
Case Report
Austin J Nanomed Nanotechnol. 2024; 12(1): 1074.
Classic Recurrent Ependymoma in Posterior Cranial Fossa Treated with Coated-Bionanocatalysts: Case Report
Tessy Lopez-Goerne1,2*; Francisco J Padilla-Godínez1,3; Daniel Álvarez1; Antonela GonzÁlez-Bondani1; Rafael Valiente1; Fernando Chico-Ponce de Leónl4; Vicente GonzÁlez-Carranza4; Pilar Dies-SuÁrez5; José Omar Navarro-FernÁndez5; Abel Santamaria1
1Autonomous Metropolitan University – Xochimilco, Department of Health Care, Mexico
2Tulane University, Department of Chemical and Biomolecular Engineering, United States
3Western Institute of Technology and Higher Education, Department of Mathematics and Physics, Mexico
4Children’s Hospital of Mexico “Federico Gomez”, Department of Neurosurgery, Mexico City 06720, Mexico
5National Institute of Cancerology, Department of Head and Neck, Mexico
*Corresponding author: Tessy López-Goerne, Laboratory of Nanotechnology and Nanomedicine, Department of Health Care, Autonomous Metropolitan University-Xochimilco, Mexico City 04960, Mexico. Email: tessy3@prodigy.net.mx
Received: September 11, 2024 Accepted: September 30, 2024 Published: October 07, 2024
Abstract
Ependymomas are genetically varied glial tumors that form in the Central Nervous System (CNS). They originate from cells in the ventricular system and can develop intracranially as well as in the spinal cord. Primary treatments, especially for intracranial ependymomas in children, frequently fail to prevent recurrence, which can happen within two years or even several years post-diagnosis. Among novel treatments, bionanocatalysts are nanostructured materials made up of oxide matrices that possess catalytic properties and biochemical characteristics. Certain bionanocatalysts can selectively induce cytotoxic effects in cancer cells while remaining harmless to healthy cells. In this regard, in this study, we present the case of a pediatric patient with classic recurrent ependymoma in the posterior cranial fossa who was previously receiving only palliative care. The patient had already received several neurosurgeries focused on the extraction of tumor tissue, which had resulted in several side effects and a significant reduction in the quality of life the patient. Due to the ineffectiveness of previous treatments, we decided to implement a protocol using oncologic bionanocatalysts for treatment. After 5 years, the patient is in remission with no significant or evident side effects. This research highlights the selective cytotoxic activity of these nanostructures on cancer cells, sparing healthy cells in surrounding tissues, unlike other chemotherapeutics such as cisplatin.
Keywords: Recurrent Ependymoma; bionanocatalyst; NPt; Posterior Cranial Fossa; Catalysis
Introduction
Ependymomas are genetically diverse glial tumors that develop in the Central Nervous System (CNS), originating from cells of the ventricular system and growing both intracranially and in the spinal cord [1,2]. These tumors affect both children and adults, accounting for approximately 5-16% of all CNS tumors, with over 3,000 deaths in the US alone over the past 16 years [3–5]. The standard treatment involves surgical resection followed by radiotherapy. Gross Total Resection (GTR) significantly improves patient survival, while adjunct radiotherapy aims to reduce recurrence [5,6]. Although chemotherapy is controversial, some pediatric patients have benefited from its use [5,7,8]. However, side effects, including severe cognitive impairments, have been noted, particularly in children [9]. Consequently, exploring novel therapeutic approaches is crucial for improving the quality of life of these patients.
Traditional diagnosis of ependymomas relies on histopathological characteristics, using WHO histologic grading and morphological patterns [10]. This classification is limited by the heterogeneous nature of tumor progression and observer variability. Significant improvements in diagnosis have been made through DNA methylation analysis, which identifies nine to ten different types of ependymomas based on genetic aberrations [5,11,12]. This advancement led the 2021 WHO Classification of Tumours of the Central Nervous System to differentiate between multiple molecularly defined types [13]. Despite these advances, treatment strategies for ependymomas continue to evolve. Posterior fossa ependymomas, which develop in the fourth ventricle or cerebellopontine angle, are associated with complex genetic changes, such as alterations in DNA methylation and overexpression of specific proteins [14]. DNA methylation analysis and sequencing have identified subtypes like PFA and PFB, recognized in the 2021 WHO classification [13]. PFB ependymomas generally have a better prognosis. Immunostaining for histone H3 p.K27 trimethylation is a crucial marker for differentiating between PFA and PFB subgroups, often used as a surrogate for DNA methylation profiling [15]. Childhood ependymomas frequently occur in the back of the skull, with prognosis influenced by tumor location [16]. MRI may reveal well-defined tumors, sometimes with hemorrhage and calcifications, located within the fourth ventricle or extending laterally through natural foramina [17].
