Review Article
Austin J Nanomed Nanotechnol. 2014;2(3): 1017.
Cells as Drug Carriers
Arun Kumar*
Department of Medical Laboratory Sciences, University of Delaware, USA
*Corresponding author: Arun Kumar, Department of Medical Laboratory Sciences, Nanomedicine Research Laboratory, College of Health Sciences, University of Delaware, USA
Received: January 06, 2014; Accepted: March 17, 2014; Published: March 24, 2014
Introduction
The biggest challenges in drug delivery are to transport the drug to the site of infection without degradation with high concentration and protect it from body’s immune system. In order to achieve these goals, it is important to have a better understanding of the cellular mechanisms and primary intracellular uptake systems and to figure out fate of nanomaterials in multifarious biological systems [1]. Most delivery systems suffer from one or another drawback such as rapid clearance by the immune system, low targeting efficiency and difficulty in crossing biological barriers [2]. The understanding of mechanisms underlying intracellular uptake is important to design nanomaterials for effective drug delivery. The most common drug delivery methods are injection, infusion, ingestion, and inhalation. The common ingestion systems are tablet, capsule or liquid formulations and inhalation systems use a dry powder inhaler, metered–dose inhaler (MDI) or a nebulizer. The challenges for both drug and drug delivery systems are to deliver a drug in such a manner that improves the benefits to the patients, healthcare personnel and the healthcare structure. The major challenges in drug delivery are: (i) Efficacy of drug and side effects, (ii) slow and sustained release, (iii) minimizing the pain from drug administration, (iv) increased ease of use, (v) increased use compliance, (vi) improved mobility, and (vii) decreased involvement of healthcare personals. Improved safety for healthcare personnel and minimization of the environmental impactby elimination of chlorofluorocarbon (CFC) is key to develop safe drug delivery methods. A number of approaches are being used tomeet these challenges [3,4]. The drug entered into cells was believed to be via diffusion through the lipid bilayer of the cell membrane, with the influence of transporter proteins. However, recent research has shown that the drug uptake is transporter–mediated [5]. This suggests that uptake transporters may be a major determinant of idiosyncratic drug response and a site at which drug–drug interactions occur. Precisely modeling of drug pharmacokinetics involves the knowledge of systems biology and transporters with which a drug interacts and where those transporters are expressed in the biological systems. The pharmacokinetic models, based on biophysical properties of the cells, allows for improved drug uptake by diffusion [6]. The incorporation of transporter protein delivery systems greatly improves cellular uptake of the drug [7]. Development of a new drug molecule is expensive and time consuming. Improving the safety and efficacy of existing drugshas been attempted using different methods such as individualizing drug therapy, dose titration, and therapeutic drug monitoring. Drug delivery at a controlled rate, with slow and sustained delivery, and targeted delivery are important to improve the cellular uptake of a drug. Nanoparticles and nanoformulations have been used by many researchers as safe and effective drug delivery systems and have greater potential for many applications including anti–tumors therapy, gene therapy, AIDS therapy, radiotherapy, protein delivery, antibiotics, virostatics, and vaccines [8].
Challenges in cellular drug delivery
In cellular systems membranes are major obstacles for drugs attempting to target intracellular structures. Drugs degrade while attempting to across biological membranes and it is directly related to the polarity of a drug molecule; nonpolar or lipophilic molecules easily bypass this complication with greater membrane penetration, generally via diffusion [9]. However, numerous other cellular processes such as endocytosis mechanisms, intracellular trafficking, and release of the drug directly affect the intracellular concentrations and effectiveness of the drug. However, many pharmaceutical agents, including many large molecules such as nucleic acids, proteins, enzymes, antibodies or drug–loaded pharmaceutical nanocarriers,need to be delivered intracellularly to exert their therapeutic action. Biological membranes prevent hydrophilic drug molecules from entering cells [10]. The intracellular transport of different biologicallyactive molecules is one of the major complications in drug delivery. Many compounds shows great potential in vitro studies but cannot succeed in vivo because of delivery problems. Several intracellular drug delivery systems were evaluated for their potential to transport therapeutic molecules inside cells and genome sequencing critically contributed in designing the drug delivery strategies. Significant advances were made with many efficient transfection reagents to study the gene functions. However, few nucleic acid intracellular delivery systems were developed for transfection, gene therapy, and delivery of other biomolecules [11].
