
Review Article
Austin J Nanomed Nanotechnol. 2023; 11(1): 1067.
Nanobots for Medicinal Applications
Smrutimedha Parida1; Anil Ramdas Bari2*
¹School of Chemical Sciences, NISER, Odisha, 752050, India
²Department of Physics, Arts, Commerce and Science College, Bodwad, Maharashtra, 425310, India
*Corresponding author: Anil Ramdas Bari Department of Physics, Arts, Commerce and Science College, Bodwad, Maharashtra, 425310, India. Tel: +91 9421523832 Email: anilbari_piyu@yahoo.com
Received: April 07, 2023 Accepted: May 11, 2023 Published: May 18, 2023
Abstract
The applications of nanotechnology have increased exponentially in the field of medicinal chemistry with the implementations of nanorobotics. Nanobots provide one of the most promising areas of nanotechnology spreading its roots to applications in various fields including medical imaging, drug delivery and even in the development of Nanobots have the advantages of small size, low weight, large thrust-to-weight ratio, high flexibility, and high sensitivity. The applications of nanobots are varied and are being explored in various fields. The aim of this review is to offer an overview to the emerging field of nanorobotics within medicinal chemistry and their applications for diagnosis, treatment and prevention of various diseases. It provides a comprehensive overview of the development of nanobots. The key components of the robots and the types of nanobots are discussed separately. The review also focuses on the disadvantages and the challenges in the development of nanobots for their specific causes. And finally, the efforts and measures that can bring us steps closer to the dream of catching up with our fantasies of developing tiny robots that could roam about inside our bodies, delivering drugs with unprecedented precision, and hunting down and destroying infected cells and most importantly science fiction becoming scientific fact are discussed.
Keywords: Nanomedicine; Nanobot; Nanomotors; Sensors; DNA nanorobot; Targeted drug delivery; Precision surgery
Introduction
Nanotechnology makes it possible to manipulate matter at the atomic and molecular scale to design materials with remarkably diverse and advanced properties. It is a rapidly expanding area of research with huge potential in many sectors, ranging from healthcare to construction and electronics. The advance in nanotechnology leads to great scientific progress in the field of medical sciences. Applications of nanotechnology in oncology have produced an emerging field of study, nanooncology and with the ease they offer in design, nanoparticles have revolutionized the drug delivery sector [1,3]. Drug loaded nanoparticles can selectively target tumor cells, thereby keeping our healthy cells safe [2]. Above all, the small size of these nanoparticles makes it possible for them to cross the physiological barrier of our body.
Nanomedicine is the field in medicine that is applied in diagnosis to treatment of diseases and nanotechnology is used for developing diagnostic systems. Technology like electrochemiluminescence makes it possible to measure the substance at nanolevel [4]. For medical diagnosis, cellular imaging by nanoprobes like quantum dots, plasmonic nanoparticles, magnetic nanoparticles, nanotubes, nanowires, and multifunctional nanomaterials can be done [5-7]. The advantage of using nanoprobes is high volume/surface ratio, surface tailorability, multifunctionality, and intrinsic properties. Nanotechnology also helps in developing drugs, improving drug formulation and distribution in the body and also in targeting the specific therapeutic site. It can even be integrated into the classical medical procedure to help improve efficacy.
The rapid growth of robot technology expanded its applications in the health and medicinal science field which led to the development of nanobots. Nanobots are nanoscale machines that can be controlled to perform specific activities in the body. They have great flexibility, adaptability and accuracy. Nanobots consist of sensors and motors. In presence of any trouble causing intruders, they undergo conformational changes that catalyses the release of a substance to act against them. The concept of nanobots was first thought of by famous physicist Richard Feyman in 1959 and he talked about that being used as a cure for heart diseases in his talk “There’s Plenty of Room at the Bottom”. Robert Frietas then did a study on medical nanobots called respirocytes; resembling red blood cells. Advances in the fields of robotics, nano structuring, medicine, bioinformatics, and computers have led to the development of nanobot drug delivery systems. Some of the examples of nanobots are respirocyte nanobots, microbivore nanobots, surgical nanobots and cellular repair nanobots.
Among various elements that are used in nanobots, carbon due to its inert nature and strength becomes the best choice. These are used as an exterior coating of the nanobots to avoid attack by the host immune system [8]. Techniques like Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM) are used to establish a visual and haptic interface to learn about the molecular structure of nanobots. The main challenges in development of these nanobots are their fabrication and controls.
Robotic systems have dramatically extended the reach of human beings in sensing, interacting, manipulating and transforming the world around us [9]. Particularly, the confluence of diverse technologies has enabled a revolution in medical applications of robotic technologies towards improving healthcare. Medical robotic devices are designed for environments and operations relevant to the treatment and prevention of diseases. They require miniaturized parts and smart materials for complex and precise operations and mating with the human body. The rapid growth in medical robotics has been driven by a combination of technological advances in motors, control theory, materials, medical imaging and increased in surgeon/patient acceptance [10,11]. Before moving on to its further applications, let us first understand how exactly it is made, the parts involved and their functioning.
Parts and Design of Nanobots
One of the important applications of nanobots is to develop treatment to target the active site by minimising the impact on other unaffected parts of the body [12]. They are designed to detect and get to the affected part of the body and send feedback. nanobots can be made from almost any type of material and can have varied manufacturing processes. The two principle manufacturing conventions are top down or bottom up. The former process involves the extreme miniaturization of existing robotic devices while the latter describes a process of building starting at the atomic level and constructing any object one atom at a time.
Current technology has employed atomic force microscopes and scanning tunneling microscopes to arrange atoms. These can resolve specimens at the atomic level and be used to move atoms and molecules. The microscope precisely locates the particle that will be moved and then a higher electron force than is normally used for imaging is targeted on the particle. This is done in a vacuum and at very low temperatures approaching four degrees Kelvin to inhibit electron excitation and spatial uncertainty caused by temperature drift in the room and between the specimen and the probe when using a scanning probe microscope.
The important parts of a nanobot are sensors, motors, power supplies, molecular computers and manipulators.