Complete surgical resection is critical for prognosis, though it is more challenging for tumors in the floor or lateral wall of the fourth ventricle, often leading to worse outcomes due to potential neurological deficits [18]. Primary therapies, particularly for intracranial ependymomas in children, often do not prevent recurrence, which can occur within two years or many years after diagnosis [16]. There is no clear consensus on the optimal treatment strategy, and apart from CAR-T cell therapy, few recent novel treatments have been developed [19]. Recurrences, especially in specific molecular subgroups, typically occur locally but can spread throughout the CNS [20]. Treatment options at this stage include surgery and radiotherapy, though re-irradiation may cause cognitive impairment: the decision to use focal or craniospinal radiation therapy depends on the patient's age and the risk of metastatic disease [21].
Bionanocatalysts are nanostructured materials composed of oxide matrices with catalytic properties and biochemical characteristics [22–24]. These materials are significant for biomedical research and clinical purposes due to their biocompatibility and selectivity for various targets. Some bionanocatalysts can selectively induce cytotoxic effects in cancer cells while being innocuous to healthy cells. The cytotoxic effects of bionanocatalysts are due to their ability to disrupt organic bonds, such as carbon-carbon and carbon-nitrogen bonds, leading to alterations in macromolecules like nucleic acids [25]. Bionanocatalysts form complexes with nucleotide chains, causing molecular degradation through combustion and dephosphorylation reactions, ultimately inhibiting replication. These catalysts also possess additional components, enhancing their biocompatibility and specificity for cancer cells, making them promising candidates for antitumor therapies. Titanium dioxide (titania)-based bionanocatalysts are noted for their thermal stability, crystalline structure, biocompatibility, and surface area [26]. Their catalytic properties have been studied for various antitumor applications [27].
This work describes a case of a recurrent ependymoma tumor in the posterior fossa of a six-year-old child initially diagnosed with frontal ependymoma and already in palliative care. The patient underwent two resection surgeries, with a third surgery following relapse, incorporating treatment with platinum-coated bionanocatalysts (1%), renamed as NPt-Ped. These nanoparticles were applied to all tumor zones during surgery. Post-treatment follow-up with Magnetic Resonance Imaging (MRI) has shown general tumor reduction since 2017.
Materials and Methods
NPt-Ped Surface-Coated Bionanocatalysts Synthesis
NPt-Ped is a type of coated bionanocatalyst with ultra-nanostructured anatase crystallinity, synthesized using previously established methods [28]. The synthesis parameters have been optimized to achieve a particle size of less than 5 nm: this preparation method is foundational for developing oncogenic platinum-coated bionanocatalysts. Platinating agents are widely used in the treatment of various cancers, as many chemical carcinogens are DNA-reactive. Alkylation is a proposed mechanism for the interaction between cancer cells and chemotherapeutic agents. Characteristics such as acid sites, nanosized particles, and platinum in different oxidation states enable platinum-based drugs to effectively interact with cancer cells [29]. Surface coating of NPt-Ped with = 5 nm metallic particles is essential to induce additive toxicity in malignant cells and enhance biocompatibility with healthy cells [24].
