
Research Article
Austin J Nanomed Nanotechnol. 2024; 12(1): 1075.
Catalytic Nanomedicine: Recurrent Pediatric Ependymoma Treated with Bionanocatalysts
Tessy López-Goerne, PhD1,2*; Francisco J Padilla-Godínez, MSc1,3; Esteban Gómez-López, PhD4; Daniel álvarez-Amador, MD5; Juan Carlos García-Beristain1; Antonela González-Bondani1; Rafael Valiente1; Vicente González-Carranza, MD5; Juan Carlos Manriquez, MD6; José Omar Navarro-Fernández, MD7; Abel Santamaría1
1Department of Health Care, Autonomous Metropolitan University-Xochimilco, Mexico
2Department of Chemical and Biomolecular Engineering, Tulane University, USA
3Department of Mathematics and Physics, Western Institute of Technology and Higher Education, Mexico
4Institute of Physics, Humboldt University of Berlin, Germany
5Department of Neurology and Neurosurgery, Children’s Hospital of Mexico “Federico Gomez”, Mexico
6Department of Cyclotron and Radiopharmacy, Doctors Hospital, Mexico
7Department of Head and Neck, National Institute of Cancerology, Mexico
*Corresponding author: Tessy López-Goerne, Department of Health Care, Autonomous Metropolitan University-Xochimilco, Mexico City 04960, Mexico. Email: tessy3@prodigy.net.mx
Received: October 18, 2024; Accepted: November 07, 2024 Published: November 14, 2024
Abstract
Background: Ependymomas, constituting about 10% of pediatric brain tumors are classified into three grades based on histology and molecular criteria. While typically arising from ependymal cells along cerebrospinal fluid pathways, exceptions exist regarding location for certain subtypes. Diagnosis relies on characteristic imaging findings and histopathological features, with intraventricular tumors often showing distinctive architectural patterns. Prognosis is significantly influenced by gross total resection and tumor location, with specific genomic alterations indicating varied outcomes. Current treatment involves surgical resection and radiotherapy, with chemotherapy’s efficacy remaining uncertain, and treatment guidelines have yet to incorporate molecular subtyping for personalized therapy. However, consensus favors microneurosurgical resection and local radiotherapy for specific molecular subtypes, notably PF-EPN-A type ependymomas in children over twelve months old.
Objective: The aim of this study was to assess the targeted eradication of tumor tissue using bionanocatalysts engineered specifically for DNA bond cleavage.
Methods: Bionanocatalysts were synthesized using established methods. These catalysts were then implanted into the tumor bed, and the progression was monitored using MRI scans.
Results: The patient showed a notable improvement in symptoms, accompanied by a considerable reduction in tumor size. Remarkably, no adverse side effects were detected. Even more impressively, after seven years, the patient, who was sent for palliative treatment, is still alive.
Conclusions: Bionanocatalysts demonstrated remarkable efficacy in eradicating a recurring pediatric ependymoma, even in a case initially slated for palliative care. Notably, no adverse effects were noted, underscoring the exceptional biocompatibility and targeted selectivity of these nanostructures exclusively towards tumorous tissue.
Keywords: Bionanocatalyst; NPt; Ependymoma; Catalytic nanomedicine
Introduction
Ependymomas comprise approximately 10% of all pediatric brain tumors [1]. Traditionally defined histologically, this entity has recently been further characterized by molecular biology, which has identified nine molecular and anatomical subtypes of ependymomas [2]. The World Health Organization (WHO) classifies ependymomas into three grades: grade I (subependymoma), grade II (ependymoma or myxopapillary ependymoma), and grade III (anaplastic ependymoma) [3]. Distinctions among tumor types are based on their neuroanatomical location, specific immunohistochemical profiles, and molecular criteria [4]. It is noteworthy that exceptions regarding location exist for subependymoma and myxopapillary ependymoma [5].