Sensors: Different types of sensors are used in nanobots like mechanical, thermal, optical, magnetic, chemical and biological sensors [13]. Sensors detect the presence of the target molecules and indirectly know the amount of damage that exists from the change in the functional properties of nanobots. Biosensors use biological reactions to detect target analytes [14]. An example of biosensors is use of nano cantilevers as Nano Electro Mechanical System (NEMS). This system utilizes biological material that will be attached by itself to a coated cantilever, causing fundamental changes in mass or its surface tension [15]. They measure cell mass, biomolecules, nucleic acids and others, and detect specific molecules or even manipulate and place nanoparticles in a predefined arrangement [16-18]. Carbon Paste Electrodes (CPE) is also a type of nanosensor used for voltammetric measurements and even in coulometry, as a renewable surface for electron transfer reactions. They are easy to fabricate, can be miniaturised, have good electrical and mechanical resistance and come at a low cost [19].
Propulsion equipment: These are needed for movement of nanobots inside the body. Nanomotors are nanodevices with their own propulsion, obtaining the energy by chemical reactions of the medium, electricity, magnetic or acoustic fields [20,21]. Challenges faced to control movement of nanobots are viscosity and Brownian motion of the medium. To facilitate movement of nanobots, MRI devices were used. The speed and direction of nanobots were controlled from an external computer, decreasing the risk [22] and the MRI was used to get real-time feedback of the behavior of the nanobots.
In 2000, bio nanomotors were introduced. In this nano-electro-mechanical device was integrated with adenosine triphosphate synthase (ATPase). Another example is Janus motors which are made by nanoparticles that have two or more sides on their surface with different properties [23]. In this, there is a chemical reaction on one side of these nanoparticles, which produces the force for the movement of these motors. There are also nanomotors based on sphere based propulsion and osmotic propulsion. Gold-nano wired ultrasound-driven motors are being developed for their utilization as drug delivery devices in cancer cells. These motors are based on the nanoporous gold segment for increasing superficial area and hence the loading capacity of the drug.
Nanocomputers: They can be electronic, biochemical, organic or quantum and have the function of controlling or directing nanobots inside the body. Computers developed at a molecular level made up of DNA, having software coded with four letters of DNA nitrogenous bases can regulate gene expression. It can also detect the type of mRNA associated with specific genes that in case of being over expressed or its opposite induce the cancer. This allows diagnosing different types of cancer and counteracting the disease with the indicated drug [24].
The nanobot design consists of integrated nano electronics and components. Binding sites of different sensors have a different affinity for distinct molecule types. Sensors detect obstacles which require a new trajectory planning and their design depends on the environment and the task. A nanobot needs transducers capabilities and smart sensors directly related to specific biomedical application. It relies on chemical contact sensors to detect them. Different nanobot sensor based actions can be evaluated by this interaction capabilities [25]. By this, we can choose the kind of low-level control to maximize the information acquired for an effective real time performance. The nanobot kinematics can be predicted using state equations, positional constraints, inverse kinematics and dynamics, while some individual directional component performance can be simulated using control system models of transient and steady state response [26]. The capacity to design, build, and deploy large numbers of medical nanobots into the human body would make possible the rapid elimination of disease and the effective and relatively painless recovery from physical trauma.
Types of nanobots: Generally nanobots can be classified into two types i.e. organic also called bionanobot and inorganic nanobots.
Nanobots in drug delivery and therapeutics can also be classified according to the applications as described below:
Pharmacyte: It is a medical nanobot used to carry a given drug in the tanks. It is controlled using mechanical systems for sorting pumps. For full targeting accuracy, it has molecular markers or chemotactic sensors. Glucose and oxygen that are extracted from the local environments such as blood, intestinal fluid and cytosol are the on board power supply. Nanobots are removed after completing their tasks by centrifuge nanapheresis [29].
Diagnosis and Imaging nanobots: These nanobots have microchips projected to send electrical signals when the human molecules on the chips detect a disease. They can also be used to monitor the sugar level in the blood. Their production cost is and they can be easily manipulated [27].
Respirocyte: It is Artificial Oxygen Carrier nanobot. Its power is obtained by endogenous serum glucose. This artificial cell is able to give 236 times more oxygen to the tissues per unit volume than RBCs (Red blood cells). It is also used to administer acidity [28].
Microbivores: It is an oblate spheroidal device for nanomedical applications. The nanobot can continually consume power up to 200pW and this power is used to digest trapped microbes. It also has the ability to phagocyte approximately 80 times more efficiently than macrophages agents, in terms of volume/sec digested per unit volume of phagocytic agent [29].
Clottocytes: This nanobot has the ability for instant hemostasis. They are also called artificial mechanical platelets that are roughly spheroidal nucleus-free blood cells. Platelets join at a place of bleeding and are activated. Then they aid in stamping the blood vessel and stop the bleeding. They also deliver substances that help promote coagulation [28].
Chromallocyte: They replace entire chromosomes in individual cells thus reversing the effects of genetic disease and other accumulated damage to our genes, preventing aging. Usually inside a cell, first the repair machine sizes up the situation by examining the cell’s contents and activity, and then takes action by working along molecule-by-molecule and structure-by structure [29].
DNA nanobots: They are used to deliver the drug to the targeted cell so as to avoid side effects. Their aim is the design and fabrication of dynamic DNA nanostructures that do specific tasks via state changes done from the hybridization/denaturing of a single base to the hybridization/denaturing of entire strands. DNA nanobots use DNA origami where one long strand of DNA is folded to produce the desired structure with the help of smaller staple strands. This method is based on the folding of the large ssDNA (usually the 7.3 kilobase genome of the M13 bacteriophage) with an excess of smaller complementary strands called staple strands (typically 32 bases). These strands are complementary to at least two distinct segments of the long ss DNA. DNA nanobots are used as a targeted drug delivery system to improve treatment of diseases [64].
