic solvent and, therefore, functionalization of graphene is necessary to enable formation of stable graphene suspension with different organic solvent [40,41]. Further treatments of the oxidised groups of GO by organic species can also introduce functional groups such as carboxylic groups, enabling graphene to be available for more applications [42,43]. According to literature, radical reaction can be used as the second route to synthesize graphene oxide and further functionalization. One example is the utilization of benzoyl peroxide to synthesize graphene oxide [44]. The preparation procedure is shown in Figure 1.
Figure 1: The procedures to prepare GO and reduced GO using BPO [32].
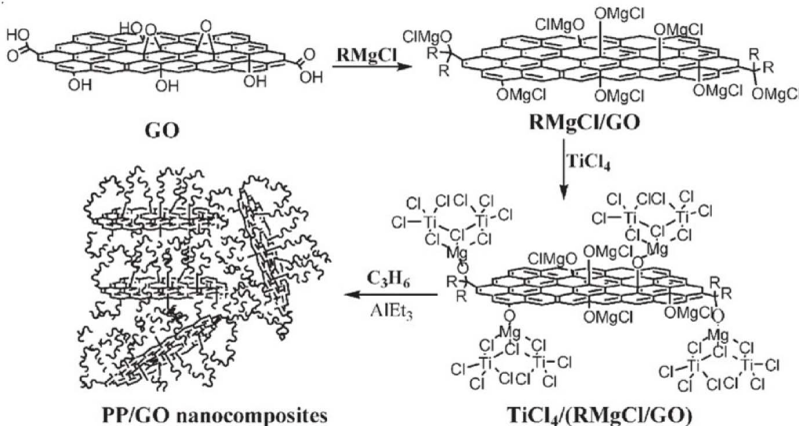
The functionalization of graphene via macromolecules is achieved by grafting macromolecules onto graphene sheets. There are two kinds of methods for this type of functionalization: “Graft from” method and “Graft to” method [40]. For “Graft from” method, initiators are immobilized on graphene network for further reaction to take place. One example of this method is the grafting of polypropylene chains onto graphene network by in situ Ziegler-Natta polymerization (Figure 2) [45]. In terms of “Graft to” method, the key point is cooperating graphene into polymer matrixes to form composites with the presence of functional groups on graphene sheet which able to form covalent bonds with polymer matrixes. One example for this kind of method is the formation of nanocomposites by ring opening reaction between epoxide and graphene pre-functionalized by amines [46] . Comparing “Graft to” and “Graft from” methods, “Graft from” method is more likely to improve the compatibility of graphene with organic solvents and polymer matrixes whilst “Graft to” method is to fabricate graphene-polymer nanocomposites by covalently bond functional groups on graphene network to polymer matrixes[40].
Figure 2: Procedure to graft PP chains on graphene via in situ Ziegla-Natta polymerization [34].
The third route to functionalize graphene is essentially the route to fabricate graphene–nanoparticles (G–NPs) composites. The nanoparticles are well investigated in these years and they are widely regarded as providing profound property enhancement in many applications. The combination of graphene and nano–particles is to achieve the synergistic effects of both materials with the goal of developing excellent properties composites. There are three main strategies to synthesize graphene–nanoparticles composites: pregraphenization, post–gaphenization and syn–graphenization [5]. For pre–graphenization, reduced graphene oxide is synthesized first and then mixed with nanoparticles to form composites. One example for this strategy is the synthesis of Pd nanoparticles on graphene oxide Sheets via electrochemical method [47]. The solubility of reduced graphene oxide in different organic solvent and the addition of second phase nano–particles are the major consideration in this strategy. In post–graphenization, nanoparticles are prepared first and, then, nanoparticles and appropriate metal salt precursors are mixed with graphene oxide suspension followed by reduction. The synthesis of RGO⁄TSCuPc Composite is an example for post–graphenization. RGO solution was prepared first and then it was mixed with TSCuPc aqueous solution for subsequent processes which deposited NPs on RGO network [48]. Syn–graphenization is a one–step approach in which the second component of the composites functions as a stabilizer to improve the composites’ properties. A good example for syn–graphenization can be the study of synthesizing graphene–CdS quantum dot nanocomposites by a one–step facial method. In this one–step facial method, solvothermal reduction of GO and deposition of CdS on GO network took place simultaneously [49].
Applications of graphene in coating
Pristine graphene based materials coatings
It may be convenience and cost effective to use pristine graphene based materials directly without further processing such as synthesize graphene on a substrate to form a coating layer with commercial available technique. The published papers indicated that this concept was possible and the coated substrates showed improved properties. However, some problems still need to be resolved before commercialization.
Coatings for electrical applications: For the utilization of graphene, the electrical properties of graphene are often the most favourable because they are the most promising and attractive properties in industry. A potential transparent conductive coating suitable for the practical touch panel application was fabricated on copper substrate via the combination of plasma CVD and roll–to–roll process [50]. Improved electrical conductivity and highly uniform transmittance and haze were reported. Although the electrical conductivity was improved, the measured sheet resistance was still so high that could not meet the requirements in electrical applications. A large conductive transparent chemical converted graphene film was fabricated via spray coating method [51]. GO was synthesized from expandable graphite according to modified Hummer’s method and mixed with hydrazine monohydrate afterward. The substrate sed for coating was quartz. The conductive film exhibited low sheet resistance of 2200 Ω sq–1 and high transmittance of 84%. However, the stabilization time of GO–hydrazine suspension is a few weeks long and this limits the conductive film to be used in commercial applications. The success of electrophoresis deposited graphene film on glass substrate was reported by Ishikawa et al. [52]. Graphene was reduced from GO, which was produced by modified Hummers’ method, after being electrical deposited on glass substrates. The lowest sheet resistant and highest transmittance measured were 4.59 x 104 Ω sq–1 and 83.8%, respectively. The sheet resistant measured here was lower than the CVD fabricated graphene film mention previously but it is still too high to be used in commercial product. Uniform reduced GO film for soft touch screen was able to be fabricated on PET substrate via large scale rod–coating [53]. The reduced GO film reported has even lower sheet resistance which is up to 1,800 Ω sq–1 and high transparency and high flexibility were also reported.
Although the most attractive property of graphene is electrical conductivity, other outstanding electrical properties such as fast electron mobility make it promising in improving other electrical properties other than electrical conductivity. A hierarchical GO⁄ MnO2 nanostructure sponge was fabricated by dipping commercial available macroporous sponge into graphene oxide and MnO2 solutions in a sequence [54]. The GO used was produced by modified Hummer’s method. The results showed that this coated sponge could be used as super capacitor or be used in batteries because of high specific capacitance, wide operation range, good energy and power density, and excellent cycling stability. Its outstanding properties included about 10% degradation after 10,000 cycles at a charge- discharge, specific current of 10 A⁄g, retaining 90% of its capacitance after 10,000 cycles under a scan rate of 10 V⁄s, maximum E of 2.08 Wh⁄ kg and highest P of 94 kW⁄kg at the operate voltage of 0.8 V. For the utilization of electron mobility of graphene, Jeon et al. reported than moderately RGO could be used as the hole transporting layer (HTL) in polymer solar cells (PSCs) [55]. The reduction of GO was performed via the thermal treatment of solution processed graphene film at about 250 under air atmosphere. The reduced graphene oxide layer was coated onto indium tin oxide (ITO) coated glass substrate by spin coating method. Compared to poly (3, 4–ethylenedioxythiophene): poly (styrene–sulfonate) HTL, the transportation efficiency of moderately reduced graphene oxide HTL was improved slightly but it was much more stable after exposing the HTL into air. In terms of transmittance properties, an example is the attempt to use RGO coating on aluminium film for an effective optoacoustic transmitter used high pressure and high frequency ultrasound generation [56].Graphene oxide was synthesized by modified Hummer’s method and then it was reduced to form RGO. RGO was spin coated on glass substrate before the aluminium film deposition on the RGO. The RGO coated aluminium transmitter generated enhanced optoacoustic pressure of 64 times the aluminium–alone transmitter under a pulsed laser excitation. As a result, this RGO coated aluminium film was very ideal for laser–induced ultrasound application. In addition, graphene based materials could be used to improve the photocatalytic activity of TiO2 film [57]. GO was synthesized through a modified Hummers’ method and it was coated on TiO2 film by spin coating method. The GO coated TiO2 film exhibited better photocatalytic activity due tothe giant p–conjunction system and two dimensions planar structure. However, the photocatalytic activity decreased on higher GO loading as a result of absorbance and scattering of photons via excess cabon in the system. There are still a lot of studies related to utilize graphene based materials as coatings in electrical applications and some of them are listed in Table 1.
Coating type
Synthesis method
Substrates
Coating methods
Materials detected
Ref
Graphene
Reduction of graphene oxide
Stainless steel sire fibre
Dip coating
Six selected pyrethroid pesticides
[78]
Graphene
-
Stainless steel wire
Sol-gel
Organochlorine pesticides (OCPs)
[79]
Graphene nano sheets
Microwave assisted reduction of graphene oxide
Stainless steel wire
Dip coating
OCPs
[80]
Graphene
Their previous work
Stainless steel wire
Dip coating
Triazines herbicides
[81]
Graphene
Reduction of graphene oxide
Stainless steel wire
Dip coating
Carbamates
[82]
C18 functionalized graphene oxide
-
Stainless steel wire
LBL
Polycyclic aromatic hydrocarbons (PAHs)
[83]
Table 1: Pristine graphene based coatings used in electrical applications.