Nanoparticles for cellular drug delivery
Nanoparticles are made up of natural or synthetic polymers ranging in size between about 10 and 1000 nm (1 mm). Drugs can be bound to nanoparticles or dispersed or adsorbed to the surface or can be chemically attached to it. Poly (butylcyanoacrylate) nanoparticles are successfully used for the in vivo delivery of drugs into the brain. This successful delivery to the brain uses hexapeptidedalargin (Tyr–DAla– Gly– Phe–Leu–Arg), and Leu–enkephalin analogue nanoparticleswith opioid activity [12]. Nanoparticles provide enormous advantages regarding drug targeting, delivery and release, and, with the potential to combine diagnosis and therapy, emerge as one of the major tools in nanomedicine. The main goals in using nanoparticles in drug delivery are to improve drug stability in the biological system, to mediate the bio–distribution, improve drug loading, targeting, transport and release, and efficiently cross the biological barriers. The major problems of nanoparticles are cytotoxicity, remaining degraded products, and biocompatibility. Liposomes are used in controlled drug delivery systems due their bio–adhesive and levonorgestrel properties. Mesophasicproliposomal systems were mostly unilamellar and some were multilamellar with zero order kinetics [13]. Alcohol as a polar molecule had greater effect on transdermal flux, and in vivo studies have shown that a significant lag phase observed before it reaches therapeutic levels. This proliposomes system is better thanPEG–based ointment systems [14] (Figure 1).
Figure 1: Multifunctional nanoparticle for drug delivery.
A liposomal reservoir system bearing local anesthetic benzocaine was developed for controlled and localized delivery via topical route. The liposomal suspension was incorporated into an ointment and gel base to deliver the drug with controlled release rate [15].
Role of cells as drug transporters
All cells are transporters of nutrients and intermediary metabolites, and the human genome codes of several types. However, the 'passive’ permeability of drugs occurs in the absence of carriers [16]. Comparison of the rate of drug transport in natural versus synthetic membranes has shown that there is 100–fold increase or more, when using cell transporters. These transporters expressed in the tissues can be determined using expression profiling data of the cells. This provides significant information and can suggest what kind of carriers are important for taking drugs into those cells, which can ultimately improve the effectiveness of the drug. Drug entry into cells was previously assumed to be by diffusion through the lipid bilayer of the cell membrane, with the minimal contribution of transporter proteins. However, recent research has shown that drug uptake is mainly transporter–mediated [17]. This suggests that uptake transporters may be a major determinant of characteristic of the drug response and a site where drug–drug interactions occur.
Accurately modelling of drug pharmacokinetics is difficult from a systems biological point of view. It requires knowledge of both the transporters and interaction of drug with transporters which are expressed in the cells and tissues [18]. The physiologically based pharmacokinetic models try to model the character of the drug from the biophysical properties of the drug and their uptake by diffusion. The incorporation of transporter proteins and drug interactions into such models will greatly improve the drug delivery process. The way by which drugs localize in tissues and interact with transporter of the drugs can be determined. The yeast–based exporter expression system can be used for the initial screening of drugs for their equivalent transporters.
Many drug molecules are transported across biological membranes through passive diffusion and rate of transportation is related to their lipophilicity. However, the types of biophysical forces of cells involved in the interaction of drugs with lipid membranes and their interactions with proteins are based on lipophilicity, which could be applied to drugs transported by membrane transporters or carriers.
The biophysical properties of cells which make them unique drug carriers are:
1. Elasticity
2. Viscoelasticity
3. Stiffness
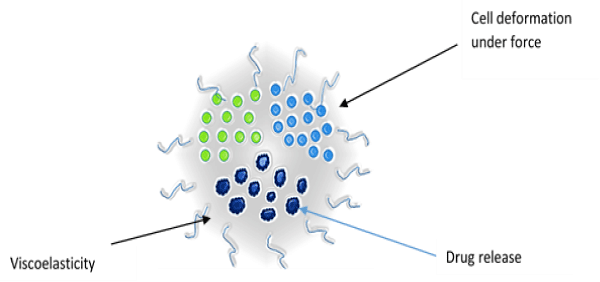
The important biophysical properties of a cell significantly involved with the drug delivery process are elasticity, visco elasticity and stiffness. These parameters provide information about the resistance of a material to deformation or strain, and they show significant differences [19] (Figure 2).