Applications of Nanobots
Owing to their small size, the nanobots have unique properties that do not exist in other larger counterparts, including increased surface area, charge, reactivity, and other physicochemical properties, all of which may affect how these nanomaterials interact with biological entities. The potential applications of nanobots are:
For Drug delivery: Nanobots having the ability of controlled navigation deliver drugs to the target or affected areas, hence treating many diseases. They can even penetrate into tissues [30]. These nanobots are usually propelled and/or guided by endogenous or exogenous stimuli towards the area of interest [31]. Wire-shaped magnetoelectric nanobots designed and fabricated can be precisely steered toward a targeted location by means of wireless magnetic fields and can perform on-demand magneto electrically assisted drug release to cells [32]. Ultrasound-powered nanowire motors has been developed based on a nanoporous gold segment and showed that the nanoporous gold structure can facilitate the Near-Infrared (NIR)-light-controlled drug release through photothermal effects [33]. A DNA nanobot was made which was capable of delivering molecular payloads to cells and was controlled by an aptamer-encoded logic gate, enabling the robot to respond to a wide array of signals such as cell surface markers [34]. Gold nanowires conjugated with a cytokine such as tumor necrosis factor-alpha can be transported along any prescribed trajectory or orientation using electrophoretic and dielectro-phoretic forces to a specific location with subcellular resolution, promoting the development of controlling signaling events on the single-cell level [35].
Figure 1: Nanobot (Courtesy-https://doctorinprogress.com/2019/09/01/nanobots-in-medicine/?locale=en).
Figure 2: Medical nanobot (Courtesy-https://www.cnet.com/news/nanobots-can-now-swarm-like-fish-to-perform-complex-medical-tasks/).
Figure 3: Nanobots with the size as small as bacteria (Credits-https://www.cnet.com/news/nanobots-can-now-swarm-like-fish-to-perform-complex-medical-tasks/).
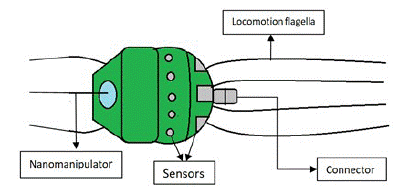
Figure 4: Parts of a nanobot.
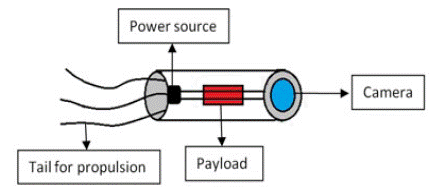
Figure 5: Parts of a Medical nanorobot.
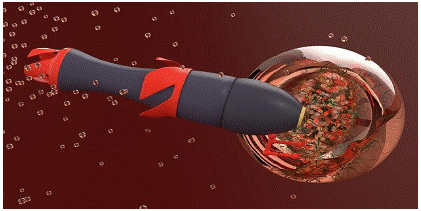
Figure 6: Nanobots delivering drugs directly to eyes (Credits:https://www.scientificeuropean.co.uk/nanorobots-that-deliver-drugs-directly-into-the-eyes/).
Figure 7: Nanobots magnifying dynamics of medical precision (Credits: https://www.analyticsinsight.net/nanobots-magnifying-the-dynamics-of-medical-precision/).
For Surgery: Robots were introduced as a solution to problems associated with complex surgical procedures, and for extending the capabilities of human surgeons. Robot-assisted surgery is a rapidly evolving field that allows doctors to perform a variety of minimally-invasive procedures with high precision, flexibility and control [36,37]. Nanobots target many specific health problems as they can navigate to tissues which are hard to reach in the human body. For addressing the limitations of the current surgical procedures and for precision surgery, recent nanobots have shown big promises [38,39]. Untethered nanorobotic tools, ranging from nanodrillers to micro-grippers and microbullets, offer unique capabilities for minimally invasive surgery. nanobots offer major advantages for high precision minimally-invasive surgery since their size is compatible with the small biological entities that they treat. The moving nanobots with nanoscale surgical components are powered by diverse energy sources and are able to directly penetrate or retrieve cellular tissues for precision surgery. They can navigate through the body’s narrowest capillaries and perform procedures down to the cellular level.
A set of responsive microgrippers have been developed that can be actuated autonomously by diverse environmental factors and used as minimally invasive microsurgical tools [40]. These can subsequently move out of the capillary tube with the captured cells in its grasp, demonstrating its strength for performing an in vitro tissue biopsy. Acoustically-triggered vaporization of perfluorocarbon fuel was used for developing tubular microscale cannons capable of loading and firing nanobullets at remarkable speeds [41]. These could be used to eject high-speed nanobullets and shoot a wide range of payloads deep into diseased tissues. By choosing appropriate propulsion methods and using real-time localization and mapping with a robust control system, surgical nanobots can be improved so that they penetrate through the tissues and sense specific targets.
For precise diagnosis: Due to their straight forward surface functionalization and flexible mobile performance nanobots have the ability of precise diagnosis of diseases. MiRNAs have been regarded as biomarker candidates in disease diagnosis. A nanomotor-based strategy for rapid single-step intracellular biosensing of a target miRNA expressed in intact cancer cells at the single-cell level [42] can allow precise and real-time monitoring of intracellular miRNA expression through the measurement of a fluorescence signal in the cells.
For sensing: Nanobots have biosensing applications since they have features like autonomous motion, easy surface functionalization, effective capture and isolation of target analytes in complex biological media. Their sensing capability relies on motility of artificial nanomotors, functionalized with different bioreceptors, through the sample to realize specific biomolecular interactions [68,69]. The continuous movement of receptor functionalized synthetic nanomotors has led to built-in solution mixing in microliter clinical samples, which highly enhances the target binding efficiency and increases sensitivity and speed of biological assays [70]. Tubular microrockets functionalized with targeting ligands helps in recognition and isolation of specific cancer cells [71]. The significant mixing induced by the motion of unmodified self-propelled motors greatly enhances analyte–bioreceptor interactions and hence the sensitivity of an immunoassay microarray [72]. The internalization and movement of nanobots within cells can also be exploited for intracellular sensing [94].
In addition to that, nanobots are used for treatment of many diseases. Due to properties such as more targeted localization in tumors and active cellular uptake, nanobots have gradually evolved into a new type of cancer therapy, offering enhanced efficacy and fewer side effects [43]. They also have dentistry applications. Nanobots are used for complete dentition replacement therapy, tooth durability and appearance, orthodontic treatment and oral prophylaxis [44]. Medical nanobots are even used for diabetes control. Nanobiosensors are embedded in the bloodstream to detect glucose levels and collect information that is transferred to a cell phone as a practical way to interface and communicate with the nanobots [45]. By comparing the molecular structures of both DNA and proteins found in the cell to known or desired reference structures, correcting any irregularities and implementing the editing of desired modifications, nanobots can be used for treating genetic diseases [50]. The wide applications of nanobots promote the development of biomedical technology and human healthcare.