However, the water vapour barrier properties were not improved. For water barrier properties, a superhydrophobic structures was fabricated by coating multi–walled carbon nanotubes (MWCNTs) and ROG onto silica colloid [69]. Graphene oxide was produced by modified Hummer’s method and then it was reduced to form RGO. RGO and MWCNTs were coated onto the colloids by LBL in which RGO sheets were negatively charged and MWCNTS were positively charged. The fabricated composites exhibited controllable surface hydrophobicity which could be readily controlled from 119 to 151°C by controlling the number of the bilayers deposited onto the colloidalparticles. In addition, graphene fluoride was adopted as transparent hydrophobic coating innovatively by Zhang et al. [70]. Graphene fluoride was produced from liquid phase exfoliation of graphite fluoride and graphene fluoride coating was spin coated on glass substrate for further characterization. The measured contact angle was 123° and the light transmittance was up to 92% both indicated that a good candidate for transparent hydrophobic coating was produced. Moreover, long service life of graphene fluoride coating was detected in water erosion experiments and ultraviolet aging tests.
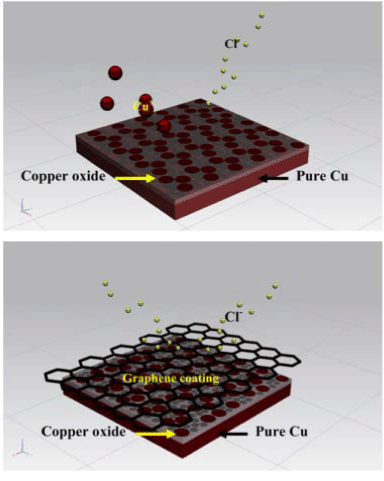
The efficiency of graphene based materials as anti–corrosion and anti–oxidation coating on different substrates with different coating methods has been reported [71-77]. Most of reported graphene coating layers was fabricated by CVD and their results were summarized in Table 2. Nilsson and his co–workers suggested the limitations of graphene coating for corrosion inhabitation on metal surfaces where graphene coated by CVD could only function as corrosion inhibitors at low gas pressure [73]. Nayak et al. indicated that graphene was a good protective coating due to its inertness to oxidizing gas and liquid solutions but its oxidation resistance was limited below 500 [74]. An anti–corrosion mechanism of CVD graphene coating on copper substrate was proposed by Singh Raman et al. and this mechanism may be used to explain the anti–corrosion behaviour of graphene film on different metal substrates (Figure 4) [75]. Apart from CVD, spray coating was used to coat graphene on gold coated cuprous substrates by Noel et al. [77]. Graphene solution was purchased from market directly. The coated substrates had less weight gain during corrosion test than pure gold coated substrates and low contact resistance and low friction coefficient were also identified. The spray coated graphene was not only be used as anti–corrosion coating but also be used as surface protection coating resulted from its low friction coefficient. Dry contact sliding was carried out by Won and his co–workers to test the durability and degradation mechanisms of graphene coating on copper substrates [78]. The graphene coating was grown on Cu substrates by CVD. The friction coefficient of graphene was obtained as about 0.18 and remained stable up to several thousand cycles. As a result of the formation of amorphous carbon layer on the wear crack, an increase of friction coefficient after a certain time was detected. From these results, pristine graphene can be a good candidate as surface protection coating.
Substrates
The performance of graphene coating
Ref
Cu and Cu/Ni alloy
Metal surfaces were well protected from oxidation even after heating at 200
in air for up to 4 h
[60]
Cu
Deterioration was not detected under vigorous flow boiling conditions for long exposure
[61]
Pt
Reconstruction of Pt could be preserved in O2 pressures as high as 10-4 mbar and CO pressures below 10-6 mbar
[62]
Ni
Oxidation resistance of the coating was effective up to post annealing of 500?
[63]
Cu
Corrosion resistance of coating is 1.5 orders of higher magnitude tan uncoted substrates
[64]
Cu and Ni
In an aerated Na2SO4 solution:
Direct coated copper films were corroded 7 times slower than bare copper.
Direct coated nickel substrates were corroded 20 times slower than bare nickel.
[65]
Table 2: Performance of graphene as anti-corrosion and anti-oxidation coating.
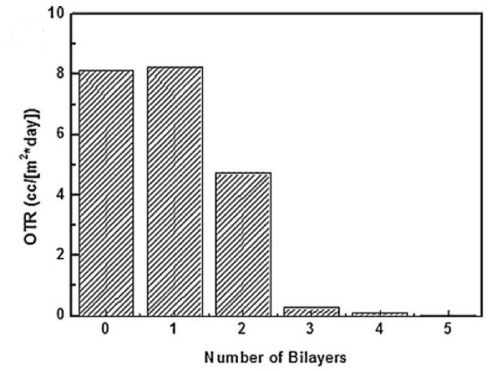
Figure 2: OTR versus number of bi layers [57].
Coatings for sensing and absorbent applications: The unique structure of graphene based materials makes it a promising candidate in sensing and absorbent applications [4]. A superhydrophobic and superoleophilic sponge was fabricated by coating graphene on melamine sponge via dip coating [79]. This graphene coated sponge exhibited high absorption capacities up to 165 times of its weight, high selectivity, good recyclability, lightweight, robustness, and inertness to corrosive environments. Biochar, a attractive material has potential applications in many environmental areas, could be coated by graphene to enhance thermal stability and absoption properties [80]. Graphene was produced by mechnical exfoliation from graphite powder and it was coated onto cotton wood by dip coating before pytolysis into biochar. Thermal decomposition temperature of graphene coated biochar was 64 higher than pure biochar and enhanced absoption ability to aqueous methylene blue was also identified.
Figure 4: Schemes of anti-corrosion mechanism of CVD graphene coating on copper [67].
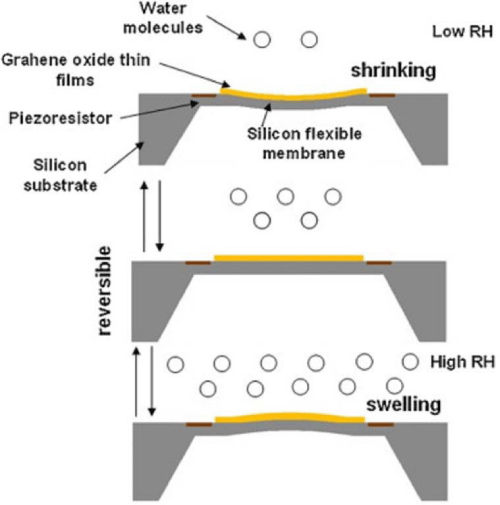
Compared to absorbent applications, graphene based materials had attracted more attention of the researchers in sensing applications. Graphene was coated on quartz crystal microbalance (QCM) by solution drop–coating method for the detection of formaldehyde [81]. The QCM–type sensor showed frequency change when exposed to different concentrations of formaldehyde. Perfect linear correlation between frequency shifts versus concentration change of HCHO was achieved during the analysis. A potential bio–sensor was produced via coating reduced graphene oxide nano ribbons (rGONRs) on Si⁄SiO2 substrates by spray coating [82]. GONRs were fabricated through chemically unzipping multi–walled carbon nanotubes and they were deposited on Si⁄SiO2 substrates before reduction. High on⁄off ratio and ability of detecting adenosine triphosphate (ATP) molecules were identified in this rGONRs network. Graphene based materials can also be used in water sensing applications. Yao et al. developed graphene oxide⁄silicon bilayers for humidity sensing applications [83]. The ultimate sensitivity could reach 79µV⁄%RH with a wide detection range of 10–98%RH and the humidity sensibility could be enhanced by increasing the thickness of the graphene oxide coating. A humidity sensing mechanism was also proposed (Figure 5). Graphene oxide coated quartz crystal microbalnce was also used for humidity detection [84]. Graphene oxide was synthsized by the modified Hummers method and spin coated on quartz crystal substrates. The GO coated quartz crystal substrates showed excellent humidity sensing properties and a linear frequency response versus RH in the wide detection range of 6.4-93.5% RH was obtained.
Figure 5: Humidity sensing mechanism of graphene oxide/silicon bilayer [73].
Apart from using graphene based materials to develop new sensing structure, graphene based materials can also be coated onto commercial available equipments such as probes to improved their performance. As reported by Zhange et.al, graphene can be coated onto a plunger–in–needle microsyring for solid–phase microextraction (SPME) device as a sorbent material toward UV filters [85]. The graphene was coated on the microsyring by sol–gel method. Compared to other commercial available SPME fibers, graphene coated mocrosyring exhibited different selectivity and showed high extraction efficiency for light polar salicylates and 4–MBC. There are a lot of related reports about the utilization of graphene based materials in SPME and they were summarized in Table 3. The performance of graphene and graphene oxide coated columns as station phase for capillary electrochromatography and capillary liquid chromatography was investigated by Qu et al. [86]. Graphene oxide was synthesized by the modified Hummers method and then was coated on a capillary column via 3–aminopropyldiethoxymethyl silane as coupling agent. Graphene coated column was reduced from GO coated capillary. Effective separation of of natural, basic and proteins were observed on the GO coated column. However, graphene coated column had poor separation performance. Graphene coated Fe3O4 nanoparticles coule also be used as adsorbent material for high–performance liquid chromatography [87]. Graphene was synthesized via the reduction of graphene oxide obtained by modified Hummer’s method. Graphene was coated on Fe3O4 nanoparticles by in situ chemical coprecipitation of Fe2+ and Fe3+ in an alkanine solution in the presence of graphene. Compared to other methods (e.g. SPME), the type of magnetic nanoparticles exhibited high adsorption capacity, rapid adsorption rates, low amount of sorbents can be used and short equilibrium time to extract triazine herbicides. Similar study was conducted for gas chromatography using GO [88]. The capillary column was pretreated with 3–AMDS toluene solution and it was then dipped into GO solution to form GO coated column. Separation of various organic compounds with good separation efficiencies was achieved.
Coating type
Synthesis method
Substrates
Coating methods
Materials detected
Ref
Graphene
Reduction of graphene oxide
Stainless steel sire fibre
Dip coating
Six selected pyrethroid pesticides
[78]
Graphene
-
Stainless steel wire
Sol-gel
Organochlorine pesticides (OCPs)
[79]
Graphene nano sheets
Microwave assisted reduction of graphene oxide
Stainless steel wire
Dip coating
OCPs
[80]
Graphene
Their previous work
Stainless steel wire
Dip coating
Triazines herbicides
[81]
Graphene
Reduction of graphene oxide
Stainless steel wire
Dip coating
Carbamates
[82]
C18 functionalized graphene oxide
-
Stainless steel wire
LBL
Polycyclic aromatic hydrocarbons (PAHs)
[83]
Table 3: Graphene based coatings used in SPME application.