Physicochemical Characterization of NPt-Ped
The particle size, grain size, morphology, and texture were examined using Transmission Electron Microscopy (TEM; JEM-2100F, JEOL, USA) operating at 200 kV. Samples were placed on copper grids coated with holey carbon films. The vibrational states and bond types were identified using Fourier-Transform Infrared Spectroscopy (FTIR). FTIR spectra were recorded at room temperature on a 40% sample–KBr transparent pellet with a Shimadzu IRAffinity-1 spectrometer, covering the 4000–400 cm-¹ range. Non-polarized Raman spectra of the powdered NPt-Ped samples were obtained in a near-backscattering geometry using a Horiba-Jobin Yvon LabRam micro-spectrometer (300 mm focal length spectrograph) equipped with an integrated Olympus BX40 microscope. Raman spectroscopy, a light scattering technique, involves scattering incident light from a high-intensity laser [30]. In this vibrational spectroscopy method, a small fraction of light (typically 0.0000001%) is scattered at different wavelengths, depending on the sample's chemical structure; this phenomenon is known as Raman scattering.
Clinical Case Story
A two-year-old male patient was admitted to the Department of Neurosurgery at the "Federico Gómez" Children's Hospital of Mexico in October 2012. His parents reported that he had experienced frequent vomiting, sleeplessness, irritability, and an increased head size over the past month. An MRI revealed a mass at the infratentorial level on the left side (Figure 1a-c). In December 2012, he underwent surgery, where a partial resection of the tumor was performed (Figure 1d-e). An intraoperative pathology study diagnosed the mass as a classic Ependymoma (Figure 1g). The patient began chemotherapy with Cisplatin, Vincristine, and adjuvant Etoposide.
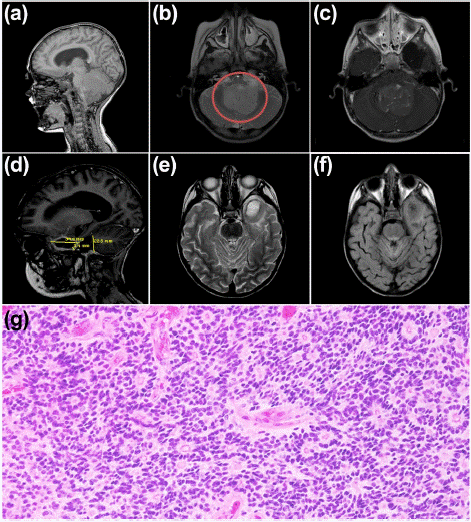
Figure 1: (a-c) Brain MRI October 11, 2012: Course and ventricular shunt system in the right frontal horn and ventriculomegaly; Galassi type II left temporal arachnoid cyst is observed. Posterior fossa with intraventricular tumour lesion measuring 4.3 x 4.5 cm isointense on T1, slightly hyperintense on T2 and FLAIR with minimal perilesional oedema towards the inferior cerebellar peduncles; in T1+Gad sequence with mild and heterogeneous uptake. The lesion causes dorsolateral displacement of cerebellar structures (vermis and cerebellar hemispheres) and ventral displacement of the floor of the fourth ventricle, brain stem (mesencephalic tectum, dorsum of the pons and cervicomedullary junction)—duplication of the longitudinal sinus as an anatomical variant. (d-f) MRI of the brain December 4, 2012: Absence of right shunt system, with ventricular tract and shunt system in the right frontal horn and persistence of ventriculomegaly. Post-surgical changes in the occipitocervical region due to suboccipital craniectomy and absence of posterior arch of C1. A cystic lesion is observed in the posterior fossa in communication with the fourth ventricle. Without abnormal uptakes in the posterior fossa or intensity change in T2 T2/FLAIR. (g) Histopathological analysis of extracted tissue confirming ependymoma.
Two years later, in 2014, at the age of four, the patient relapsed, presenting with weakness in the right lower limb, resulting in a claudicant gait. This was later accompanied by weakness in the ipsilateral upper limb, headaches, nocturnal vomiting, and an unquantified fever. A resection of the residual tumor was performed, followed by external radiation therapy at a dose of 39.6 Gy in 22 sessions targeting the tumor area (Figure 2a-c). In March 2017, the patient experienced another recurrence, this time with symptoms of vertigo, bladder incontinence, ataxia, and tonic-clonic seizures, necessitating an emergency room visit.
