Editorial
Austin J Nanomed Nanotechnol. 2014;2(3): 1020.
The “Nano” World in Photodynamic Therapy
Huang-Chiao Huang1 and Tayyaba Hasan1,2*
1Harvard Medical School, Massachusetts General Hospital, USA
2Division of Health Sciences and Technology, Harvard- MIT, USA
*Corresponding author: Tayyaba Hasan, Division of Health Sciences and Technology, Harvard Medical School, Massachusetts, USA
Received: May 22, 2014; Accepted: May 27, 2014; Published: May 29, 2014
Abstract
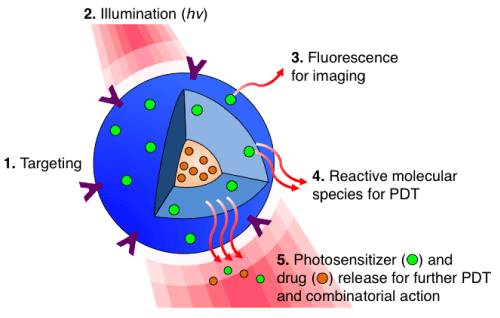
Photodynamic Therapy (PDT) is an externally activated, photochemistrybased approach that generates cytotoxic reactive molecular species (RMS), which kill or modulate biological targets. PDT provides unique opportunities for applications of nanotechnology where light activation can trigger both direct RMS–mediated cytotoxic activity and the release of contents within the nanoconstructs (Figure 1). This process allows several species, working via different mechanisms and molecular targets to be activated or released in the right place and time, thus providing a distinctive approach to combination therapy. With advances in the development of miniaturized, even–biodegradable light sources and delivery systems, exciting possibilities of anatomical reach with PDT are being made possible. This brief article introduces aspects of interfaces of PDT and nanotechnology but, due to space constraints, makes no attempt to be a comprehensive review.
Introduction
The concept of combining light and chemicals for therapy is thousands of years old [1,2]. PDT, in its present form, can be dated back to Raab's accidental discovery in 1900 that Paramecia combined with acridine orange and exposed to sunlight resulted in cytotoxicityto the organism [3]. Contemporary PDT was developed by contributions from many investigators, notably of Lipson, Schwartz and Dougherty [4–8] and is approved for several cancer and noncancer applications [2]. PDT involves the light activation of certainchemicals called photosensitizers (PS) to elicit photochemistry that is cytotoxic or deleterious to biologic targets. Inherent to PDT is thedual selectivity imparted by preferential accumulation of the typical photosensitizing agents and by the confinement of light to defined volumes. This photochemistry–based approach is distinct from the more frequently reported laser–activated photothermal approaches where high intensity, often using pulse lasers, is required to generatethermal effects. PDT typically requires low irradiances in the mW⁄ cm2 ranges and does not depend on thermally induced “burning” oftissues but rather on the induced photochemistry. It is thus a “kinder,gentler” approach to phototoxicity allowing biological effects tocontinue after the light trigger has been switched off. Combined withnanotechnology, PDT–Nano provides exceptional opportunities for delivery of therapeutic reagents and newer approaches tocombination treatments grounded in cellular mechanisms [9–13] andother advances in photomedicine [14,15]. In addition to enhancedPS delivery, it allows modification of PS physiochemical properties, development of novel, even resorbable light sources, establishment of personalized predictive dosimetry, and the evolution of novel combinatorial therapeutic approaches.
Figure 1: Targeted, multi-compartment nanoconstruct (blue) co-delivers photosensitizer (PS) and drug to the cell. (1) Surface modification with stabilizers and targeting moieties improves stability and selectively of the nanoconstructs. (2) Upon light irradiation (hv), the (3) fluorescence signal generated from the excited PS can be used for imaging. (4) Light activation of PS results in reactive molecular species production for PDT, and (5) facilitates PS and drug release, allowing for an interactive combination therapy.