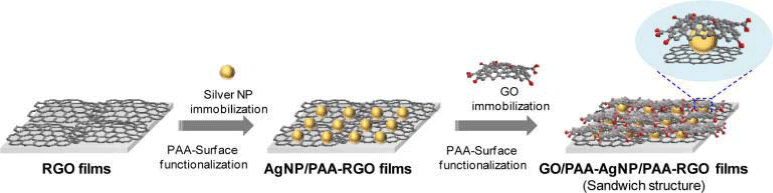
Signal enhancement is also an area that can be benefited from graphene based materials. According to Hao and his coworkers, graphene can also be cated onto conventional metallic surfaceenhanced Raman scattering (SERS) substrates to improve the sensitivity of SERS detection [95]. Graphene was synthsized by CVD on copper foil and then was transferred to gold nano structures (nanoparticles or nanohole arrays). The graphene coated substrates exhibited 3–fold or 9–fold enhancement in Raman signal of methylene blue compared to bare nanohole or nanoparticle substrates, respectively. Kim et al. developed a GO⁄polyallylamine hydrochloride (PAA) - Ag nanoparticles (AgNPs)⁄PAA–RGO three layers films for SERS enhancer and anti–corrosion coating [96]. The preparation procedure is shown in (Figure 6). Graphene oxide was synthesized by their method and GO was reduced by hydrazine monohydrate. Ramansignal on R6G on was enhanced by 6 fold on GO⁄PAA–AgNPs⁄PAARGO whilst AgNPs⁄PAA–RGO film was 1.67 fold. The GO⁄PAAAgNPs⁄ PAA–RGO films exhibited prolonged lifetime up to 72 days against oxidation under ambient conditions. Cobalt nanoparticles coated by graphene surface functionalized with benzylamine groups were nanomagnets that were able to broaden the range of analytes for surface–assisted laser desorption⁄ionization mass spectrometry(SALDI–MS) analysis [97]. This G coated CoNPS was prepared flame spray pyrolysis using metal containing organic precursors. The nanomagnets were also able to extract perfluorooctanesulfonate from large volumes of aqueous solutions by magnetic separation.
Figure 6: Preparation procedure of G/PAA-AgNps/RGO sandwich structure [85].
Graphene based nanocomposites coatings
As reported previously, pristine graphene based materials coating exhibited excellent properties in different applications. However, it is still challenging when proceeds to large scale commercialization. Adhesion is the critical factor of coating durability and applicability. Some surface treatments and graphene functionalization routes can help to achieve good adhesion but these may not good enough for long term durability. In addition, the pristine graphene based coating layer may not dense and many micro channels may exist between graphene sheets. The existence of micro channels is detrimental to long term durability as these micro channels allow erosion and corrosion to take place easily. A porous coating layer is impossible to be commercialized. The technologies nowadays are difficult to produce perfect defect free graphene based materials. Therefore, the development of graphene based nanocomposites is an alternative route to trigger the commercialization of graphene based materials. In coating applications, researchers mainly focus on polymeric graphene based nanocomposites and graphene–nanoparticles composites (G–NPs).
Graphene–nanoparticles composites as coatings: In G–NPs, graphene based particles are usually coated onto nanoparticles first and then the coated nanoparticles can be used as coating materials. There are some published articles related to electrical properties improvement by G–NPs. Wang et al. reported that graphene oxide wrapped sulphur particles could be used to modify the capacity and cycling stability of rechargeable Lithium–Sulfur battery cathode material [98]. The resulted composites exhibited high and stable specific capacities up to about 600 mAhg–1 over more than 100 cycles. Another example is the attempt to adopt SnO2–SiC⁄G nanocomposite for high performance Lithium–ion storage [99]. SnO2–SiC⁄Gnanocomposite was in situ generated by simple ball milling method and it was graphene coated onto SnO2–SiC core–shell structure in which SnO2 nanoparticles were uniformly deposited onto SiC core. Graphene was produced from reduction of graphene oxide. The nanocomposite exhibited a high reversible capacity of 810 mA h g–1 and 83% capacity retention over 150 charge⁄discharge cycles between1.5 and 0.01 V at a rate of 0.1 A g–1. A high reversible capacity of 425 mA h g–1 was obtained at a rate of 2 A g–1as well. A reversible capacity of 1451 mA h g–1 and good cyclability were measured when discharged to a higher potential at 3.0 V. G–NPs were also used to improve photoactivity in their related applications. A graphene– TiO2 nanoparticles composite coating was prepared and coated onto a conducting support medium for enhanced photoelectrocatalytic activity [100]. Graphene was reduced from GO. The composite coating was applied onto F–doped tin oxide (FTO) via dip coating method to form a film electrode. Compared to pure TiO2 film electrode, graphene–TiO2 composite exhibited better photoelectrocatalytic capacity. In addition, a TiO2–dextran–Graphene oxide nanocomposite, which was prepared by an environmental friendly strategy, could be used to enhance photocatalytic activity [101]. This composite coating was applied onto the substrates via spin–coating method. The results showed that TiO2– dextran–GO composite exhibited excellent photocatalytic activity and photovoltaic response than pure TiO2 because the electron–hole pairs in the composite had longer life time. Graphene based TiO2 nanocomposites were mostly studied in photocatalytic activity but there were many other systems could be used as potential coating on a substrate to improve photocatalytic activity as suggested by Zhang et al. and Yang et al. [102,103]. In their group, the utilization of G–NPs in both ‘selective’ and ‘non–selective’ processes were studied extensively [104-106], and the effect of different carbon based materials for photocatalytic activity was investigated as well [107].
In terms of barrier properties, there is only one related report. Kou et al. have developed a facial strategy to synthesize GOSiO 2 nanohybrids as general building blocks for large area superhydrophilic coating [108]. Go was synthesized according to their own method and SiO2 nanoparticles were deposited onto GO nanosheets via in situ hydrolysis of TEOS. Ceramic, PP and lotus leaf were used as substrates. Compared to bare substrates, all the coated substrates had much smaller contact angles which indicated excellent wettability of water was achieved. In addition, GO–SiO2 sheets could be made intopaper film by a filtering technique and exhibited high conductivity of 103-104 Sm–1 after reduction by hydrazine vapour. G–NPs can also be used in SPME application to improve extraction performance. ZnO⁄ graphene nanocomposite for SPME silica fiber coating was developed by Zhang et al. [109]. Graphene was produced by reduction from GO. The SPME fiber coated by ZnO⁄graphene nanocomposite exhibited enhanced durability, enlarged surface area, higher extraction selectivity and sensitivity toward sulphur compounds.
The challenges of G–NPs commercialization are similar to pristine graphene based materials coatings. A dense layer G–NPs coating layer without any pores is difficult to achieve. The process of coating graphene based materials on nanoparticles is complicated and is not cost–effective. G–NPs may have better properties but the cost to produce this type of composites is much higher than using pristine graphene based materials.
Polymeric graphene based nanocomposites as coatings: The incorporation of graphene based materials into polymer not only can improve one property of a polymer but also enhance several properties at the same time such as electrical properties and mechanical properties. Polyurethane acrylate (PUA) coating reinforced by graphene was synthesized by Liao et al. using in–situ polymerization on a TEFLON plate [110]. Their results showed that the electrical conductivity of the composite increased with increasing graphene loading. The cured composites exhibited lower percolation concentration than uncured composite and the addition of graphene could reinforce the mechanical properties of PUA coating in rubbery region. A graphene reinforced poly ((butylene terephthalate) (PBT) composite was synthesized by Fabbri et al. and they suggested that this composite could be a potential conductive coating [111]. The key results of this study are shown in Table 4. The decomposition temperature under oxygen atmosphere exhibited optimum value at 0.75 wt% graphene loading while the melting temperature decreased with the increase of graphene loading. The Percolation threshold appears between 0.5 wt% and 0.75 wt% graphene loadings. The composite with 0.5 wt% graphene loading has optimum hardness but the hardness decreased with the increase of graphene loading. The performance of DSSCs was also improved by using Graphene⁄Nafion® composites as counter electrode [112]. Graphene was reduced from GO synthesized according to Staudenmaier’s method and graphene⁄ Nafion® was coated on ITO glass by drop coating method. A solarto– electricity conversion efficiency (η) of 8.19% was achieved by using this type of composite. Although it was only a little smaller the η of s–Pt film (8.89%), the cost is much lower. A transparent conductive graphene oxide⁄poly (ethylene glycol) diacrylate coating was developed for magnetic shielding and anti–static applications [113]. The coating was applied onto glass substrates and cured by UV light. The percolation threshold was 0.02 wt% and the lowest sheet resistance measured was 6300 Ω sq–1.
Samples
Ti. ox (?)
Tm(?)
Electrical resistance (MO)
Hardness (HV)
PBT
389
228.4
-
15.1
1.2
PBT/G 0.5 wt%
388
223.6
760
26.6
1.8
PBT/G 0.75 wt%
404
214.2
200
23.1
1.7
PBT/G 1.0 wt%
390
212.3
50
9.5
2.6
Table 4: Comparison of pure PBT and PBT/Graphene coatings.