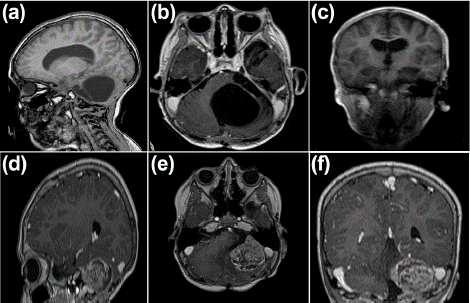
Figure 2: (a-c) Simple and contrasted MRI of the brain March 7, 2014: Course and ventricular shunt system in the right frontal horn, with average supratentorial ventricular size. Post-surgical changes due to suboccipital craniectomy. In the posterior fossa, cystic lesions were observed, the largest measuring 4x5 and the smallest measuring 1x1 cm, all located sagittally and left parasagittally (left cerebellopontine angle), septate, with a volume effect and displacement of the adjacent cerebellar parenchyma. No abnormal uptake of contrast medium was observed in the cysts’ interior or the septa’s walls. (d-f) Simple and contrasted MRI of the brain March 28, 2017 (Recurrence of the lesion): The tumour lesion recurs in the cerebellar hemisphere, left cerebellopontine angle, and posterior surface of the petrous apex. It is 4 x 3.3 cm, isointense on T1, T2, and FLAIR, with irregular uptake and a reticular pattern of the contrast medium.
A new MRI confirmed the recurrence (Figure 2d-f) and he was then placed in palliative care. Given the recurrence, the parents were offered treatment with NPt-Ped. During neurosurgery, a complete resection of the tumor was performed, followed by infiltration of the area with NPt-Ped nanoparticles.
Results
Physicochemical Properties of NPt-Ped
Figure 3a presents Transmission Electron Microscopy (TEM) images of NPt-Ped. The bionanocatalysts exhibit distinctive characteristics, demonstrating that coating platinum is integrated in the surface of the titania lattice, which possesses an anatase crystalline phase. TEM analysis reveals that these particles are assemblies of atoms with well-defined geometries. By examining a few nanoparticles at the edges of the agglomerates, the particle size can be estimated, showing titania particle diameters of approximately 3 nm. The electron diffraction pattern further confirms the presence of the anatase phase. Evaluation of light scattering vibrations through Raman spectroscopy confirm anatase chemical structure (Figure 3b). The vibration peak corresponding to the Pt-O bond is not appreciated because the metal oxide copies the titania network.
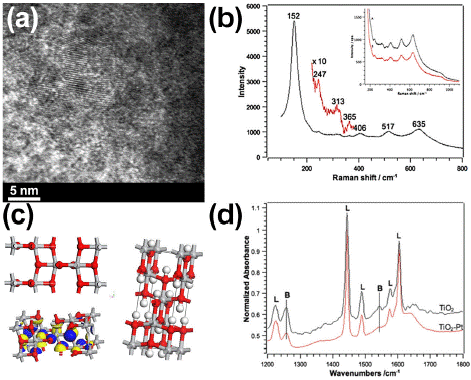
Figure 3: (a) TEM micrographs of NPt-Ped showing anatase crystalline structure. (b) Raman spectra confirming anatase
chemical signalling. (c) 3D structure of NPt-Ped calculated with density functional theory. (d) FTIR spectra with adsorbed pyridine for acid site determination.
In Figure 3c the 3D structure of NPt-Ped calculated with density functional theory can be observed for the identification of main structures composing the oxide matrix. Finally, in Figure 3d, the FTIR spectrum of NPt-Ped with adsorbed pyridine is shown, an analysis used to determine the type of acidity present. This spectrum suggests the formation of a physical bond between the platinum complex and the oxide surface. The interaction occurs through superficial OH groups, leading to a reduction in Brönsted acid sites. FTIR spectroscopy with adsorbed pyridine molecules is a highly effective tool for evaluating the acidic properties of a nanomaterial surface [31,32]. This technique provides evidence of both Brönsted and Lewis acid sites, which are relevant to tissue/cell interactions with NPt-Ped; it also indicates metal-support interactions with cellular receptors in tumors. In summary, the nitrogen atom in pyridine, with its two free electrons, allows for the determination of the nature, strength, and number of Lewis acid sites in the biocatalytic nanomaterial. At Lewis acid sites, the interaction with pyridine occurs through the donation of these electrons to a Lewis acid. If the acidic site is Brönsted-type, characterized by H+-labile surfaces, the formation of the pyridinium cation (C5H5NH+) is observed. Conversely, platinum with a low coordination number creates electron-accepting sites that can coordinate with pyridine molecules, classifying these sites as Lewis’s type. The nature of these acidic sites (Lewis or Brönsted) is confirmed by studying the vibrations of the ν(C-C) ring in the 1400-1700 cm-¹ range. The absence of a band at 1540 cm-¹ in Figure 4c indicates the lack of strong Brönsted sites.