Figure 2: Biophysical properties of cell.
Elasticity: The cell deforms under external forces and returns to its original shape when the external force is removed. This property of the cell can be exploited for drug delivery. The relationship between force and deformation is linear and this parameter plays an important role in cellular physiology. The principle of elasticity can be used to develop force–indentation model for drug delivery [20].
Viscoelasticity: When cells undergo deformation they exhibit both viscous and elastic characteristics, known as viscoelasticity. Viscosity is a measurement of the resistance of a fluid to being deformed by either shear stress or extensional stress. This is due to the result of diffusion and interaction of molecules inside of an amorphous material. The reciprocal of viscosity is fluidity. The relationship between stress and strain is non–linear for viscoelastic material and the deformation energy is not returned completely. The amount of this lost energy is represented by the hysteresis of loading and unloading of forces [21].
Stiffness: Stiffness is the resistance of a solid body to deformation by an applied force. In general, elastic modulus is not the same as stiffness. Elastic modulus is a property of the constituent material; stiffness is a property of a solid body. The elastic modulus does not depend on the size, shape, amount of material and boundary conditions of the material; stiffness, on the other hand, is an extensive property and it depends on the size, shape, amount of material and boundary conditions of the solid body [22].These biophysical properties play significant roles in designing the drug delivery model for cells.
Cell mediated drug delivery
The major challenge in functionalizing nanoparticles is the difficulty with the complex surface chemistry of a biological cell. The recent research has made significant progress in reducing macrophage engulfment of polystyrene beads and their conjugation with an immunosuppressive RBC–membrane protein, CD47. The bioconjugation techniques which involve chemical processes frequently lead to protein denaturation [23].
Cell–mediated nanotechnology has shown the way to use the cell itself as a delivery vehicle to deliver the drug in high concentration at the site of infection without degradation Encouraged by the concept of delivering nanoparticles, Arun Kumar and Don F Cameron et al [24] has shown that nanoencapsulated drugs can be delivered into deep lung using Sertoli cells as vehicles for delivery of nanoencapsulated drugs. In this approach they have shown that isolated rat Sertoli cells preloaded with chitosan nanoparticles can be used to obtain a high–density distribution and concentration of the nanoparticles in the lungs of mice by way of the peripheral venous vasculature rather than the more commonly used pulmonary route. There was a marked positive therapeutic effect achieved 24 h following curcumin treatment delivered by this Sertoli cell nanoparticle protocol (SNAP). These results identify a novel and efficient protocol for targeted delivery of drugs to the deep lung mediated by extratesticularSertoli cells. Utilization of SNAP cell–mediated delivery may optimize drug therapy for conditions such as ARDS, status asthmaticus, pulmonary hypertension, lung cancer, and complications following lung transplantation where the use of high concentrations of antiinflammatory drugs is desirable, but often limited by risks of systemic drug toxicity. This novel cell–mediated drug delivery protocol to the deep lung offers a new and potential way to treat lung pathologies far more effectively than current protocols, opening new ways to deliver a drug of choice.
References
- Liboiron BD, Mayer LD. Nanoscale particulate systems for multidrug delivery: towards improved combination chemotherapy. Ther Deliv. 2014; 5: 149-171.
- Freeman A, Mayhew E. Targeted drug delivery. Cancer. 1986; 58: 573-583.
- Meyer Zu Schwabedissen HE, Begunk R, Hussner J, Juhnke BO, Gliesche D. Cell-Specific Expression of Uptake Transporters-A Potential Approach for Cardiovascular Drug Delivery Devices. Mol Pharm. 2014.
- Fumoto S, Kawakami S. Combination of nanoparticles with physical stimuli toward cancer therapy. Biol Pharm Bull. 2014; 37: 212-216.