In terms of protective coatings, there are a few researches published in recent years. An anti–corrosion polyaniline⁄graphene composite coating for steel has been prepared by Chang and his co–workers [114]. The graphite powders were exfoliated and functionalized with4–aminobenzonic acid (ABA), and the graphene and graphene–like sheet were obtained from a direct electrophilic substitution reaction in a PPA⁄P2O5 medium. Compared to bare steel, PANI coating and clay reinforced PANI coating, graphene reinforced PANI coatings exhibited the best electrochemical corrosion resistance, oxygen barrier efficiency and water barrier efficiency (Table 5). Graphene reinforced polyphenylene sulphide (PPS) was reported that exhibited seven times higher wear life than pure PPS coating [115]. GO was synthesized by the Hummer’s method and it was functionalized to yield organophilic graphene. The as–produced solution was coated on steel substrates through spray coating. The major wear form was identified as abrasive wear for graphene reinforced PPS coating whilst the wear form of pure PPs coating was adhesive wear. In addition, polyamide 11 (PA11)⁄graphene coating could also be used to improve tribological properties of PA11 [116]. GO was functionalized to produce organophilic graphene. The PA11 and organophilic graphene composites were spray coated onto steel substrates. The wear life of the composite coating increased by 460%-880% compared with that of pure PA11 coating but the values of friction coefficients had hardly changed. Higher performance polyurethane (PU)⁄functionalized graphene nanocomposites were developed by Cai and his co–workers for mechanical and thermal stability improvement and it could be a potential surface protection coating [117]. The morphology of resulted composites is shown in Figure 6 and a good dispersion of GO was observed. Improved scratch resistant, thermal stability, hardness indentation and Young’s modulus were reported. A study suggested that graphene could be used as nano–additives in galvanic corrosion protection coating [118]. Graphene was purchased from a company. ITO and graphene were used as additives of primer epoxy coating which spray coated on the surfaces of carbon fiber composites. The results showed that both ITO and graphene could be used for lighting strike protection but ITO had better protection efficient.
Samples
Electrochemical corrosion measurements
Oxygen Permeability (barrer)
Vapour Permeability Rate ( g/hr.m2)
Ecorr (mV)
Icorr (μA/cm2)
Steel
-789
14.71
N/A
N/A
PANI
-647
307
0.75
170
0.5 wt% Graphene reinforced PANI
-537
0.38
0.13
20
0.5 wt% Clay reinforced PANI
-584
1.38
0.35
60
Table 5: Comparison of PANI coating, Steel and composite coatings.
Conductive coatings and protective coatings receive a lot of attraction but the study toward mechanical properties and thermal stability of a coating is important as well. A graphene–reinforced waterborne polyurethane nanocomposite coating was fabricated by sol–gel method using silane–functionalized graphene nanosheets [119]. The graphene oxide powder was synthesized according to Hummers method and the graphene oxide powder was chemicallyreduced to form graphene nanosheets suspension. With increasing the weight percent of graphene in the coating (from 0 to 5 wt%), the tensile strength increased from 11.8 MPa to 20.2MPa, the Young’s modulus increased from 69.8 MPa to about 118 MPa and the elongation at break reduced from 324% to 138%. In addition, the thermal properties of the waterborne coating were improved by graphene where the decomposition temperature increased from about 258 to about 270. Polyurethane acrylate coating could be reinforced by functionalized GO to obtain improved thermal stability and mechanical properties according to Yu et al. [120]. GO was functionalized to be UV curable with PUA. The initial degradation temperature of the PUA composite with 1.0 wt% functionalizedgraphene oxide was increased to 316 °C from 299 °C (pure PUA). The storage modulus and tensile strength of the PUA composite with 1.0 wt% functionalized GO were increased by 37% and 73%, respectively, compared with those of neat PUA. A functionalized graphene sheets⁄UV cured epoxy nanocomposite coating was developed to seek better mechanical properties by Martin–Gallego et al. [121]. The coating was coated on glass substrates and cured by UV–light for subsequent characterizations. Compared to pure epoxy resin, graphene reinforced epoxy nanocomposite exhibited higher glass transition temperature, higher stiffness and higher storage modulus for high temperature. The toughening effect of graphene platelets toward epoxy resin, which is widely used in coating application, was investigated [122]. The graphene platelets were produced from direct mechanical exfoliation and chemical modified for interfacial strength study. The glass transition temperature had a 14.7% increase compared to pure DGEBA and the highest fracture energy release rate G1c obtained was 613.4 Jm–2. The toughening mechanisms were identified as voiding, micro–cracking and breakage of graphene platelets. The possibility of polymeric graphene based composites being used in SPME application was studied by Zou et al. [123]. Graphene⁄Polypyrrole composite was coated onto stainless steel wire by in situ polymerization. The prepared graphene⁄polypyrrole coated fiber showed the highest extraction efficiency toward five phenols compared to a number of commercial fibers and polypyrrole or GO⁄polypyrrole coated fibers. The graphene⁄polypyrrole composite coating on fiber also exhibited good thermal and mechanical stability, excellent adhesion and long lifetime.
The published papers about graphene based materials reinforced composites are numerous and they may be used in coating applications if proper processing techniques are used. Yoo et al. summarized a list of graphene based materials reinforced polymers nanocomposites which could be used in all sorts of applications. Corrosion resistance and erosion resistance are vital for the commercialization of a coating because it relate to the life time of a coating system. It is worthy to commercialize a coating system only when it process both good properties and long life time. Orientation, particle size, aspect atios, number and winkles of graphene based materials in polymer matrix are the major factors that affect the corrosion and erosion resistant. The process of corrosion and erosion is basically a process of substances, which can deteriorate the substrate and coating, diffuse hrough the coating layer and damage the substrate. The utilization of fillers and produce defect free composites is to diminish or lengthen the diffusion paths of these substances. In order to achieve maximized barrier efficiency of the graphene based materials: 1) good dispersion of graphene based materials must be achieved; 2) the orientation of graphene sheets should be parallel to coating layer; 3) the aspect ratios of graphene sheets are high and the particle size of graphene particles is large; 4) aggregation and wrinkles of graphene sheets should be prevented; and 5) larger number of graphene sheets presented in the polymer matrix can help to increase the diffusion paths [15]. The most attractive property of graphene is electrical property. Although the graphene based materials can improve the electrical properties of a polymer, the achieved electrical properties still cannot meet the requirements of electrical applications. In general, the resistant of a filler reinforced polymer composite is determined by the tunnelling resistant between the particles and the contact resistant between fillers [124]. The electrical conductivity of polymers is generally low. Although graphene can improve their electrical conductivity, it is still not high enough to fulfill the industrial requirements. The combination of small particle size and large particle size conductive fillers is a way to further improve the electrical conductivity of polymer nanocomposites because small particle size fillers can act as a bridge between two large particle size particle to reduce the tunnelling resistance between them [124].
Coating methods
Different coating methods have been used recently and they were summarized in this section. Their possible advantages and limitations are discussed and their availability for different types of graphenebased coatings is suggested briefly.
CVD: Chemical vapour deposition method is a method to synthesize graphene layers on a substrate and transfer to other substrates. When utilizing this method in coating, graphene can be deposited onto the target substrates without the transfer process. More detailed graphene growth mechanisms were stated in the former graphene synthesis method section. CVD is a method with great possibility to produce graphene in large scale and this also suggests that large scale coating is also foreseeable. In addition, pure and dense coating can be produced by CVD method, and the CVD parameters can be adjusted to produce coatings with different surface morphology, thickness and even crystal structure. The major disadvantages of CVD are safety and hazard issues caused by the precursor gases, difficulty of depositing multicomponent materials and high equipment cost [125]. In general, CVD is suitable to apply highly dense and pure graphene based coatings such as pristine graphene on a substrate. The coating structure can be controlled at the atomic level or nanometer level.
Dip coating: Dip coating is a convenient method to coat thin film on a substrate and it is very frequently used for research purposes. However, it is not suitable for industrial processes due to the inconsistent coating quality. Compared to dip coating, spin coating is often used in industrial processes. The dip coating process generally has five steps [126]:
1. The substrate is dipped into the solution of the coating material at a constant speed. Before this step, pre–treatment process may be conducted according to different substrates.
2. The substrate remains in the solution for a designated time and is starting to be pulling up.
3. The coating thin film begins to deposit on the substrate while the substrate is pulled up. The thickness of the coating depends on the speed used in pulling up process. Slower pulling up gives thinner coating layer.
4. Excess liquid will be drained from the substrate surface.
5. Solvent evaporates from substrate surface to from a thin film. The evaporation may begin in step 3 if the solvent is volatile.
In research, dip coating is more convenience and feasible than other methods because it is a very simple method and do not require sophisticated equipment. Because of its simplicity, the coating layer produced by this method may not have good quality. For graphene based composite coating, the thickness distribution can be a problem. In terms of pristine graphene based materials coating, the coating layer produced may not be dense enough to provide extraordinary properties. In addition, the substrate cannot be too large and complicated. However, the biggest advantage of dip coating is simplicity. Although dip coating is more suitable in lab, it still can be a potential large scale coating method to produce products that can satisfy the applications for low standard requirements with a competitive price. This approach is possibly more suitable to produce graphene based composite coating than pristine graphene based coating because the viscosity of the graphene based composite coating is much higher. Therefore, it can develop a better interfacial adhesion toward a substrate and a more uniform coating layer. A subsequent treatment process may need to form a solid coating layer.
Spin coating: Spin coating is a process widely used for applying uniform thin film on a flat substrate. The applied materials are usually polymeric coatings and they are applied onto flat substrates in the form of solution. The solvents used are usually volatile. The driving force that drives the solution rapidly of the substrate is centrifugal force and a machine used for spin coating is called spin rotator spinner The centrifugal force is applied continuously until desired film thickness is achieved [126,127]. Several factors can affect the final film thickness: 1. kinematic viscosity; 2. coefficient of solvent diffusion; 3. the critical shear rate for onset of shear thinning of the viscosity; 4. the rotational speed; 5. the radius of the substrate; 6. the coating solution concentration. The two major influenced factors are the rotational speed and the coating solution concentration [126-128]. A typicalspin coating process is a four steps process [126,128,129]:
1. Polymer solution is deposited on the substrate with an excess amount to cover the substrate completely.
2. The substrate spins rapidly to displace the polymer solution with centrifugal force.
3. Laminar radial flow of the liquid layer of uniform thickness remaining on the substrate.
4. Removal of solvent via evaporation until the film stops flowing and is dried completely.
Some treatments were used to assist the coating process. For example, N2 gas was used to blow the graphene oxide coated siliconchip to accelerate the evaporation of water during the process of spin coating [83]. All the assisted treatments during or after the spin coating process are to obtain uniform continuous graphene based materials thin film. Compared to dip coating, the coating layer produced by this approach is denser and more uniform. The substrateshape and size is limited by the spin coating apparatus. Only flat surface can be spin coated. The spin coating method is not suitable for complicate shape product. In addition, this method is more suitable to coat pristine graphene based coatings or graphene–nanoparticles composite coatings on a substrate.