Types
Manufactured using
Toxicity
Organic
Viruses and bacteria DNA cells
Less toxic
Inorganic
Diamond structures, synthesized proteins
More toxic
Table 1: Types of nanobots.
Field of medicine
Nanorobotic application
References
Microbiology
Magnetotactic bacteria is used to transport and navigate nanobots
[98]
Hematology
Ultrasound-powered nanobots swim through blood and remove harmful bacteria, nanobots is used for haemostasis
[99]
Dentistry
Nanobot penetrating dentinal tubules is used for administration of targeted analgesic
[100]
Neurosurgery
Nanobot is used for the monitoring of intracranial aneurysm development and progression
[101]
Oncology
DNA nanobots can do precise drug delivery for cancer therapy.
[102,103]
Screening nanobot can circulate and monitor for detection of neoplasia
Table 2: Recent Nanobots.
Recent Nanobots
In 2012, Diagnostic nanosensor was invented for diagnosis in medicine based on metallic semiconductors and magnetic nanoislands. The surface of these nanoparticles can be modified to achieve interaction with the molecule of interest or recognize a specific substrate surface as a cell membrane. So these can be used for the detection of pathogens or toxins inside the body [65]. In 2016, a nanobot was developed to create more efficient cancer markers and to reduce side effects from cancer treatment. This robot is composed of a loading component, a power component and a connecting component. The nanobot releases a labeling reagent at the specific point where there is a tumor. The motor is propelled by ATP [66]. Nanobots called Nano Bee that destroy brain cancer cells were simulated in 2016 [67]. These can detect cancer cells and destroy them. They emit an acoustic signal when the tumor is detected.
Nanorobots containing therapeutic compounds integrated into magnetotactic bacteria would be the bacterial cell component. Magnetotactic cocci are used for intravascular functions and travel in consistent and predictable patterns following established geomagnetic lines. These robots function in and navigate through blood vessels. The magnetosomes in magnetotactic bacteria are guided by magnetic fields [98]. Tiny ultrasound-powered robots can offer a safe and efficient way to detoxify and decontaminate biological fluids. These nanobots are built by coating gold nanowires with a hybrid of platelet and red blood cell membranes. The platelets bind pathogens and red blood cells absorb and neutralize the toxins produced by these bacteria. The gold coating of the nanorobots responds to ultrasound, which gives them the ability to swim around rapidly without chemical fuel and speed up detoxification [99]. Nanorobots can be used in almost every aspect of dentistry. These are administered orally and they enter the gingival sulcus, and travel through the dental tubules to reach the pulp. These nanorobots allow activation of analgesic activity in highly specific areas in proximity to where the treatment will be done. Nanorobots can also be used for routine cleaning, cosmetics and teeth whitening, hypersensitivity, and orthodontics [100]. There are many benefits of neurosurgery including improved detection of pathology, minimally invasive intracranial monitoring, and pharmaceutical delivery. Intravascular nanorobots are designed with the capability to detect aneurysm formation by detecting increased levels of nitric oxide synthase protein within the affected blood vessel. These nanorobots wirelessly communicate information about pertinent vascular changes [101]. DNA robots can be programmed to transport payloads and present them specifically in tumors. The outer side is functionalized with a DNA aptamer that binds nucleolin and the inside with blood coagulation protease thrombin. The nucleolin-targeting aptamer acts as a targeting domain and a molecular trigger for the mechanical opening of the DNA nanorobot. The thrombin inside is thus exposed and activates coagulation at the tumor site and that induces intravascular thrombosis resulting in tumor necrosis and inhibition of tumor growth [102,103].
Nanobots have diverse applications in medicinal chemistry like molecular machines [73-75], self-propelled nanomotors [76-78], and DNA nanorobotics [79-81]. Nanobots are also being used in the current world for transporting biological substances (e.g., ions [82], molecules [83], drugs [84]), in vivo treating diseases (e.g., cancers [85], renal damage [81]), handling different types of biological samples (e.g., single cells [86,87], organelles [88], exosomes [89], single molecules on cells [90]) and also characterizing biomaterials for regulating cellular behaviors [91-93]. However, the challenges that are faced put a limit to their use.
Challenges for Handling of Nanobots
With the advancements in the fields of nanotechnology, robotics, biomedicine, and electromechanical science, nanobots have made considerable improvements. nanobots possess unique and multivalent functionalities that include fast motion in complex biological media, large cargo-towing force for directional and long-distance transport, easy surface functionalization for precise capture and isolation of target subjects, and excellent biocompatibility for in vivo operation. To accommodate the growing demands of nanobots, we need to address the challenges associated with them.
Though nanobots have unrivalled advantages due to their size, they also face some challenges. When the dimensions are reduced to nano scale, the nature of certain physical laws is altered due to changes in the surface area-to-volume ratio and surface area. Perimeter-related forces tend to predominate. The behavior of nanobots is also relatively susceptible to temperature, humidity, and fluids [46,47].
One of the challenges faced by nanobots is multi-functionality. Due to their size, existing nanobots have a single function, and it is challenging to integrate multiple functions into one robot. It is important for smart nanobots to incorporate signal perception, acquisition, processing and transmission in them. To grasp the real-time position of the robot and for preventing the nanobot from losing contact with external controls, the feedback mechanism needs to be improved. The operation and intelligence of nanobots rely primarily on their materials and surface properties. Biomedical nanobots are designed for environments like unanticipated biological events, changing physiological conditions, and soft tissues. Diverse smart materials, such as biological materials, responsive materials, or soft materials are expected to provide the necessary actuation and multi-functionality and avoid irreversible malfunctions in complex physiologically-relevant body systems. Synthetic nanomachines coupled with natural biological materials can minimize undesired immune evasion and biofouling effects experienced in complex biological fluids, leading to enhanced mobility and lifetime in them [60]. For designing configurable nanobots for adaptive operation under rapidly changing conditions, we need responsive materials. Nanobots need to be soft and deformable to ensure maneuverability and mechanical compliance to human body and tissues [61,62]. They should be made of transient biodegradable materials that disappear upon completing their tasks [63]. For large-scale, high-quality, and cost-efficient fabrication of biomedical nanobots, new synthesis and cutting-edge fabrication methods should be explored.