- Lai Y, Hsiao P. Beyond the ITC White Paper: Emerging Sciences in Drug Transporters and Opportunities for Drug Development. Curr Pharm Des. 2014; 20: 1577-1594.
- Arduini A, Holme S, Sweeney JD, Dottori S, Sciarroni AF. Addition of L-carnitine to additive solution-suspended red cells stored at 4 degrees C reduces in vitro hemolysis and improves in vivo viability. Transfusion. 1997; 37: 166-174.
- Lundquist P, Lööf J, Sohlenius-Sternbeck AK, Floby E, Johansson J. The impact of solute carrier (SLC) drug uptake transporter loss in human and rat cryopreserved hepatocytes on clearance predictions. Drug Metab Dispos. 2014; 42: 469-480.
- Canfarotta F, Piletsky SA . Engineered magnetic nanoparticles for biomedical applications. Adv Healthc Mater. 2014; 3: 160-175.
- Baik J, Rosania GR. Modeling and Simulation of Intracellular Drug Transport and Disposition Pathways with Virtual Cell. J Pharm Pharmacol (Los Angel). 2013; 1.
- Nam J, Won N, Bang J, Jin H, Park J. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv Drug Deliv Rev. 2013; 65: 622-648.
- Warth A, Muley T, Meister M, Herpel E, Pathil A. Loss of aquaporin-4 expression and putative function in non-small cell lung cancer. BMC Cancer. 2011; 11: 161.
- Lin Y, Pan Y, Shi Y, Huang X, Jia N. Delivery of large molecules via poly(butyl cyanoacrylate) nanoparticles into the injured rat brain. Nanotechnology. 2012; 23: 165101.
- Deo MR, Sant VP, Parekh SR, Khopade AJ, Banakar UV. Proliposome-based transdermal delivery of levonorgestrel. J Biomater Appl. 1997; 12: 77-88.
- Wang S, Ye T, Yang B, Yi X, Yao H. 7-Ethyl-10-hydroxycamptothecin proliposomes with a novel preparation method: optimized formulation, characterization and in-vivo evaluation. Drug Dev Ind Pharm. 2013; 39: 393-401.
- Spera R, Petralito S, Liberti M, Merla C, d'Inzeo G, et al. Controlled release from magnetoliposomes aqueous suspensions exposed to a lowintensity magnetic field. Bioelectromagnetics. 2014; 35: 309-312.
- Biswas S, Torchilin VP. Nanopreparations for organelle-specific delivery in cancer. Adv Drug Deliv Rev. 2014; 66C: 26-41.
- Doss CG, Debottam S, Debajyoti C. Glutathione-responsive nano-transporter-mediated siRNA delivery: silencing the mRNA expression of Ras. Protoplasma. 2013; 250: 787-792.
- Hall AJ, Chappell MJ, Aston JA, Ward SA. Reprint of "Pharmacokinetic modellingof the anti-malarial drug artesunate and its active metabolitedihydroartemisinin". Comput Methods Programs Biomed. 2014.
- Su J, Zhang L, Zhang W, Choi DS, Wen J, et al. Targetingthe biophysical properties of the myeloma initiating cell niches: apharmaceutical synergism analysis using multi-scale agent-based modeling. PLoS One. 2014; 27: 9.
- Verma P, Wong IY, Melosh NA. Continuum model of mechanical interactions between biological cells and artificial nanostructures. Biointerphases. 2010; 5: 37-44.
- Pelton M, Chakraborty D, Malachosky E, Guyot-Sionnest P, Sader JE. Viscoelastic flows in simple liquids generated by vibrating nanostructures. Phys Rev Lett. 2013; 111: 244502.
- Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA. Microvesicles and exosomes for intracardiac communication. Cardiovasc Res. 2014; .
- Wetzel DM, McMahon-Pratt D, Koleske AJ. The Abl and Arg kinases mediate distinct modes of phagocytosis and are required for maximal Leishmania infection. Mol Cell Biol. 2012; 32: 3176-3186.
- Kumar A, Glaum M, El-Badri N, Mohapatra S, Haller E. Initial observations of cell-mediated drug delivery to the deep lung. Cell Transplant. 2011; 20: 609-618.