Layer by layer self–assembling (LBL): In multi–film production, layer–by–layer self–assembling method is very ideal. This method utilize the attraction between positive and negative charges to make the films self–assemble on a substrate and the this method is also called layer–by–layer deposition method [130,131]. In layer–by–layer self–assembling process, the films are deposited onto a substrate by immerging the pre–treated substrate into graphene solutions and polymer solution cyclically to form multi–layers films [130]. The substrate is pre–treated into positively charged by introducing positive charged functionalities such as amine functionalities [131]. The graphene solution can be directly used as negatively charged dipping particles but the polymer solution may need to be changed the pH to for subsequent dipping process [130,131]. This method is a method suitable for producing two components multi–layers coating. However, only some research papers investigated its applicability for graphene based materials. There is no direct indication of utilizing this method for coating applications. This method is similar to dip coating method where the substrates are dipped into the coating solution and are withdrawn to form coating layer, but the coating layer produced by LBL is denser due to the compact force originated from the attractive force between negative and positive charges.
Sol–gel approach: The sol–gel process is a widely used wetchemical process and it has been studied extensively since its discovery. Sol refers to the colloidal solution which acts as the precursor and it will evolve to gel which refers to the integrated network. The precursor sol can be deposited onto a substrate (e.g. via dip coating), casted into a mould with the designated shape or used to synthesize powders. The gel is generally a gel–like biphasic system containing both solid and liquid phases. Large quantity of liquid needs to be removed for sol turn into gel and the simplest method is pour off the liquid after sedimentation which requires a certain amount of time to take place. Drying process is required in sol–gel method to remove the liquid phase in the system. Severe densification and shrinkage always accompany the drying process. After the drying process, a thermal treatment is usually necessary for further polycondensation, mechanical properties enhancement and structural stability. The advantages of sol–gel process are cheap, low temperature and fine control of the final product’s chemical composition [132,133]. The coating film produced by sol–gel method tends to crack and there is a thickness limitation of each layer (about 1 micron) [125]. This method is suitable to apply graphene based composite coating onto a substrate and the substrate can have complicate shape.
Direct apply and curing: This method is a very simple coating method that a coating mixture is coated onto a substrate directly and cured under ambient conditions. The coatings that can be cured under ambient conditions such as curing at room temperature are suitable to use this method, UV curable nanocomposite coatings, for example. The interfacial bonding between the substrate and the coating may be weak because this method is applying a coating directly without any treatments or subsequent processes. The substrate shape and size are not limited and coating can be applied onto some vital parts of a component directly. This method is more suitable to apply graphene based composite coatings.
Spray drying: Spray drying is a technique to produce dry powder from solution or suspension by rapid drying with the aid of a hot gas. The heating gas used in spray drying unique is usually air. However, nitrogen is used when the liquid is flammable solvent or the product is oxygen sensitive [134]. The liquid dried is dispersed into a controlled drop size spray by atomizer or spray nozzle. The drop size rages from 10 µm to 500 µm according to different process needs [135]. Spray drying technique is only suitable to produce powder form product and one vital requirement to produce graphene coated powder is that the particle size of graphene must be much smaller than the target powder. The graphene is coated onto a powder form substrate during the spraying process. Powder agglomeration and uneven coating may be problems when using this method. This method is more suitable to process pristine graphene based materials and graphenenanoparticles composite coating.
Spray coating: Spray coating is a technique for depositing thin film on a substrate. This technique is widely used in industrialcoating, painting and graphic art [136]. This large output technique is able to deposit a broad range of materials on various substrates with different morphologies and it is usually used in in–line production. In addition, it can be easily scaled to larger substrates or roll–to–roll manufacturing. The substrates with different shape can be processed and the fluids with different characteristics can be used. As a result, solutions with different properties can be readily deposited onto different shapes’ substrates to obtain films with desired properties [136,137]. The apparatuses for spray coating employ compressed gas (airbrush gun) to plasma torch (plasma spray coating) to fulfill different product requirements. Different apparatuses should be selective carefully to process graphene based coatings. Both pristine graphene based coatings and graphene based composites coatings can be processed by this method.
In–situ polymerization coating: The in–situ polymerization method is suitable for the unstable reactants that must be synthesized in–situ. The polymerization happens in a continuous phase to make all the unstable reactants in the reaction mixture but not isolated on their own. The reaction mixture can be applied onto a substrate as coating before the viscosity of the mixture becomes too high. However, therestill no study about applying the mixture on a substrate as coating right after the mixture is in–situ polymerized. This method may not suitable to be used in coating industries.
Electrophoretic deposition (EPD): EPD is a technique that attracts lot of attentions because of its high versatility usage withdifferent materials and their combinations. It is also a cost–effective process and requires very simple equipment. This method was first widely used in ceramic coating and it began to be used in other aterials when more and more interests were received. This process is a colloidal process and its advantages are short deposition time, simple apparatus, low coast, little restriction of the shape of substrate and easy control of deposited layer thickness and surface morphology. The film produced also exhibited good microstructure homogeneity and high packing density. The only intrinsic limitation of EPD compared to other colloidal process is that water cannot be used as its liquid medium because the applied voltage in water triggers thegeneration of hydrogen and oxygen gases at the electrodes which have negative effects on the quality of the deposited films and potential safety threats [138].
In this process, the solid particles are charged under electric filed. Then, the charged particles are attracted to move toward thesubstrates with opposite charge to form deposited layer via particle coagulation[138,139]. There are two types of EPD process: cathodic and anodic. When the particles are positively charged, they are attracted to deposition onto negative electrode (cathode). The reason for anodic EPD is similar where the particles are negatively charged. The substrates used in EPD must be conductive and the required conductance of liquid medium is lower than electroplating [140]. The principle driving force of EPD process is the charge on the particles and the electrophoretic mobility of the particles in the solvent under applied voltage. There are two major groups of parameters can influence film quality and film characteristics: (1) those parametersrelated to the suspension and (2) those parameters related to the process. In terms of the parameters relate to the suspension, many of them must be considered. However, particle size, dielectric constantof liquid, conductivity of suspension, viscosity of suspension, zeta potential and stability of suspension are more influencing among those suspension parameters. Regarding the parameters relate to the process, deposition time, applied voltage, concentration of solid suspension and conductivity of substrate [138]. To ensure a successful EPD process, powder washing, which removes any impurities on the powder, is very vital because it contribute to the careful control of a defined chemistry of particle suspension [141]. One problem must be prevented in EPD process is the formation of drying cracking. Several approaches have been published such as careful control of the EPD process with moderate control of subsequent drying [138,142].
EPD has been adopted to fabricate carbon nanotube coatings [143]. The carbon nanotube coatings produced via EPD can be usedin biomedical, structural and functional applications [139,143]. For carbon nanotubes coatings, the suspension for EPD is usually prepared by adding carbon nanotubes, Triton X100 as an ionic surfactant and iodine 99.9% as a charger to an aqueous solution. The resulted suspension mixture is centrifuged to remove large carbon nanotubes agglomerations [139]. EPD has been widely used to coat graphene based materials on different substrates [144]. It is believed to be a promising method to produce high quality graphene based coatings. There are still a lot of works needed to be done to find out suitable conditions for different coating applications. This method is more suitable to produce pristine graphene based coatings and graphene–nanoparticles composite coatings.
Conclusions and future perspectives
It is evidenced that graphene exhibit many extraordinary properties from many published characterization results. Graphenebased materials have wide range of potential applications such as flexible transparent electrode, sensors and electronic components. Graphene based polymeric nanocomposites also show very lowpercolation threshold of electrical conductivity, and improved mechanical, thermal, and barrier properties. However, there are still lots of challenges lie on the path toward mature graphene utilization. Defect–free graphene is the perfect materials for being used in all kinds of applications but the fabrication techniques are still not mature. Moreover, the scale–up of fabricating graphene based materials with acceptable coast is still extremely challenging.Essentially, the potential health risks of graphene based materials need to be evaluated before large scale utilization.
The potential electrical properties, barrier properties, mechanical properties and thermal properties were identified when coated pristine graphene based materials and graphene based composites on a substrate. In addition, other properties were also identified such as catalytic activity, sensing sensitivity, and barrier efficiency. However, most of the published articles on utilizing graphene in coating focuson electrical properties. Much more efforts need to be given in for fully understanding the potential applications of graphene based coatings. The influence of graphene coated surface morphology on improvement efficiency needs to be investigated as well. No matter how the graphene is synthesized, the ease for subsequent coating processing needs to be considered. If the synthesized graphene can be readily used without any treatment, the cost can be lowered and a good quality coating may be produced. In order to optimize the properties of polymer graphene based composites, the control of graphene based materials orientation and dispersion during processing is critical. Much more efforts in nano–engineering are needed to understand the behaviour of graphene based materials in processing. In coating applications, coating methods have a profound effect on the properties and morphology of the resulted coating. Two or more coating techniques may be used at the same time to produce a multilayer graphene based coating system to meet the industrial requirements as the current techniques are difficult to satisfy industrial standards when they are used separately. The development of new technologies for graphene based materials fabrication and processing is still essential to face the demands and Challenges in industries nowadays.
References
- Chattopadhyay DK, Raju KVSN. Structural engineering of polyurethane coatings for high performance applications. Prog Polym Sci 2007; 32:352- 418.