Biomedical nanobots need to cooperate with one another for performing tasks like effective delivery of large therapeutic payloads or large-scale detoxification processes, which are not possible using a single robot. Mimicking the natural swarm intelligence communication and synchronized coordination, from one to many, is a challenging task. It is highly important for enhancing their precision treatment capability. With the conventional optical microscopy techniques, it is difficult for high-resolution simultaneous localization and mapping of nanobots in the human body. Future biomedical nanobots will require their coupling with modern imaging systems and feedback control systems for arbitrary four-dimensional navigation of many-nanobot systems.
Another challenge is problems faced by nanobots in clinical applications and crossing the gap between scientific research results and the market demand. Though there are many models of nanobots out there, they are rarely used. For in vivo applications things like biocompatibility, reliability and biodegradability need to be considered. The nanobots entering the living body should have the ability to cross barriers like blood vessels and tissues. They also should not be rejected by the body when they enter, should be harmless to normal tissues, and should have no side effects. The material of the robot should be biodegradable or it should be equipped with an integrated recycling mechanism. The nanoparticles involved in certain medical devices interact with both the outer environment and the human body. The risks humans have with it are in the adsorption of biomolecules and oxidative stress, causing DNA damage. The inability to control the movement of the nanoparticles throughout the body creates a risk that they can reach undesirable locations and lead to side effects. One of the main characteristics of nanoparticles is that they can cross biological barriers [48]. This can be a disadvantage since the unnecessary cross of barriers may be a trigger for some inflammatory reactions. The ethical aspects involved in the diagnosis of diseases should also be evaluated. When patients are known to be prone to a disease through gene analysis, things like who will possess such information, how fundamental rights can be protected, how responsible use of nanomedicine can be promoted should be considered [48]. The risk nanobots pose for the environment is much more complex by the large number of interactions that are involved [49].
For getting the full potential of the nanobots, great efforts and innovations are needed. Future nanobots are expected to mimic the natural intelligence of their biological counterparts with great mobility, adaptable and sustainable operation, precise control, self-evolving and self-replicating capabilities. We need good energy sources for prolonged, biocompatible in vivo operations. For locomotion of nanobots in aqueous media, many chemical fuels and external stimuli have been explored [51]. nanobots have already demonstrated great performance in viscous biological fluids such as gastric fluid or whole blood [55-57]. Enzyme-functionalized nanomotors can be powered by bodily fluid constituents, such as blood glucose or urea [52-54]. The power and stability of these motors need more improvements for practically implementing them. Magnetic and acoustic nanomotors can provide fuel-free and on-demand speed regulation for surgery at nanoscale but they might hinder autonomous therapeutic interventions.
For working in human tissues and organs nanobots have an advantage of size. Nanoscale magnetic propellers display a significant advantage for propulsion in viscoelastic hyaluronan gels as they are of the same size range as the openings in the gel’s mesh, compared to the impeded motion of larger propellers [58]. So, nanobots can achieve efficient motion in tissues by their nanoscale size and optimized design. They can overcome cellular barriers and internalize into cells [59].
Current nanobots have drawbacks in energy conversion mechanisms, control methods and fabrication technologies. The magnetic drive needs an external magnetic field, the electric field drive requires external electrodes, and the light drive needs light to penetrate the tissue. Most existing methods control the nanobots to move in the 2D plane and lack movement control in the third dimension. Many nanobots are driven by corrosive chemical fuels which creates problems when used in vivo. For better diagnosis and treatment, the nanobot should load more drugs. For commercialization of nanobots, the manufacturing costs should be reduced. Instead of expensive metals, low-priced metals need to be used [95]. and water can be used instead of other chemical reagents to drive nanobots [96]. Bio-syncretic robots have both living components and nonliving components [97]. Some living microorganisms can be directly used to assemble nanobots since they have the capability to act as sensing or driving elements for the robots. Biomaterials have higher sensitivity than inorganic materials. But when these nanobots are used in the air, the long-term survival of living biological components is a challenge. So, they must be supplied with nutrients and gases and should be operated in a controlled-humidity environment. This is why innovative materials with better environmental adaptability are needed.
Discussion
Over the past few years, a number of theoretical and experimental studies have led to the development of various nanobots that are propelled by different mechanisms. Currently, the clinical translation of nanobots is very limited because of the challenges faced by them. So, nanorobotics has been a reality to some extent for medicinal chemistry applications but we have a room for its advancement for better applications. The present nanobots mainly act as drug carriers and their movements rely on the blood flow and once the drug inside is released at the target site, the nanobots are cleared by our immune system. Reusing nanobots and communication and coordination between nanobots inside the body is needed to expand the applications. Pathological changes in the cell, alters its physical properties. So, applying physical property detection based on nano manipulators to drug efficacy prediction will increase their precision. Automated hybrid nanorobotic systems integrating different types of nano manipulators can combine their advantages which will benefit handling biological samples of patients with high throughput and reliability. The biological environments are very different from the fluids on which clinical testing is done which leads to some complexities. Though there has been significant progress to achieve efficient locomotion and propulsion of nanobots in body fluids like blood, there still remains some challenges to be faced. Due to the introduction of technologies like MRI, OCT, etc deep tissue imaging and tracking of nanobots can be possible using various external controls via magnetic, ultrasound or optical fields. During the manufacturing of nanobots, many factors like biocompatibility, biodegradability, toxicity should also be kept in mind. With continuous technological innovations, the nanobots will prove to be of great importance for numerous medicinal chemistry applications.
Conclusion
In the coming years, the development and application of nanobots in medicinal chemistry will become a robust research area. To realize the full potential of the nanobots in this field, researchers need to study the biocompatibility, retention, toxicity, biodistribution, and therapeutic efficacy of nanobots. With nanobots that can do surgery, targeted drug delivery, and gene therapy, our interventions will become more efficient and proactive, helping to treat diseases in ways that are frankly not possible today. nanobots can also contribute to a world where people do not get sick in the first place because of monitoring and preventive medicine that nips diseases and disorders in the bud. Considering the promising results achieved in the past years in varied fields from drug delivery to treatment of diseases, addressing the challenges posed by them should be looked into. There is a great deal of work that still must be done to bring nanobots from a more theoretical realm to a practical one. Nevertheless, nanobots will contribute to the ever brightening horizon of medicine’s future.