Ependymomas originate from ependymal cells surrounding the pathways of cerebrospinal fluid (CSF) circulation [5]. Diagnosis is typically more straightforward when intraventricular tumors are detected in brain imaging, particularly if architectural characteristics such as perivascular pseudorosettes or true rosettes are present. Diagnosis of all ependymomas requires the presence of typical morphological and immunohistopathological features. Pediatric ependymomas predominantly occur intracranially, with 60% of cases being infratentorial. Gross Total Resection (GTR) of the tumor is the most effective predictive factor for prognosis. Additionally, the location within the brain is associated with prognosis, with tumors originating from specific areas showing different growth patterns. Genomic analysis has revealed various cytogenetic patterns with different prognostic implications. Tumors with partial genomic alterations tend to have a worse prognosis, while those without chromosomal alterations are associated with a better prognosis, especially in very young children.
Surgical resection followed by adjuvant radiotherapy has been linked to increased survival rates, while the role of chemotherapy remains uncertain. Present treatment guidelines do not yet incorporate molecular subgroups to guide treatment decisions. However, consensus recommends micro-neurosurgical resection and local radiotherapy for patients with PF-EPN-A type ependymoma over twelve months of age.
This study presents the clinical case of an 8-year-old pediatric patient diagnosed with recurrent ependymoma. The patient underwent initial treatment with the NPt-Ped bionanocatalyst and subsequent surgical intervention. The utilization of NPt-Ped nanoparticles alongside surgical intervention is detailed, and a comprehensive follow-up on the patient's progress to date is provided.
Materials and Methods
Nanoparticle Synthesis
The synthesis of bionanocatalysts (NPt-Ped) employed previously reported techniques. In summary, matrix precursors were blended with surface functionalizing agents to craft cell-analog structures with remarkable catalytic capabilities. Following this, the resultant sample underwent meticulous vacuum drying to facilitate subsequent processing steps.
Bionanocatalysts Characterization
The bionanocatalysts were meticulously transferred under the safeguard of a high vacuum environment into the Transmission Electron Microscope (TEM), specifically the JEOL 2010 model, operating at a voltage of 120 kV and outfitted with an energy dispersive spectroscopic (EDS) microanalysis system from OXFORD. Imaging was facilitated using a CCD Mega Vision (III) camera. Infrared investigations were conducted using a Fourier Transform Perkin- Elmer spectrometer, Paragon 1000 model. Self-supported samples were meticulously prepared for analysis, with the study encompassing the spectral range from 4000 to 400 cm-1.
Clinic Story
A 6-year-old female patient with no significant history began her current illness with nausea, vomiting, intermittent headache that did not resolve with the administration of drugs, transient horizontal diplopia, and neck pain. Subsequently, the symptoms evolved, so they decided to go to their Health Center. During the physical examination, the following were ataxia, dysarthria, hearing loss, unstable gait and rightward movement. Due to the findings being compatible with intracranial hypertension, a fundoscopy was performed, in which papilledema was found, so the patient was referred to a third level.
Wavenumber (cm-1)
Assignment
3800
OH groups of occluded ethanol, and water from the environment.
3432
This band shift to lower energy when the gel is formed.
2800
Th C-H bond vibration of the residual ethoxy groups.
2300
Ti-OH vibration due to superficial hydroxylation.
1600
An OH flexion band caused by environmental water.
1080
A Ti-O stretching band, which correspond to the titania support.
960
A Ti-OH stretching band.
790 and 468
Flexion bands corresponding to Ti-O and -O-Ti-O- nucleophile structures formed in the first stage when pH was basic.
590
A Pt-O stretching vibration.
Table 1: FTIR Spectra of NPt-Ped Bionanocatalysts.
Upon arrival at the emergency room of the "Federico Gómez" Children's Hospital of Mexico due to persistent neurological deterioration (hyporeflexia in the pelvic limbs, myasthenia generalized paresthesias and loss of balance) without clinical improvement, it was decided to admit him to the neurology service. Based on what was observed in his clinical history and physical examination, a magnetic resonance imaging scan was requested for suspected hydrocephalus.
MRI of the neuraxis showed hydrocephalus and an infratentorial tumour in the posterior fossa (Figure 4), with no evidence of supratentorial or spinal lesions. An urgent ventriculoperitoneal shunt was performed, and 48 hours later, a suboccipital craniectomy with subtotal tumour resection was performed. The pathological anatomy reported the diagnosis of grade II classic ependymoma, NOS (no other indication). Subsequently, a Cerebrospinal Fluid (CSF) cytology was indicated, which obtained results within normal parameters.