Clinical Case Outcomes
The patient underwent another tumor resection surgery. This time, unlike previous procedures, a palliative treatment approach was employed, involving the topical application of NPt-Ped over the tumor bed following removal (Figure 4a). Remarkably, the NPt-Ped paste was absorbed into the tissue within seconds (Figure 4b, t=0; and Figure 4c, t=3 seconds). Immediately after surgery, a new MRI was performed, showing NPt-Ped deposits as lighter signals against the dark background (Figure 4d-f). Importantly, no uptake of the bionanocatalysts was observed in other tissues. The patient was kept under continuous observation to monitor his progress, and no side effects were noted after the surgery. A new MRI performed one month after surgery shows the progression of improvement in the patient (Figure 4g-i).
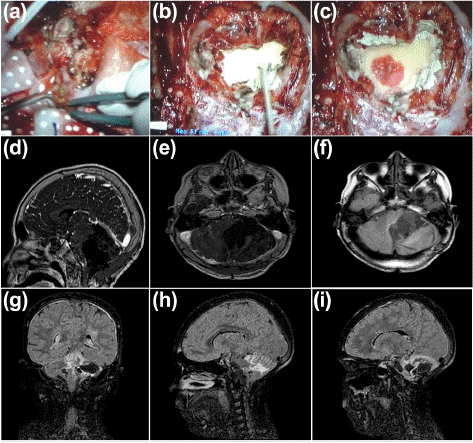
Figure 4: (a) Surgical resection of tumor with (b,c) NPt-Ped topical administration: comparison of tissue absorption at time 0 (b) and at 3 seconds after (c). (d-f) Simple brain MRI April 22, 2017 (early post-surgical MRI, with impregnation of NPt-Ped): Post-surgical changes in the left suboccipital and mastoid region. In the cerebellar hemisphere and left cerebellopontine angle, a surgical bed with hemosiderin deposits is observed in its periphery. Diffuse hyperintensity is observed on T1 and FLAIR VIEW in the midbrain, right superior and middle cerebellar peduncle, and cerebellar foliae bilaterally with right predominance. No abnormal uptakes or residual tumours were observed upon application of contrast medium. (g-i) Simple brain MRI May 23, 2017: Course and ventricular shunt system in the right frontal horn, with standard supratentorial ventricular size. Post-surgical changes due to suboccipital craniectomy. A septate cystic lesion persists in the cerebellar hemisphere and left cerebellopontine angle, hyperintense (isointense to CSF) on T2 in FLAIR VISTA sequence, hyperintensity is observed in the entire cerebral convexity in both cerebral hemispheres and in the ependyma of the entire supratentorial ventricular system. Diffuse hyperintensity in cerebellar vermis foliae and walls of cystic lesion.
Five years later, during a follow-up examination, the patient was declared in remission. He was in good general condition, asymptomatic, and a control MRI showed no signs of recurrence.
Discussion
The medical applications of nanotechnology for brain cancer represent a multidisciplinary field that leverages highly specific materials and devices to target malignant cells. This approach allows nanometric-level chemotherapy to directly reach the affected area, sparing healthy cells and thereby avoiding the side effects commonly associated with traditional chemotherapy [33]. Cancer remains one of the leading causes of death worldwide, with less than half of diagnosed patients surviving [34]. Platinum complexes are among the most widely used drugs in cancer treatment: the ongoing search for improved platinum-based drugs aims to reduce toxic side effects and enhance effectiveness, particularly against cisplatin-resistant tumors [35]. The chemical structures of these derivatives confirm their relationship to cisplatin, a cornerstone of cancer therapy. Oncologically active Pt-based drugs typically involve the coordination of the Pt2+ ion with two non-leaving/carrier groups [36]. An activation step is required for efficient DNA platination. Among platinum-based drugs, oxaliplatin is notably less ototoxic and nephrotoxic compared to cisplatin, which has a 2+-oxidation state [37]. Satraplatin, with a coordination number of 6 and an oxidation state of +4, also demonstrates fewer side effects. The pharmaceutical industry is actively addressing these issues, aiming to ensure that more than 20% of the drug reaches the targeted area while minimizing the impact on healthy cells.