Author Statements
Acknowledgements
The author would like to thank the Young Science Leaders - Virtual Internship with Science Leaders program for giving me the opportunity to work under an eminent scientist and utilize my time productively. The author would like to express his heartfelt gratitude to the supervisor Dr. Anil Ramdas Bari for guiding the way throughout and acting as constant support.
Competing Interests
The authors hereby declare that there is no competing interest among the authors.
References
- Jain KK. Advances in the field of nanooncology. BMC Med. 2010; 8: 83.
- Misra R, Acharya S, Sahoo SK. Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug DiscovToday. 2010; 15: 842-850.
- Bharali DJ, Mousa SA. Emerging nanomedicines for early cancer detection and improved treatment: current perspective and future promise. Pharmacol Ther. 2010; 128: 324-335.
- Rhyne PW, Wong OT, Zhang YJ, Weiner RS. Electrochemiluminescence in bioanalysis. Bioanalysis 2009; 1: 919-35
- Matsue T. Bioimaging with micro/nanoelectrode systems. Anal Sci. 2013; 29: 171-9.
- Chi X, Huang D, Zhao Z, Zhou Z, Yin Z, et al. Nanoprobes forin vitro diagnostics of cancer and infectious diseases. Biomaterials. 2012; 33: 189-206.
- Choi YE, Kwak JW, Park JW. Nanotechnology for early cancer detection. Sensors (Basel). 2010; 10: 428-55.
- Sivasankar M, Durairaj RB. Brief Review on Nano Robots in Bio Medical Applications. ARA. 2012; 1: 101.
- Mehra P, Nabhi K. A Nanorobotics - The Changing Face of Dentistry. IJSR. 2016; 5: 192-197.
- Nandkishor K, Swapnil P, Rajeshwar K, et al. Review on application of nanorobots in health care. World J pharmacy and pharmaceutical sciences. 2014; 3: 472-480.
- Ummat A, Dubey A, Sharma G, et al. Nanorobotics: 1-43.
- Khanna VK. Nanosensors, physical, chemical and biological. USA: CRC Press; 2011. 665.
- Gao W, Sattayasamitsathit S, Wang J. Catalytically propelled micro-/nanomotors: how fast can they move? The Chemical Record. 2011; 12: 224–231.
- Mavroidis C, Ferreira A. Nanorobotics: past, present and future. Nanorobotics, Current Approaches and Techniques. 2013; 467.
- Varadan VK, Chen L, Xie J. Magnetic Nanoparticles. Nanomedicine, design and applications of magnetic nanomaterials. Nanosensors and nanosystems. USA: Wiley. 2008; 484.
- Zhu A, Yi Y, Reed F, Zhu H, Cubukcu E. Optoelectromechanical multimodal biosensor with graphene active region. Nano letters. 2014; 14: 5641–5649.
- Sattler KD. Handbook of nanophysics: nanomedicine and nanorobotics. USA: CRC press. 2010; 1–18.
- Johnson BN, Mutharasan R. Biosensing using dynamic-mode cantilever sensors: a review. Biosensors and bioelectronics. 2012; 32: 1–18.
- Gutierrez B, Bermúdez CV, Ureña YRC, Chacon SV. Nanobots: development and future. Int J Biosen Bioelectron. 2017; 2: 146–151.
- Chomoucka J, Drbohlavova J, Masarik M, Ryvolova M, Huska D, Prasek J, et al. Nanotechnologies for society. New designs and applications of nanosensors and nanobiosensors in medicine and environmental analysis. International Journal of Nanotechnology. 2012; 9: 746–783.
- Chalupniak A, Morales Narváez E, Merkoçi A. Micro and nanomotors in diagnostics. Adv Drug Deliv Rev. 2015; 95: 104–116.
- Sánchez S, Soler L, Katuri J. Chemically powered micro-and nanomotors. Angew Chem Int Ed Engl. 2015; 54: 1414–1444.
- Wheat PM, Marine NA, Moran JL, Posner JD. Rapid fabrication of bimetallic spherical motors. Langmuir. 2010; 26: 13052–13055.
- Gao W, Wang J. Synthetic micro/nanomotors in drug delivery. Nanoscale. 2014; 6: 10486–10494.
- Hill C, Amodeo A, Joseph JV, Patel HRH. Nano and microrobotics: how far is the reality? Expert review of anti-cancer therapy. 2008; 8: 1891-1897.
- NANOROBOTICS-A REVIEW. Sci J Phar and Pharmaceu Sci. 2019; 1: 01-09.
- Rohit K, Omprakash B, Sanat K, et al. Applications of Nanorobotics. International J of Scientific Res Eng & Tech (IJSRET). 2014; 3: 1131-1136.
- GléciaVirgolino dS, Kleber VGB, Fábio Vladimir CdA, Fabio Vladimir Calixto de Araujo, Gabriela Barbosa da Silva, et al. Nanorobotics in Drug Delivery Systems for Treatment of Cancer: A Review. J Mat Sci Eng A. 2016; 6: 167-180.
- Sarath KS, Beena PN, Elessy A. Nanorobots a future Device for Diagnosis and Treatment. J Pharm Pharmaceutics. 2018; 5: 44-49.
- Zhang MJ, Tarn TJ, Xi N. Micro/nano-devices for controlled drug delivery. In: 2004 IEEE International Conference on Robotics and Automation. IEEE. 2004. 2068–2073.
- Medina-Sánchez M, Xu HF, Schmidt OG. Micro-and nano-motors: The new generation of drug carriers. Ther Deliv. 2018, 9: 303–316.
- Chen X Z, Hoop M, Shamsudhin N, Huang T, Ozkale B, et al. Hybrid magnetoelectric nanowires for nanorobotic applications: Fabrication, magnetoelectric coupling, and magnetically assisted in vitro targeted drug delivery. Adv Mater. 2017; 29: 1605458.
- Garcia-Gradilla V, Sattayasamitsathit S, Soto F, Kuralay F, Yardimci C, et al. Ultrasound-propelled nanoporous gold wire for efficient drug loading and release. Small. 2014; 10: 4154–4159.