The patient improved clinically, so radiotherapy and adjuvant chemotherapy were indicated; since the tumor was recurrent and there was a remaining tumor mass after surgery (carboplatin 150 mg and cyclophosphamide 500 mg, both once a week for 12 cycles, after the completion of radiotherapy) on an outpatient basis. The patient presented a slow and progressive clinical worsening over the next two years, so she underwent surgery a second time, achieving an almost total resection of the tumour. She had a deteriorated recovery, and due to the gradual neurocognitive adverse effects that greatly affected his quality of life (delay in learning, attention deficit, immaturity in growth and development for age) due to previous therapeutic chemoradiation, it was decided to perform close monitoring and surveillance. After three craniectomies and having tried all the existing alternatives, she was finally referred to palliative care in 2017, he was offered the experimental treatment (applying NPt-PED nanoparticles to the tumour bed). With full understanding of the risks involved, they provided informed consent as a final recourse. The patient underwent an MRI on November 12, 2014, once the symptoms and signs of intracranial hypertension were identified and before the nanoparticles were placed, which we will use as a reference (Figure 1). The National Institute of Cancerology (INCAN) declares to be the owner of a Cyclotron & Radiopharmacy Unit with a license for the operation of radioisotopes with Cyclotron issued on December 13, 2017, under number A000.200/1417/2017.
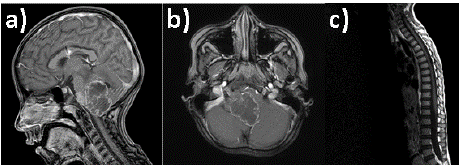
Figure 1: Brain MRI T1WI+Gad showing a fourth ventricle tumor with
invasion to right CPA through lateral recess (Luschka foramen) and severe
displacement of lower brainstem (A and B). Spine axis MRI showing no
lesions or abnormal enhancement in spinal canal (C).
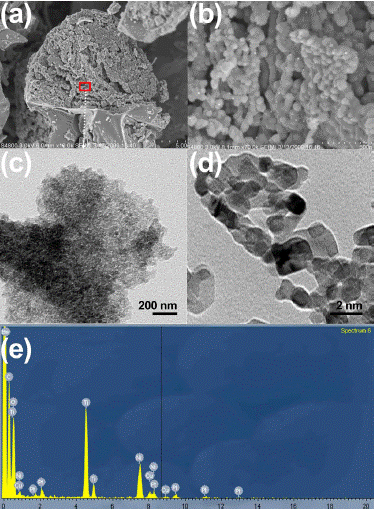
Figure 2: (A,B) SEM micrographs at low (A) and high (B) magnification.
(C,D) TEM evaluation showing individual particle size. (E) Elemental
composition through EDS.
Ethics and Consent
The authors affirm that all aspects of the research involving human patients in this study were carried out with the appropriate ethical approval from relevant bodies, and such approvals are duly acknowledged within the article. The study adhered to the guidelines set forth by the National Institutes of Health and was specifically approved by the Ethics Committee of the Children’s Hospital of Mexico under Protocol Number HIM 2017-072 entitled “Estudio del Efecto Antineoplásico del Nanomaterial Organoplatino al 1% Soportado en Titania Funcionalizada (NPt-Ped) en Niños con Tumores de Sistema Nervioso Central con Pronóstico Reservado en el Hopsital Infantil de México ‘Federico Gómez’ y la Universidad Autónoma Metropolitana”.
Results
Bionanocatalyst Physicochemical Composition
To analyze the functional groups present on the material's surface, Fourier Transform Infrared Spectroscopy (FTIR) was employed. In the lower energy range, a prominent broad band at 3800 cm-1 signifies the presence of OH groups from intermediate silanols, along with occluded ethanol and water from the ambient environment. Upon gel formation, this band shifts to a lower energy level, settling at 3432 cm-1. Additionally, a flexion band attributed to environmental water is discernible at 1600 cm-1. The vibration of residual ethoxy groups manifests at 2800 cm-1, a feature absents in powdered NPt-Ped. Notably, at 1080 cm-1, a Ti-O stretching band indicative of the titania support is observed, alongside a Ti-OH stretching band around 960 cm-1, signaling complete polycondensation in the preparation process. Flexion bands corresponding to nucleophiles, formed during the initial basic pH stage, are evident at 790 and 468 cm-1. A Pt-O stretching vibration is discerned at 590 cm-1. Upon calcination, infrared spectra exhibit remarkable similarity, with only the Ti-OH vibration attributable to surface hydroxylation remaining prominent; vibrations at 2300 cm-1 and 1600 cm-1 diminish, and the Ti-OH band at 960 cm-1 undergoes a frequency shift towards lower values.
Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM) techniques were employed to analyze the sample in terms of morphology, size, and texture. TEM operates on the principle of transmitted electrons, wherein electrons pass through the sample before being detected. This method yields detailed insights into the inner structure, including crystal morphology and stress states. Conversely, SEM provides surface and compositional information. Notably, TEM boasts superior spatial resolution, with recent advancements achieving resolutions finer than 50 picometers, whereas SEM is limited to around 0.5 nanometers. Both TEM and SEM utilize electrons for imaging, employing an electron source, electromagnetic and electrostatic lenses, and electron apertures within a high vacuum chamber to control the electron beam's trajectory and shape. By utilizing focused electron beams, both techniques surpass the resolution capabilities of light-based microscopes. Figure 1 depicts SEM (Figure 1a,b) and TEM (Figure 1c,d) analysis. The bionanocatalyst conglomerates exhibit a spherical regular structure with particle size around 500 nm that tend to conform bigger structures (Figure 1a). However, when analyzed by TEM, the individual bionanocatalysts were observed as semi-spherical 2-nm structures in an amorphous distribution.
Bionanocatalyst Implantation
Despite the pharmacological and surgical interventions, the patient continued to worsen, so on February 3, 2017, the protocol for the placement of the nanoparticles began. Patient F was reoperated achieving near gross total resection and local application of nanoparticles in the resection cavity (Figure 3). After the placement of the nanoparticles, an MRI was performed every week for followup In Figure 3C-E an MRI of the brain in the coronal and axial sagittal section after placement of the NPt-PED nanoparticles can be observed, with the treatment identified by a lighter section.
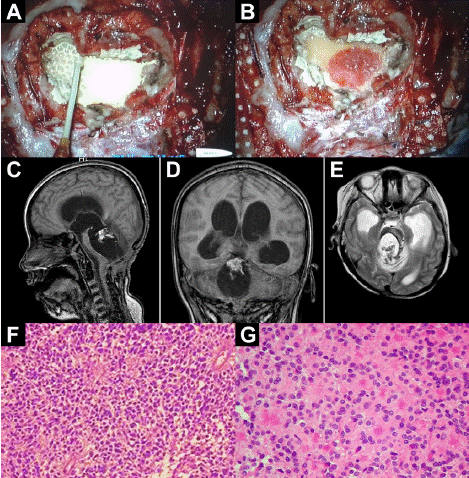
Figure 3: (A) After resection of the ependymoma with a cannula, nanoparticle
suspension is added. (B) The NPt bionanocatalyst after 30 sec. Almost all of
it has been adsorbed due to its affinity for cancer cells. (C-E) MRI followup
analyses after NPt bionanocatalyst administration. (F, G) H-E-stained
histopathology of recurrent ependymoma tissue extracted from patient. (F)
General appearance. (G) 40X magnification, the irregular distribution of the
nuclei is striking, with enucleated spaces, generally around the vessels.
After the placement of the nanoparticles, an MRI was performed every week for follow-up (Figure 3). In Figure 3C, D, E, an MRI of the brain can be seen in sagittal, coronal and axial sections, respectively, showing a tumor of the fourth ventricle with invasion of the right CPA through the lateral recess (foramen of Luschka) and severe displacement. of the lower part of the brain stem. Follow-up MRIs were performed weekly, one month after nanoparticle placement on March 18, 2017 (Figure 4A, B). A significant decrease is observed in the tumor area because these bionanocatalysts are programmed to attack only the DNA of malignant cells, catalytically converting the nitrogenous bases into CO2 + H2O + N2 + M3(PO4)n where n is equal to 2 if it binds to calcium and equal to 1 if it binds to sodium or potassium, this is because it breaks the C-C, C-N and C-O bonds. A space is created that would seem empty but is full of CO2, H2O and N2 (gases), Figure 4C. These gases will be excreted from the brain and will pass to the spleen kidneys and exit through urine, which can be verified by doing a clinical analysis of free nitrogen in urine and the result is always a significant excess of nitrogen.