Developing new methodologies with excellent bioactivity and selectivity to attack cancer while reducing tumor size is a significant challenge that has gained momentum in the 21st century with the advent of colloids and nanomedicine [38]. The size of blood capillaries in tumors (400–800 nm) facilitates the extravasation of colloidal particles into cancerous tissue [39]; furthermore, because cancerous tissues have fewer lymphatic capillaries, their drainage to healthy tissues is limited, causing colloidal particles to remain in the targeted area—a phenomenon known as "enhanced permeability and retention." Nanoparticles created through the self-assembly of amphiphilic copolymers have been utilized as carriers for cisplatin [40]. Additionally, various nanoscale delivery carriers, such as dendrimers, micellar silica-coated nanoparticles, ceramic nanoparticles, and targeted cross-linked liposomes, have been developed [41]. These carriers are used with different chemotherapeutics, including Doxorubicin polybutylcyanoacrylate nanoparticles (Dox-PBCA-NP), Doxorubicin liposomal nanoparticles, Paclitaxel-loaded PEG-PLA micelles, and PEG-PGlu micelles loaded with cisplatin [42]. Organic oxide nanoparticles offer a suitable method for controlled drug release to tissues or cells [43]. Their nanometric size facilitates uptake by cells through endocytosis/phagocytosis, while their hydrophilic surfaces promote recognition by the reticuloendothelial system and their intrinsic stability prevents degradation in the bloodstream.
NPt-Ped is an oncological bionanocatalyst comprising 1% platinum coating an oxide matrix. Unlike existing antineoplastic agents, NPt-Ped particles range from 1 to 10 nm in size. This nanomaterial is designed to break the carbon-nitrogen bonds of mitochondrial and nuclear DNA, selectively eliminating neoplasms without affecting healthy cells [24]. The large surface area and tailored acid-base properties of these particles enhance their site-specific activity. The term "bionanocatalyst" was coined by López-Goerne in 2013 [23]. The stabilization of nanoparticulate transition metals on the oxide network surface is a crucial component of bionanocatalysts. Coating the matrix improves the selective breaking of C-N bonds and produces synergistic effects due to the nature of platinum [25]. Research has shown that coating increases effectiveness, as smaller amounts of metal are needed to achieve equivalent results. The dispersion of platinum in the titania network’s surface approaches 100%.
López-Goerne et al. observed that nanocatalysts internalize into cancer cells through endocytosis [25]. Real-time transmission electron microscopy provided detailed insights into the cell incorporation process. These molecules improve the physicochemical structure of the external surface. Surface coating is vital in synthesizing bionanocatalysts, optimizing production, efficiency, and selectivity to specific molecules and surface functionalization. The coating and acidity are directly related to the internalization mechanism of pediatric NPt in cancer cells, with selectivity due to receptor recognition. This innovation is transforming current approaches to cancer treatment and prevention.
Conclusions
In the present work we report the case of a pediatric patient with classic recurrent ependymoma in posterior cranial fossa who was already receiving only palliative care. Given the inefficiency of the treatments received, it was decided to implement a protocol for the use of oncologic bionanocatalysts for his treatment. After 5 years, the patient is in remission without the presence of significant or evident side effects. This study demonstrates the selective activity of this type of nanostructures to exert cytotoxicity on cancer cells without affecting healthy cells in the surrounding tissues, as observed in other types of chemotherapeutics (such as cisplatin). It is necessary to continue evaluating new cases to determine factors to optimize this branch of science and to continue offering evidence of its effectiveness and safety, particularly in pediatric cases.
Highlights
• Recurrent ependymoma in posterior cranial fossa is identified in paediatric patient.
• Palliative treatment includes total resection and administration of bionanocatalysts.
• Patient is declared in remission five years post-surgery.