- Douglas SM, Bachelet I, Church GM. A logic-gated nanorobot for targeted transport of molecular payloads. Science. 2012; 335: 831-834.
- Fan D, Yin Z, Cheong R, Zhu FQ, Cammarata RC, et al. Subcellular-resolution delivery of a cytokine through precisely manipulated nanowires. Nat Nanotech. 2010; 5: 545-551.
- Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery a current perspective. Ann Surg. 2004; 239: 14–21.
- Barbash GI, Glied SA. New technology and health care costs – the case of robot-assisted surgery. N Engl J Med. 2010; 363: 701–704.
- Ullrich F, Fusco S, Chatzipirpiridis G, Pané S, Nelson BJ. Recent progress in magnetically actuated microrobotics for ophthalmic therapies. Eur Ophthalmic Rev. 2014; 8: 120–126.
- Beasley RA, Medical robots: current systems and research directions, Journal of Robotics. 2012; 401613: 1–14.
- Leong TG, Randall CL, Benson BR, Bassik N, Sterna GM, et al. Tetherless thermobiochemically actuated microgrippers. PNAS. 2009; 106: 703–708.
- Soto F, Martin A, Ibsen S, Vaidyanathan M, Garcia-Gradilla V, et al. Acoustic microcannons: toward advanced microballistics. ACS Nano. 2016; 10: 1522–1528.
- Esteban-Fernández de Á B, Martín A, Soto F. Single cell real-time mirnas sensing based on nanomotors. ACS Nano. 2015; 9: 6756-6764.
- Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discov. 2008; 7: 771-782.
- Verma SK, Chauhan R. Nanorobotics in dentistry – A review. Ind J Dentistry, 2014, 5: 62-70.
- Cavalcanti A, Shirinzadeh B, Kretly LC. Medical nanorobotics for diabetes control. NanoMed-Nanotechnol Biol Med. 2008; 4: 127-138.
- Zhou Q, Chang B, Koivo HN. Temperature and humidity effects on micro/nano handling. Mater Sci Forum. 2006; 532-533: 681-684.
- Tambe NS, Bhushan B. Scale dependence of micro/nano-friction and adhesion of MEMS/NEMS materials, coatings and lubricants. Nanotechnology. 2004; 15: 1561-1570.
- Loscri V, Natalizio E, Mannara V, et al. A novel communication technique for nanobots based on acoustic signals; 2012.
- Sung J, Kuk E, Nam K, Ho-Kim J, Park SJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine: Nanotechnology, Biology, and Medicine. 2007; 3: 95–100.
- Khulbe P. Nanorobots: A review. IJPSR. 2014; 5: 2164–2173.
- Wang J, Nanomachines: fundamentals and applications. Wiley-VCH; Weinheim, Germany. 2013.
- Ma X, Jannasch A, Albrecht UR, Hahn K, Miguel-López A, et al. Enzyme-Powered Hollow Mesoporous Janus Nanomotors. Nano Lett. 2015; 15: 7043-50.
- Dey KK, Zhao X, Tansi BM, Mendez-Ortiz WJ, Cordova-Figueroa UM, et al, Micromotors powered by enzyme catalysis. Nano Lett. 2015; 15: 8311–8315.
- Abdelmohsen LKEA, Nijemeisland M, Pawar GM, Janssen G-JA, Nolte RJM, et al. Dynamic loading and unloading of proteins in polymeric stomatocytes: formation of an enzyme-loaded supramolecular nanomotor. ACS Nano. 2016; 10: 2652–2660.
- Li J, Thamphiwatana S, Liu W, Esteban-Fernández de Ávila B, Angsantikul P, et al. Enteric Micromotor Can Selectively Position and Spontaneously Propel in the Gastrointestinal Tract. ACS Nano. 2016; 10: 9536-9542.
- Zhao G, Viehrig M, Pumera M. Challenges of the movement of catalytic micromotors in blood. Lab Chip. 2013; 13: 1930-6.
- Venugopalan PL, Sai R, Chandorkar Y, Basu B, Shivashankar S, et al. Conformal cytocompatible ferrite coatings facilitate the realization of a nanovoyager in human blood. Nano Lett. 2014; 14:1968-75.
- Schamel D, Mark AG, Gibbs JG, Miksch C, Morozov KI, et al. Nanopropellers and their actuation in complex viscoelastic media. ACS Nano. 2014; 8: 8794-801.
- Esteban-Fernández de Ávila B, Angell C, Soto F, Lopez-Ramirez MA, Báez DF, et al. Acoustically Propelled Nanomotors for Intracellular siRNA Delivery. ACS Nano. 2016; 10: 4997-5005.
- Wu Z, Li J, Esteban-Fernández de Ávila B, Li T, Gao W, et al. Water-powered cell-mimicking janus micromotor. Adv Funct Mater. 2015; 25: 7497–7501.
- Huang HW, Sakar MS, Petruska AJ, Pané S, Nelson BJ. Soft micromachines with programmable motility and morphology. Nat Commun. 2016; 7: 12263.
- Hines L, Petersen K, Zhan Lum G, Sitti M. Soft Actuators for Small-Scale Robotics. Adv Mater. 2017; 29: 1603483.
- Chen C, Karshalev E, Li J, Soto F, Castillo R, et al. Transient micromotors that disappear when no longer needed. ACS Nano. 2016; 10: 10389–10396.
- Mazumder S, Biswas GR, Majee SB. Applications of nanorobots in medical techniques. Int J Pharm Sci & Res. 2020; 11: 3138-47.
- Al Arif SMR, Quader N, Shaon AM, et al. Sensor based autonomous medical nanorobots: a cure to demyelination. Journal of Selected Areas in Nanotechnology. 2011.
- Fritsch M, Fritsch J. Contact lens materials, designs, substances, and methods. 2015.
- Sensor, molecular machine and controller added to programmable nano-robot. 2016.
- Guix M, Mayorga-Martinez CC, A Merkoci. Nano/micromotors in (bio)chemical science applications. Chem Rev. 2014; 114: 6285–6322.
- Duan W, Wang W, Das S, Yadav V, Mallouk TE, et al. Synthetic nano- and micromachines in analytical chemistry: sensing, migration, capture, delivery, and separation. Annu Rev Anal Chem. 2015; 8: 11–33.