- Liao B, Song M, Liang H, Pang Y. Polymer-layered silicate nanocomposites. 1. A study of poly (ethylene oxide )/ Na + -montmorillonite nanocomposites as polyelectrolytes and polyethylene-block-poly ( ethylene glycol ) copolymer / Na+ -montmorillonite nanocomposites as illers for reinf. Polymer (Guildf) 2001; 42: 10007-10011.
- Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y. Dubonos SV, et al. Electric ield effect in atomically thin carbon ilms. Science. 2004; 306: 666-669.
- Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007; 6: 183-191.
- Singh V, Joung D, Zhai L, Das S, Khondaker SI, Seal S. Graphene based materials: Past, present and future. Prog Mater Sci 2011; 56: 1178-1271.
- Sanjinés R, Abad MD, Vâju C, Smajda R, Mionic M, Magrez A. Electrical properties and applications of carbon based nanocomposite materials: An overview. Surf Coatings Technol. 2011; 206: 727-733.
- Lee C, Wei X, Kysar JW, Hone J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science. 2008; 321: 385-388.
- Lee C, Wei X, Li Q, Carpick R, Kysar JW, Hone J. Elastic and frictional properties of graphene. Phys Status Solidi 2009; 246: 2562-2567.
- Ni ZH, Yu T, Lu YH, Wang YY, Feng YP, Shen ZX. Uniaxial strain on graphene: Raman spectroscopy study and band-gap opening. ACS Nano. 2008; 2: 2301-2305.
- Mohiuddin TMG, Lombardo A, Nair RR, Bonetti A, Savini G, Jalil R, et al. Uniaxial strain in graphene by Raman spectroscopy: G peak splitting, Grüneisen parameters, and sample orientation. Phys Rev B Condens Matter Mater Phys 2009; 79: 205433.
- Balandin AA, Ghosh S, Bao W, Calizo I, Teweldebrhan D (2008) Superior thermal conductivity of single-layer graphene. Nano Lett 8: 902-907.
- Zhang H, Lee HK. Plunger-in-needle solid-phase microextraction with graphene-based sol-gel coating as sorbent for determination of polybrominated diphenyl ethers. J Chromatogr A. 2011; 1218: 4509-4516.
- Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, et al. Graphene and graphene oxide: synthesis, properties, and applications. Adv Mater. 2010; 22: 3906-3924.
- Young RJ, Kinloch IA, Gong L, Novoselov KS. The mechanics of graphene nanocomposites: A review. Compos Sci Technol. 2012; 72: 1459-1476.
- Yoo BM, Shin HJ, Yoon HW, Park HB. Graphene and graphene oxide and their uses in barrier polymers. J Appl Polym Sci. 2014; 131: 39628(1-23).
- Hsieh C Te, Chen W. Water/oil repellency and work of adhesion of liquid droplets on graphene oxide and graphene surfaces. Surf Coatings Technol. 2011; 205: 4554-4561.
- Lin L, Kim DE, Kim WK, Jun SC. Friction and wear characteristics of multi- layer graphene ilms investigated by atomic force microscopy. Surf Coatings Technol. 2011; 205: 4864-4869.
- Kirkland NT, Schiller T, Medhekar N, Birbilis N. Exploring graphene as a corrosion protection barrier. Corros Sci. 2012; 56: 1-4.
- Hernandez Y, Nicolosi V, Lotya M, Blighe FM, Sun Z, De S, et al. High- yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol. 2008; 3: 563-568.
- Green AA, Hersam MC. Solution phase production of graphene with controlled thickness via density differentiation. Nano Lett. 2009; 9: 4031- 4036.
- Eda G, Fanchini G, Chhowalla M. Large-area ultrathin ilms of reduced graphene oxide as a transparent and lexible electronic material. Nat Nanotechnol. 2008; 3: 270-274.
- Becerril HA, Mao J, Liu Z, Stoltenberg RM, Bao Z, Chen Y. Evaluation of solution-processed reduced graphene oxide ilms as transparent conductors. ACS Nano. 2008; 2: 463-470.
- Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon NY. 2007; 45: 1558-1565.
- Somani PR, Somani SP, Umeno M. Planer nano-graphenes from camphor by CVD. Chem Phys Lett. 2006; 430: 56-59.
- Cao H, Yu Q, Colby R, Pandey D, Park CS, Lian J, et al. Large-scale graphitic thin ilms synthesized on Ni and transferred to insulators: Structural and electronic properties. J Appl Phys. 2010; 107: 044310 - 0443107-7.
- Kim KS, Zhao Y, Jang H, Lee SY, Kim JM, Kim KS, et al. Large-scale pattern growth of graphene ilms for stretchable transparent electrodes. Nature. 2009; 457: 706-710.
- Reina A, Jia X, Ho J, Nezich D, Son H, Bulovic V, et al. Large area, few-layer graphene ilms on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009; 9: 30-35.
- Li X, Cai W, Colombo L, Ruoff RS. Evolution of graphene growth on Ni and Cu by carbon isotope labeling. Nano Lett. 2009; 9: 4268-4272.
- Malesevic A, Vitchev R, Schouteden K, Volodin A, Zhang L, Tendeloo GV, et al. Synthesis of few-layer graphene via microwave plasma-enhanced chemical vapour deposition. Nanotechnology. 2008; 19: 305604.
- O'Hern SC, Stewart CA, Boutilier MS, Idrobo JC, Bhaviripudi S, Das SK, et al. Selective molecular transport through intrinsic defects in a single layer of CVD graphene. ACS Nano. 2012; 6: 10130-10138.
- Brodie BC. Sur le poids atomique du graphite. Ann Chim Phys 1860; 59: 466-472.
- Staudenmaier L. Verfahren zur darstellung der graphitsäure. Berichte Der Dtsch Chem Gesellschaft. 1898; 31: 1481-1487.
- Hummers Jr WS, Offeman RE. Preparation of Graphitic Oxide. J Am Chem Soc 1958; 80: 1339-1339.
- Potts JR, Dreyer DR, Bielawski CW, Ruoff RS. Graphene-based polymer nanocomposites. Polymer (Guildf) 2011; 52: 5-25.
- Paredes JI, Villar-Rodil S, Martínez-Alonso A, Tascón JM. Graphene oxide dispersions in organic solvents. Langmuir. 2008; 24: 10560-10564.
- Wang G, Yang J, Park J, Gou X, Wang B, Liu H, et al. Facile Synthesis and Characterization of Graphene Nanosheets. J Phys Chem C. 2008; 112: 8192-8195.
- Si Y, Samulski ET. Synthesis of water soluble graphene. Nano Lett. 2008; 8: 1679-1682.
- Dua V, Surwade SP, Ammu S, Agnihotra SR, Jain S, Roberts KE, et al. All- organic vapor sensor using inkjet-printed reduced graphene oxide. Angew Chem Int Ed Engl. 2010; 49: 2154-2157.
- Dubin S, Gilje S, Wang K, Tung VC, Cha K, Hall AS, et al. A one-step, solvothermal reduction method for producing reduced graphene oxide dispersions in organic solvents. ACS Nano. 2010; 4: 3845-3852.
- Song M, Cai D. Graphene functionalization? A Review. In: Mittal V, editor. Polym. Nanocomposites, Royal Society of Chemistry; 2012; p: 1-51.
- Titelman GI, Gelman V, Bron S, Khalin RL, Cohen Y, Bianco-Peled H. Characteristics and microstructure of aqueous colloidal dispersions of graphite oxide. Carbon N Y. 2005; 43: 641-649.
- Veca LM, Lu F, Meziani MJ, Cao L, Zhang P, Qi G, et al. Polymer functionalization and solubilization of carbon nanosheets. Chem Commun (Camb): 2009; 2565-2567.
- Salavagione HJ, Martínez G, Gómez MA. Synthesis of poly (vinyl alcohol)/ reduced graphite oxide nanocomposites with improved thermal and electrical properties. J Mater Chem 2009; 19: 5027-5032.
- Shen J, Hu Y, Shi M, Lu X, Qin C, Li C, et al. Fast and Facile Preparation of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets. Chem Mater. 2009; 21: 3514-3520.
- Huang Y, Qin Y, Zhou Y, Niu H, Yu Z, Dong J. Polypropylene/Graphene Oxide Nanocomposites Prepared by In Situ Ziegler-Natta Polymerization. Chem Mater. 2010; 22: 4096-4102.
- Fang M, Zhang Z, Li J, Zhang H, Lu H, Yang Y. Constructing hierarchically structured interphases for strong and tough epoxy nanocomposites by amine-rich graphene surfaces. J Mater Chem. 2010; 20: 9635-9643.
- Sundaram RS, Gómez-Navarro C, Balasubramanian K, Burghard M, Kern K. Electrochemical Modiication of Graphene. Adv Mater 2008; 20: 3050-3053.
- Chunder A, Pal T, Khondaker SI, Zhai L. Reduced Graphene Oxide/Copper Phthalocyanine Composite and Its Optoelectrical Properties. J Phys Chem C. 2010; 114: 15129-15135.
- Cao A, Liu Z, Chu S, Wu M, Ye Z, Cai Z, et al. A facile one-step method to produce graphene-CdS quantum dot nanocomposites as promising optoelectronic materials. Adv Mater. 2010; 22: 103-106.
- Yamada T, Ishihara M, Hasegawa M. Large area coating of graphene at low temperature using a roll-to-roll microwave plasma chemical vapor deposition. Thin Solid Films 2013.
- Pham VH, Cuong TV, Hur SH, Shin EW, Kim JS, Chung JS, et al. Fast and simple fabrication of a large transparent chemically-converted graphene ilm by spray-coating. Carbon N Y. 2010; 48: 1945-1951.
- Singh BP, Jena BK, Bhattacharjee S, Besra L. Development of oxidation and corrosion resistance hydrophobic graphene oxide-polymer composite coating on copper. Surf Coatings Technol. 2013; 232: 475-481.