Author Statements
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
The authors confirm their contribution to the paper as follows: study conception and design: TLG; data collection: TLG, DA, FCPL, VGC, PDS; analysis and interpretation of results: TLG, DA, JONF, AS; draft manuscript: TLG, FJPG, AGB, RV. All authors reviewed the results and approved the final version of the manuscript.
References
- Wu J, Armstrong TS, Gilbert MR. Biology and management of ependymomas. Neuro Oncol. 2016; 18: 902–13.
- Zamora EA, Alkherayf F. Ependymoma. 2024.
- Ostrom QT, Price M, Ryan K, Edelson J, Neff C, Cioffi G, et al. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2022; 24: iii1–38.
- Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro Oncol. 2022; 24: v1–95.
- Pohl LC, Leitheiser M, Obrecht D, Schweizer L, Wefers AK, Eckhardt A, et al. Molecular characteristics and improved survival prediction in a cohort of 2023 ependymomas. Acta Neuropathol. 2024; 147: 24.
- Cage TA, Clark AJ, Aranda D, Gupta N, Sun PP, Parsa AT, et al. A systematic review of treatment outcomes in pediatric patients with intracranial ependymomas. J Neurosurg Pediatr. 2013; 11: 673–81.
- Merchant TE, Bendel AE, Sabin ND, Burger PC, Shaw DW, Chang E, et al. Conformal Radiation Therapy for Pediatric Ependymoma, Chemotherapy for Incompletely Resected Ependymoma, and Observation for Completely Resected, Supratentorial Ependymoma. Journal of Clinical Oncology. 2019; 37: 974–83.
- Ritzmann TA, Chapman RJ, Kilday J-P, Thorp N, Modena P, Dineen RA, et al. SIOP Ependymoma I: Final results, long-term follow-up, and molecular analysis of the trial cohort—A BIOMECA Consortium Study. Neuro Oncol. 2022; 24: 936–48.
- Morrall MCHJ, Reed-Berendt R, Moss K, Stocks H, Houston AL, Siddell P, et al. Neurocognitive, academic and functional outcomes in survivors of infant ependymoma (UKCCSG CNS 9204). Child’s Nervous System. 2019; 35: 411–20.
- Ellison DW, Kocak M, Figarella-Branger D, Felice G, Catherine G, Pietsch T, et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011; 10: 7.
- Pajtler KW, Witt H, Sill M, Jones DTW, Hovestadt V, Kratochwil F, et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell. 2015; 27: 728–43.
- Ramaswamy V, Hielscher T, Mack SC, Lassaletta A, Lin T, Pajtler KW, et al. Therapeutic Impact of Cytoreductive Surgery and Irradiation of Posterior Fossa Ependymoma in the Molecular Era: A Retrospective Multicohort Analysis. Journal of Clinical Oncology. 2016; 34: 2468–77.
- Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021; 23: 1231–51.
- Bertero L, Ricci AA, Tampieri C, Cassoni P, Modena P. Ependymomas. Pathologica. 2022; 114: 436–46.
- Bayliss J, Mukherjee P, Lu C, Jain SU, Chung C, Martinez D, et al. Lowered H3K27me3 and DNA hypomethylation define poorly prognostic pediatric posterior fossa ependymomas. Sci Transl Med. 2016; 8.
- Rudà R, Bruno F, Pellerino A, Soffietti R. Ependymoma: Evaluation and Management Updates. Curr Oncol Rep. 2022; 24: 985–93.
- Castro FD de, Reis F, Guerra JGG. Lesões expansivas intraventriculares à ressonância magnética: ensaio iconogrÁfico - parte 1. Radiol Bras. 2014; 47: 176–81.
- Winkler EA, Birk H, Safaee M, Yue JK, Burke JF, Viner JA, et al. Surgical resection of fourth ventricular ependymomas: case series and technical nuances. J Neurooncol. 2016; 130: 341–9.
- Hu C, Liu M, Li Y, Zhao Y, Sharma A, Liu H, et al. Recent advances and future perspectives of CAR-T cell therapy in head and neck cancer. Front Immunol. 2023; 14.
- Van Ommeren R, Garzia L, Holgado BL, Ramaswamy V, Taylor MD. The molecular biology of medulloblastoma metastasis. Brain Pathology. 2020; 30: 691–702.