- Wang J. Self-propelled affinity biosensors: moving the receptor around the sample. Biosens. Bioelectron. 2016; 76: 234–242.
- Balasubramanian S, Kagan D, Hu C, Campuzano S, Lobo-Castañon MJ, et al. Micromachine-enabled capture and isolation of cancer cells in complex media. Angew Chem Int Ed. 2011; 50: 4161–4164.
- Morales-Narváez E, Guix M, Medina-Sánchez M, Mayorga-Martinez CC, Merkoçi A. Micromotor enhanced microarray technology for protein detection. Small. 2014; 10: 2542–2548.
- S Chen, Wang Y, Nie T, Bao C, Wang C, et al. An artificial molecular shuttle operates in lipid bilayers for ion transport. J Am Chem Soc. 2018; 140: 17992-17998.
- G Rapenne, C Joachim. The first nanocar race. Nat Rev Mater. 2017; 2: 17040.
- C Joachim, G Rapenne, Molecule concept nanocars: chassis, wheels, and motors?. ACS Nano. 2013; 7: 11-14.
- Y Wang, RM Hernandez, DJ Bartlett, JM Bingham, TR Kline, et al. Bipolar electrochemical mechanism for the propulsion of catalytic nanomotors in hydrogen peroxide solutions. Langmuir. 2006; 22: 10451-10456.
- W Wang, TY Chiang, D Velegol, T Mallouck. Understanding the efficiency of autonomous nano and microscale motors. J Am Chem Soc. 2013; 135: 10557-10565.
- W Gao, A Uygun, J Wang. Hydrogel-bubble-propelled zinc-based microrockets in strongly acidic media. J Am Chem Soc. 2012; 134: 897-900.
- SM Douglas, I Bachelet, G Church. A logic-gated nanorobot for targeted transport of molecular payloads. Science. 2012; 335: 831-834.
- S Li, Q Jiang, S Liu, Y Zhang, Y Tian, et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat Biotechnol. 2018; 36: 258-264.
- D Jiang, Z Ge, HJ Im, CG England, D Ni, et al. DNA origami nanostructures can exhibit preferential renal uptake and alleviate acute kidney injury. Nat Biomed Eng. 2018; 2: 865-877.
- S Chen, et al. An artificial molecular shuttle operates in lipid bilayers for ion transport. J Am Chem Soc. 2018; 140: 17992-17998.
- C Cheng, PR McGonigal, ST Schneebeli, H Li, NA Vermeulen, et al. An artificial molecular pump. Nat Nanotechnol. 2015; 10: 547-553.
- DA Wilson, RJM Nolte, JCM van Hest. Autonomous movement of platinum-loaded stomatocytes. Nat Chem. 2012; 4: 268-274.
- S Li, Q Jiang, S liu, Y Zhang, Y Tian, et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat Biotechnol. 2018; 36: 258-264.
- X Li, H Yang, J Wang, D Sun. Design of a robust unified controller for cell manipulation with a robot-aided optical tweezers system. Automatica. 2015; 55: 279-286.
- Y Shen, M Nakajima, Z Yang, H Tajima, Z Najdovski, et al. Single cell stiffness measurement at various humidity conditions by nanomanipulation of a nano-needle. Nanotechnology. 2013; 24: 145703.
- X Wang, C Ho, Y Tsatskis, J Law, Z Zhang, et al. Intracellular manipulation and measurement with multiple magnetic tweezers. Sci Robot. 2019; 4: eaav6180.
- M Wu, Y Ouyang, Z Wang, R Zhang, PH Huang, et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci USA. 2017; 114: 10584-10589.
- M Li, X Xiao, L Liu, N Xi, Y Wang, et al. Nanoscale mapping and organization analysis of target proteins on cancer cells from B-cell lymphoma patients. Exp Cell Res. 2013; 319: 2812-2821.
- M Li, N Xi, Y Wang, L Liu. Composite nanostructures and adhesion analysis of natural plant hydrogels investigated by atomic force microscopy. IEEE Trans Nanobiosci. 2019; 18: 448-455.
- M Li, N Xi, Y Wang, L Liu. Nanoscale multiparametric imaging of peptide-assembled nanofibrillar hydrogels by atomic force microscopy. IEEE Trans Nanotechnol. 2019; 18: 315-328.
- M Li, N Xi, Y Wang, L Liu. Tunable hybrid biopolymeric hydrogel scaffolds based on atomic force microscopy characterizations for tissue engineering. IEEE Trans Nanobiosci. 2019; 18: 597-610.
- Li J, Esteban-Fernández de Ávila B, Gao W, Zhang L, Wang J. Micro/Nanorobots for Biomedicine: Delivery, Surgery, Sensing, and Detoxification. Sci Robot. 2017; 2: eaam6431.
- Teo WZ, Wang H, Pumera M. Beyond platinum: Silver-catalyst based bubble-propelled tubular micromotors. Chem Commun. 2016, 52: 4333-4336.
- Gao W, Pei A, Wang J. Water-driven micromotors. ACS Nano. 2012; 6: 8432-8438.
- Zhang C, Wang W, Xi N. Development and future challenges of bio-syncretic robots. Engineering. 2018, 4: 452-463.
- Lefèvre CT, Schmidt ML, Viloria N, Trubitsyn D, Schüler D, et al. Insight into the evolution of magnetotaxis in Magnetospirillum spp., based on mam gene phylogeny. Appl Environ Microbiol. 2012; 78: 7238-48.
- University of California - San Diego. Cell-like nanorobots clear bacteria and toxins from blood. Science Daily. 2018.
- Mantri SS, Mantri SP. The nano era in dentistry. J Nat Sci Biol Med. 2013; 4: 39-44.
- Cavalcanti A, Shirinzadeh B, Fukuda T, Ikeda S. Nanorobot for brain aneurysm. The International Journal of Robotics Research. 2009; 28: 558-570.
- Douglas SM, Bachelet I, Church M. A logic-gated nanorobot for targeted transport of molecular payloads. GM Science. 2012; 335: 831-4.
- Li S, Jiang Q, Liu S, Zhang Y, Tian Y, et al. A DNA nanorobot functions as a cancer therapeutic in response to a molecular trigger in vivo. Nat Biotechnol. 2018; 36: 258–264.