- Wang J, Liang M, Fang Y, Qiu T, Zhang J, Zhi L. Rod-coating: towards large- area fabrication of uniform reduced graphene oxide ilms for lexible touch screens. Adv Mater. 2012; 24: 2874-2878.
- Ge J, Yao H, Hu W, Yu X, Yan Y, Mao L, et al. Facile dip coating processed graphene/MnO2 nanostructured sponges as high performance supercapacitor electrodes. Nano Energy. 2012; 1-8.
- Jeon YJ, Yun JM, Kim DY, Na SI, Kim SS. High-performance polymer solar cells with moderately reduced graphene oxide as an eficient whole transporting layer. Sol Energy Mater Sol Cells. 2012; 105: 96-102.
- Lee SH, Park M, Yoh JJ, Song H, Jang EY, Kim YH, et al. Reduced graphene oxide coated thin aluminum ilm as an optoacoustic transmitter for high pressure and high frequency ultrasound generation. Appl Phys Lett. 2012; 101: 2419091-4.
- Yoo DH, Cuong TV, Pham VH, Chung JS, Khoa NT, Kim EJ, et al. Enhanced photocatalytic activity of graphene oxide decorated on TiO2 ilms under UV and visible irradiation. Curr Appl Phys. 2011; 11: 805-808.
- He Z, Wang Z, Guo H, Li X, Wu X, Yue P, et al. A simple method of preparing graphene-coated Li [Li0.2Mn0.54Ni0.13Co0.13]O2 for lithium-ion batteries. Mater Lett. 2012; 91: 261-264.
- Shi Y, Chou S, Wang J, Wexler D, Li H, Liu H, et al. Graphene wrapped LiFePO4/C composites as cathode materials for Li-ion batteries with enhanced rate capability. J Mater Chem. 2012; 22: 16465-16470.
- Jian Z, Zhao L, Wang R, Hu Y, Li H, Chen W, et al. The low-temperature (400 °C) coating of few-layer graphene on porous Li4Ti5O12via C28H16Br2 pyrolysis for lithium-ion batteries. RSC Adv. 2012; 2: 1751-1754.
- Park JH, Seo SW, Kim JH, Choi CJ, Kim H, Lee DK, et al. Improved Eficiency of Dye-Sensitized Solar Cell Using Graphene-Coated Al 2 O 3-TiO 2 Nanocomposite Photoanode. Mol Cryst Liq Cryst. 2011; 538: 285-291.
- Wang D, Gentle IR, Lu GQ. Enhanced electrochemical sensitivity of PtRh electrodes coated with nitrogen-doped graphene. Electrochem Commun. 2010; 12: 1423-1427.
- Mendes RG, Bachmatiuk A, Melkhanova S, Klingeler R, Bu B, Ru MH. A Facile Route to Coat Iron Oxide Nanoparticles with Few-Layer Graphene. J Phys Chem C. 2012; 116: 23749-23756.
- Zhang WL, Liu YD, Choi HJ. Graphene oxide coated core-shell structured polystyrene microspheres and their electrorheological characteristics under applied electric ield. J Mater Chem 2011; 21: 6916-6921.
- Li X, Li GQ, Zhao SZ, Wang XM, Yin L, Huang H, et al. Diode-pumped Nd: YVO4 laser passively Q-switched with graphene oxide spin coated on ITO substrate. Laser Phys. 2012; 22: 673-677.
- Jung SW, Park JH, Seo SW, Kim JH, Choi CJ, Kim H, et al. Enhanced Photoelectrochemical Response of Graphene-Coated Al 2 O 3 -TiO 2 Nanocomposite Photoanodes. Mol Cryst Liq Cryst. 2011; 538: 272-277.
- Gupta RK, Alahmed ZA, Yakuphanoglu F. Graphene oxide based low cost battery. Mater Lett. 2013; 112: 75-77.
- Yu L, Lim YS, Han JH, Kim K yun, Kim JY, Choi SY, et al. A graphene oxide oxygen barrier ilm deposited via a self-assembly coating method. Synth Met. 2012; 162: 710-714.
- Hong J, Kang SW. Hydrophobic properties of colloidal ilms coated with multi-wall carbon nanotubes/reduced graphene oxide multilayers. Colloids Surfaces A Physicochem Eng Asp. 2011; 374: 54-57.
- Zhang M, Ma Y, Zhu Y, Che J, Xiao Y. Two-dimensional transparent hydrophobic coating based on liquid-phase exfoliated graphene luoride. Carbon N Y. 2013; 63: 149-156.
- Chen S, Brown L, Levendorf M, Cai W, Ju SY, Edgeworth J, et al. Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano. 2011; 5: 1321-1327.
- Kousalya AS, Kumar A, Paul R, Zemlyanov D, Fisher TS. Graphene: An effective oxidation barrier coating for liquid and two-phase cooling systems. Corros Sci. 2012; 5-10.
- Nilsson L, Andersen M, Balog R, Lægsgaard E, Hofmann P, Besenbacher F, et al. Graphene coatings: probing the limits of the one atom thick protection layer. ACS Nano. 2012; 6: 10258-10266.
- Nayak PK, Hsu CJ, Wang SC, Sung JC, Huang JL. Graphene coated Ni ilms: A protective coating. Thin Solid Films. 2013; 529: 312-316.
- Singh Raman RK, Chakraborty Banerjee P, Lobo DE, Gullapalli H, Sumandasa M, Kumar A, et al. Protecting copper from electrochemical degradation by graphene coating. Carbon N Y. 2012; 50:4040-4045.
- Prasai D, Tuberquia JC, Harl RR, Jennings GK, Rogers BR, Bolotin KI. Graphene: corrosion-inhibiting coating. ACS Nano. 2012; 6: 1102-1108.
- Noel S, Baraton L, Alamarguy D, Jaffre A, Hauquier F, Viel P, et al. Graphene ilms for corrosion protection of gold coated cuprous substrates in view of an application to electrical contacts. Inst Electr Electron Eng 2012.
- Won MS, Penkov OV, Kim DE. Durability and degradation mechanism of graphene coatings deposited on Cu substrates under dry contact sliding. Carbon N Y. 2012; 1-10.
- Nguyen DD, Tai NH, Lee SB, Kuo WS. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ Sci. 2012; 5: 7908-7912.
- Zhang M, Gao B, Yao Y, Xue Y, Inyang M. Synthesis, characterization, and environmental implications of graphene-coated biochar. Sci Total Environ. 2012; 435-436: 567-72.
- Ying Z, Zheng L, Song K, Hu W. Characterization of Quartz Crystal Microbalance Sensors Coated with Graphene Films. Procedia Eng. 2012; 29: 2448-2452.
- Dong X, Long Q, Wang J, Chan-Park MB, Huang Y, Huang W, et al. A graphene nanoribbon network and its biosensing application. Nanoscale. 2011; 3: 5156-5160.
- Yao Y, Chen X, Guo H, Wu Z, Li X. Humidity sensing behaviors of graphene oxide-silicon bi-layer lexible structure. Sensors Actuators B Chem. 2012; 161:1053-1058.
- Yao Y, Chen X, Guo H, Wu Z. Graphene oxide thin ilm coated quartz crystal microbalance for humidity detection. Appl Surf Sci. 2011; 257: 7778-7782.
- Zhang H, Lee HK. Simultaneous determination of ultraviolet ilters in aqueous samples by plunger-in-needle solid-phase microextraction with graphene- based sol-gel coating as sorbent coupled with gas chromatography-mass spectrometry. Anal Chim Acta. 2012; 742: 67-73.
- Qu Q, Gu C, Hu X. Capillary coated with graphene and graphene oxide sheets as stationary phase for capillary electrochromatography and capillary liquid chromatography. Anal Chem. 2012; 84: 8880-8890.
- Zhao G, Song S, Wang C, Wu Q, Wang Z. Determination of triazine herbicides in environmental water samples by high-performance liquid chromatography using graphene-coated magnetic nanoparticles as adsorbent. Anal Chim Acta. 2011; 708: 155-159.
- Qu Q, Shen Y, Gu C, Gu Z, Gu Q, Wang C, et al. Capillary column coated with graphene oxide as stationary phase for gas chromatography. Anal Chim Acta. 2012; 757: 83-87.
- Chen J, Zou J, Zeng J, Song X, Ji J, Wang Y, et al. Preparation and evaluation of graphene-coated solid-phase microextraction iber. Anal Chim Acta. 2010; 678: 44-49.
- Ke Y, Zhu F, Zeng F, Luan T, Su C, Ouyang G. Preparation of graphene- coated solid-phase microextraction iber and its application on organochlorine pesticides determination. J Chromatogr A. 2013; 1300: 187-192.
- Ponnusamy VK, Jen JF. A novel graphene nanosheets coated stainless steel iber for microwave assisted headspace solid phase microextraction of organochlorine pesticides in aqueous samples followed by gas chromatography with electron capture detection. J Chromatogr A. 2011; 1218: 6861-6868.
- Wu Q, Feng C, Zhao G, Wang C, Wang Z. Graphene-coated iber for solid- phase microextraction of triazine herbicides in water samples. J Sep Sci. 2011
- Zhao G, Song S, Wang C, Wu Q, Wang Z. Solid-phase microextraction with a novel graphene-coated iber coupled with high-performance liquid chromatography for the determination of some carbamates in water samples. Anal Methods. 2011; 3: 2929-2935.
- Xu L, Feng J, Liang X, Li J, Jiang S. C18 functionalized graphene oxide as a novel coating for solid-phase microextraction. J Sep Sci. 2012; 35: 1531- 1537.
- Hao Q, Wang B, Bossard JA, Kiraly B, Zeng Y, Chiang IK, et al. Surface- Enhanced Raman Scattering Study on Graphene-Coated Metallic Nanostructure Substrates. J Phys Chem C. 2012; 116:7249-7254.