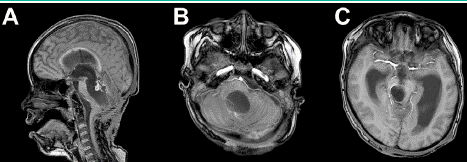
Figure 4: Brain MRI T1WI+Gad showing a nodular enhancing on the roof
of the fourth ventricle with no residual tumor and ventriculomegaly without
hydrocephalus.
In general, the patient exhibited an uneven recovery afnd because of neurocognitive side effects of previous chemo-radiation the decision was close follow-up. Remarkably, the patient responded positively to the NPt-PED treatment, marking a notable turn in her clinical course. Notably, after seven years, the patient is still alive.
Limitations
Case reports may suffer from bias, which can limit their relevance to broader patient populations since they only represent the case of one patient since the protocols accepted by the health organisms (like Level 3 Hospitals) require these conditions. However, despite this limitation, insights gained from analyzing Patient F's response to NPt- Ped bionanocatalysts offer valuable groundwork for hypothesizing about the selective cytotoxic mechanisms these therapies may exert on pediatric ependymomas considering that this is the first time that neurosurgeons apply a nanoceramic biocompatible material in the brain of a child. This study details the case of a pediatric patient treated with bionanocatalysts, which operate by catalytically breaking bonds. As such, direct comparisons with previous studies, particularly those involving controlled drug release compounds, are not feasible. Further research is essential to elucidate the precise mechanisms of action of bionanocatalysts in treating this type of tumor. It is noteworthy that no adverse effects were observed, underscoring the biocompatibility of these nanostructures. This investigation provides an updated perspective on the potential of bionanocatalysts in treating various tumor types, hinting at their potential application in future pathologies.
Conclusions
This study presents an investigation into the physicochemical attributes and therapeutic applications of a novel nanostructured compound comprising titania and platinum, renowned for its antineoplastic properties. Our prior studies have elucidated that these NPt-Ped bionanocatalysts penetrate cells via endocytosis, subsequently targeting both mitochondria and the nucleus. Within these cellular domains, the nanoparticles catalyze the cleavage of crucial C-C and C-N bonds within the DNA chain. We propose that the functionalization agents incorporated within the bionanocatalyst engage in specific ligand-receptor interactions with receptors on the plasma membrane of malignant cells, initiating the endocytosis process. This case underscores the potential for tumor elimination through bionanocatalyst administration, underscoring the necessity for ongoing research in catalytic nanomedicine. Concurrently, we are rigorously conducting clinical, radiological, and laboratory monitoring of our patients, diligently observing recurrence patterns and any unanticipated side effects that may arise.
Author Statements
Author Contribution
All authors contributed equally. All authors have given approval to the final version of the manuscript.
Acknowledgment and Funding
No acknowledgement nor funding to be declared.
References
- Eve Purdy, Donna L Johnston, Ute Bartels, Chris Fryer, Anne-Sophie Carret, Bruce Crooks, et al. Ependymoma in children under the age of 3 years: a report from the Canadian Pediatric Brain Tumour Consortium. Journal of Neuro-Oncology. 2014; 117: 359-364.
- Elisabeth J Rushing, Patrick B Cooper, Martha Quezado, Maria Begnami, Ana Crespo, James G Smirniotopoulos, et al. Subependymoma revisited: clinicopathological evaluation of 83 cases. Journal of Neuro-Oncology. 2007; 85: 297-305.
- Jens-Martin Hübner, Marcel Kool, Stefan M Pfister, Kristian W Pajtler. Epidemiology, molecular classification and WHO grading of ependymoma. J Neurosurg Sci. 2018; 62: 46-50.
- Elizabeth Vera-Bolanos, Kenneth Aldape, Ying Yuan, Jimin Wu, Khalida Wani, Mary Jo Necesito-Reyes, et al. Clinical course and progression-free survival of adult intracranial and spinal ependymoma patients. Neuro Oncol. 2015; 17: 440-7.
- Amit Jain, Anubhav G Amin, Punya Jain, Peter Burger, George I Jallo, Michael Lim, et al. Subependymoma: clinical features and surgical outcomes. Neurol Res. 2012; 34: 677-84.