- Arora RD, Agarwal MS, Maani EV, Cascella M. Palliative Radiation Therapy for Brain Metastases. 2024.
- Manríquez ME, López T, Gómez R, Navarrete J. Preparation of TiO2–ZrO2 mixed oxides with controlled acid–basic properties. J Mol Catal A Chem. 2004; 220: 229–37.
- López Goerne TM. Nanomedicina catalítica: ciencia y cÁncer. 1st ed. Ciudad de México: Arkhé Ediciones; 2013.
- López-Goerne T, Padilla-Godínez FJ. Catalytic Nanomedicine as a Therapeutic Approach to Brain Tumors: Main Hypotheses for Mechanisms of Action. Nanomaterials. 2023; 13: 1541.
- López-Goerne TM, Padilla-Godínez FJ, Castellanos M, Perez-Davalos LA. Catalytic nanomedicine: a brief review of bionanocatalysts. Nanomedicine. 2022; 17: 1131–56.
- López T, Ortiz E, Guevara P, Gómez E, Novaro O. Physicochemical characterization of functionalized-nanostructured-titania as a carrier of copper complexes for cancer treatment. Mater Chem Phys. 2014; 146: 37–49.
- Peterson A, Lopez T, Islas EO, Gonzalez RD. Pore structures in an implantable sol–gel titania ceramic device used in controlled drug release applications: A modeling study. Appl Surf Sci. 2007; 253: 5767–71.
- López T, Alvarez M, GonzÁlez RD, Uddin MJ, Bustos J, Arroyo S, et al. Synthesis, characterization and in vitro cytotoxicity of Pt-TiO2 nanoparticles. Adsorption. 2011; 17: 573–81.
- Abed A, Derakhshan M, Karimi M, Shirazinia M, Mahjoubin-Tehran M, Homayonfal M, et al. Platinum Nanoparticles in Biomedicine: Preparation, Anti-Cancer Activity, and Drug Delivery Vehicles. Front Pharmacol. 2022; 13.
- Jones RR, Hooper DC, Zhang L, Wolverson D, Valev VK. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res Lett. 2019; 14: 231.
- PARRY E. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J Catal. 1963; 2: 371–9.
- López T, Asomoza M, Gómez R, Bosch P, Rodríguez-Izquierdo J, Cauqui MA. Thermal decomposition and FTIR study of pyridine adsorption on Pt SiO2 sonogel catalysts. Thermochim Acta. 1995; 255: 319–28.
- Chehelgerdi M, Chehelgerdi M, Allela OQB, Pecho RDC, Jayasankar N, Rao DP, et al. Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation. Mol Cancer. 2023; 22: 169.
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71: 209–49.
- Zhang C, Xu C, Gao X, Yao Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics. 2022; 12: 2115–32.
- Lucaciu RL, Hangan AC, Sevastre B, Oprean LS. Metallo-Drugs in Cancer Therapy: Past, Present and Future. Molecules. 2022; 27: 6485.
- Dalian D, Haiyan J, Yong F, Yongqi L, Salvi R, Someya S, et al. Ototoxic Model of Oxaliplatin and Protection from Nicotinamide Adenine Dinucleotide. J Otol. 2013; 8: 63–71.
- Nirmala MJ, Kizhuveetil U, Johnson A, G B, Nagarajan R, Muthuvijayan V. Cancer nanomedicine: a review of nano-therapeutics and challenges ahead. RSC Adv. 2023; 13: 8606–29.
- Zhang M, Gao S, Yang D, Fang Y, Lin X, Jin X, et al. Influencing factors and strategies of enhancing nanoparticles into tumors in vivo. Acta Pharm Sin B. 2021; 11: 2265–85.
- Xiao X, Teng F, Shi C, Chen J, Wu S, Wang B, et al. Polymeric nanoparticles—Promising carriers for cancer therapy. Front Bioeng Biotechnol. 2022; 10.
- Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules. 2020; 25: 2193.
- Yan L, Shen J, Wang J, Yang X, Dong S, Lu S. Nanoparticle-Based Drug Delivery System: A Patient-Friendly Chemotherapy for Oncology. Dose-Response. 2020; 18: 155932582093616.
- Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers (Basel). 2023; 15: 1596.