- Kim YK, Han SW, Min DH. Graphene oxide sheath on Ag nanoparticle/ graphene hybrid ilms as an antioxidative coating and enhancer of surface- enhanced Raman scattering. ACS Appl Mater Interfaces. 2012; 4: 6545- 6551.
- Kawasaki H, Nakai K, Arakawa R, Athanassiou EK, Grass RN, Stark WJ. Functionalized graphene-coated cobalt nanoparticles for highly eficient surface-assisted laser desorption/ionization mass spectrometry analysis. Anal Chem 2012; 84: 9268-9275.
- Wang H, Yang Y, Liang Y, Robinson JT, Li Y, Jackson A, et al. Graphene- wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 2011; 11: 2644- 2647.
- Chen Z, Zhou M, Cao Y, Ai X, Yang H, Liu J. In situ generation of few- layer graphene coatings on SnO2-SiC core-shell nanoparticles for high-performance lithium-ion storage. Adv Energy Mater 2012; 2:95-102.
- Wang P, Ao Y, Wang C, Hou J, Qian J. Enhanced photoelectrocatalytic activity for dye degradation by graphene-titania composite ilm electrodes. J Hazard Mater. 2012; 223-224: 79-83.
- Shi M, Shen J, Ma H, Li Z, Lu X, Li N, et al. Preparation of graphene- TiO2 composite by hydrothermal method from peroxotitanium acid and its photocatalytic properties. Colloids Surfaces. A Physicochem Eng Asp. 2012; 405: 30-37.
- Zhang N, Zhang Y, Xu YJ. Recent progress on graphene-based photocatalysts: current status and future perspectives. Nanoscale. 2012; 4: 5792-5813.
- Yang MQ, Xu YJ. Selective photoredox using graphene-based composite photocatalysts. Phys Chem Chem Phys. 2013; 15: 19102-19118.
- Zhang N, Zhang Y, Pan X, Yang M, Xu Y. Constructing Ternary CdS - Graphene - TiO 2 Hybrids on the Flatland of Graphene Oxide with Enhanced Visible-Light Photoactivity for Selective Transformation. J Phys Chem C. 2012; 116: 18023-18031.
- Zhang Y, Zhang N, Tang ZR, Xu YJ. Graphene transforms wide band gap ZnS to a visible light photocatalyst. The new role of graphene as a macromolecular photosensitizer. ACS Nano. 2012; 6: 9777-9789.
- Zhang Y, Tang ZR, Fu X, Xu YJ. TiO2-graphene nanocomposites for gas- phase photocatalytic degradation of volatile aromatic pollutant: is TiO2- graphene truly different from other TiO2-carbon composite materials? ACS Nano. 2010; 4: 7303-7314.
- Zhang Y, Tang ZR, Fu X, Xu YJ. Engineering the unique 2D mat of graphene to achieve graphene-TiO2 nanocomposite for photocatalytic selective transformation: what advantage does graphene have over its forebear carbon nanotube? ACS Nano. 2011; 5: 7426-7435.
- Kou L, Gao C. Making silica nanoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale. 2011; 3: 519-528.
- Zhang S, Du Z, Li G. Graphene-supported zinc oxide solid-phase microextraction coating with enhanced selectivity and sensitivity for the determination of sulfur volatiles in Allium species. J Chromatogr A. 2012; 1260: 1-8.
- Liao K, Qian Y, Macosko CW. Ultralow percolation graphene/polyurethane acrylate nanocomposites. Polymer (Guildf). 2012; 53: 3756-3761.
- Fabbri P, Bassoli E, Bon SB, Valentini L. Preparation and characterization of poly (butylene terephthalate)/graphene composites by in-situ polymerization of cyclic butylene terephthalate. Polymer (Guildf). 2012; 53: 897-902.
- Yeh MH, Sun CL, Su JS, Lin LY, Lee CP, Chen CY, et al. A low-cost counter electrode of ITO glass coated with a graphene/Naion® composite ilm for use in dye-sensitized solar cells. Carbon N Y. 2012; 50: 4192-4202.
- Sangermano M, Marchi S, Valentini L, Bon SB, Fabbri P. Transparent and Conductive Graphene Oxide/Poly(ethylene glycol) diacrylate Coatings Obtained by Photopolymerization. Macromol Mater Eng. 2011; 296: 401- 407.
- Chang CH, Huang TC, Peng CW, Yeh TC, Lu HI, Hung WI, et al. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon N Y. 2012; 50: 5044-5051.
- Pan B, Zhao J, Zhang Y, Zhang Y. Wear Performance and Mechanisms of Polyphenylene Sulide/Polytetraluoroethylene Wax Composite Coatings Reinforced by Graphene. J Macromol Sci Part B. 2012; 51: 1218-1227.
- Pan B, Xu G, Zhang B, Ma X, Li H, Zhang Y. Preparation and Tribological Properties of Polyamide 11/Graphene Coatings. Polym Plast Technol Eng. 2012; 51: 1163-1166.
- Cai D, Jin J, Yusoh K, Raiq R, Song M. High performance polyurethane/ functionalized graphene nanocomposites with improved mechanical and thermal properties. Compos Sci Technol. 2012; 72:702-707.
- Zhang B, Patlolla VR, Chiao D, Kalla DK, Misak H, Asmatulu R. Galvanic corrosion of Al/Cu meshes with carbon ibers and graphene and ITO-based nanocomposite coatings as alternative approaches for lightning strikes. Int J Adv Manuf Technol. 2012; 67: 1317-1323.
- Wang X, Xing W, Song L, Yang H, Hu Y, Yeoh GH. Fabrication and characterization of graphene-reinforced waterborne polyurethane nanocomposite coatings by the sol-gel method. Surf Coatings Technol. 2012; 206: 4778-4784.
- Yu B, Wang X, Xing W, Yang H, Song L, Hu Y. UV-Curable Functionalized Graphene Oxide/Polyurethane Acrylate Nanocomposite Coatings with Enhanced Thermal Stability and Mechanical Properties. Ind Eng Chem Res. 2012; 51: 14629-14636.
- Martin-Gallego M, Verdejo R, Lopez-Manchado M a., Sangermano M. Epoxy-Graphene UV-cured nanocomposites. Polymer (Guildf). 2011; 52: 4664-4669.
- Zaman I, Phan TT, Kuan H-C, Meng Q, Bao La LT, Luong L, et al. Epoxy/ graphene platelets nanocomposites with two levels of interface strength. Polymer (Guildf). 2011; 52: 1603-1611.
- Zou J, Song X, Ji J, Xu W, Chen J, Jiang Y, et al. Polypyrrole/graphene composite-coated iber for the solid-phase microextraction of phenols. J Sep Sci. 2011; 34: 2765-2772.
- Jin J, Lin Y, Song M, Gui C, Leesirisan S. Enhancing the electrical conductivity of polymer composites. Eur Polym J. 2013; 49:1066-1072.
- Choy KL. Chemical vapour deposition of coatings. Prog Mater Sci. 2003; 48:57-170.
- Scriven LE. Physics and applications of dip coating and spin coating. MRS Proc. 1988; 121: 717-729.
- Lawrence CJ, Zhou W. Spin coating of non-Newtonian luids. J Nonnewton Fluid Mech. 1991; 39: 137-187.
- Le Roux JD, Paul DR. Preparation process of composite membranes by a spin coating. J Memb Sci. 1992; 74: 233-252.
- Bornside DE, Macosko CW, Scriven LE. On the modeling of spin coating. Imaging Technol J. 1987; 13: 122-130.
- Szabó T, Szeri A, Dékány I. Composite graphitic nanolayers prepared by self-assembly between inely dispersed graphite oxide and a cationic polymer. Carbon N Y. 2005; 43: 87-94.
- Sheng K, Bai H, Sun Y, Li C, Shi G. Layer-by-layer assembly of graphene/ polyaniline multilayer ilms and their application for electrochromic devices. Polymer (Guildf). 2011; 52: 5567-5572.
- Brinker CJ, Scherer GW. Sol-gel science: the physics and chemistry of sol-gel processing. Academic Press. 1990.
- Hench LL, West JK. The sol-gel process. Chem Rev. 1990; 90: 33-72.
- Mujumdar AS. Handbook of industrial drying. 3rd ed. CRC Press. Taylor & Francis Group. 2007.
- Niessen WR. Combustion and incineration processes. 3rd ed. CRC Press. Taylor & Francis Group. 2002.
- Girotto C, Rand BP, Genoe J, Heremans P. Exploring spray coating as a deposition technique for the fabrication of solution-processed solar cells. Sol Energy Mater Sol Cells. 2009; 93: 454-458.
- Maa YF, Ameri M, Rigney R, Payne LG, Chen D. Spray-coating for biopharmaceutical powder formulations: beyond the conventional scale and its application. Pharm Res. 2004; 21: 515-523.
- Besra L, Liu M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog Mater Sci. 2007; 52: 1-61.
- Li X, Liu X, Huang J, Fan Y, Cui F. Biomedical investigation of CNT based coatings. Surf Coatings Technol. 2011; 206: 759-766.
- Binner JJGP. Advanced ceramic processing and technology. Noyes da corporation/Noyes Publications. 1990.
- Basu RN, Randall CA, Mayo MJ. Fabrication of Dense Zirconia Electrolyte Films for Tubular Solid Oxide Fuel Cells by Electrophoretic Deposition. J Am Ceram Soc. 2001; 84: 33-40.
- Sarkar P, De D, RHO H. Synthesis and microstructural manipulation of ceramics by electrophoretic deposition. J Mater Sci. 2004; 39: 819-823.
- Boccaccini AR, Cho J, Roether JA, Thomas BJC, Jane Minay E, Shaffer MSP. Electrophoretic deposition of carbon nanotubes. Carbon N Y. 2006; 44: 3149-3160.
- Chavez-Valdez A, Shaffer MS, Boccaccini AR. Applications of graphene electrophoretic deposition. A review. J Phys Chem B. 2013; 117: 1502-1515.