A wide variety of organic and inorganic nanoconstructs (e.g. liposomal, micellar, polymeric, silica and gold nanoparticles) [16] have been introduced to deliver high payloads of PS to the desired sites when combined with targeting moieties. Thus far, the most studied and clinically used nanoconstructs for PS delivery is the liposome [17,18]. Jori demonstrated liposomal delivery of porphyrins and phthalocyanines to the tumors in the 80–90’s [19–21]. In 2001, collaborative work from several laboratories including ours led to the first clinical approval of PDT as a frontline treatment, using Visudyne®, a liposomal verteporfin formulation, and red light for the treatment of age–related macular degeneration [22]. Visudyne is now featured in the listing of nanomaterials approved by the US Food and Drug Administration then followed by (FDA). Micelles, with a single layer of polar–nonpolar molecules, are also commonly used to delivery PS [23–25]. TOOKAD delivered in Cremophor micelles (WST09) was in phase II⁄III clinical trials for the PDT of prostate cancer (ClinicalTrials.gov) [26,27]. Francis et al. Modified Bacteriophage MS2 virus capsids with porphyrins and Jurkat–specific aptamers to selectively target and photo–damage Jurkat leukemia T cells in vitro [28]. Packaging of PS into the nanoconstructs can significantly impact the photophysiochemical properties of PS. Pioneered by Zheng, Lovell and colleagues, porphysomes are extraordinarily tightly packed porphyrins within the confined space of the liposomal lipid bilayer [29,30]. These extensively self–quenched porphyrins nanoconstructs (over 1,200–fold greater than the monomerized porphyrins) can be light activated for in vivo photothermal but–not–PDT tumor ablation. Wang demonstrated that reactive oxygen species (ROS) production from protoporphyrin IX can be enhanced up to 4.7 fold when conjugated to gold nanoparticles, thus improving the efficacy of PDT against breast cancer cell in vitro [31]. This enhancement of ROS is likely due to an enhanced electromagnetic field as a result of the localized surface plasmon resonance of gold nanoparticle upon light exposure.
In the context of PDT, nanoconstructs may be built with enough flexibility to be responsive to a broad spectrum of microenvironmental barriers and be more than simple drug carriers. They become photoresponsive entities that act directly, release enclosed materials and yet home in or accumulate at the desired sites preferentially [32]. Polyethylene–glycol (PEG) or monosialoganglioside molecules are widely used to sterically stabilize nanoconstructs and, therefore, can reduce PS uptake by the macrophages in the reticuloendothelial system, prolong circulation half–life, and allow passive accumulation of PS at tumor sites (e.g. via the enhanced permeability and retention effect) [33–36]. Actively targeted nanoconstructs have been developed with the following broad goals:
- Inherent targets: where nanoconstructs are driven by moieties (e.g. aptamers, monoclonal antibodies etc.) to direct against specific cancer–associated molecules.
- Therapeutically induced targets: where a given treatment triggers or sensitizes the aberrant expression of biomarkers that can serve as molecular targets (e.g. with molecular inhibitors or PDT) [37–40].
- Combinations of targets: where cooperatively targeting of both inherent targets and therapeutically induced targets maximizes the treatment benefits by activating several cell death pathways via nanoconstructs.
Photo immunoconjugates are probably the best examples of focusing on inherent targets. Using non–quenching PS associated with tumor–targeting monoclonal antibodies or antibody fragments, Levy first reported this approach [41]. This has been subsequently developed by several groups including ours in the 80s and the 90s [42– 47] and more recently by others [48,49] in different tumor models. We recently reported a “tumor–activatable” photoimmunoconjugate to further improve the safety and selectivity of photo immunotherapy [50]. In this study, verteporfin was covalently linked to the EEGFRtargeting cetuximab at a high payload, which resulted inselfquenching of PS (non–phototoxic). Upon cancer cell internalization, verteporfin phototoxicity and fluorescence was activated via lysosomal proteolysis resulting in de–quenching of verteporfin followed by light irradiation. This enabled the fluorescence imaging of ovarian cancer micrometastases nodules as small as 30 μm and the tumor–specific photo cytotoxicity in a disseminated model of peritoneal cancer micrometastases [50].
The ability to target and control the spatiotemporal release of the PS (e.g. via external activation) has been suggested [51–53]. This approach is extremely useful for applications to tumor where the genetically complex and heterogeneous tumor cells develop multiple mechanisms of survival and resistance to treatments. It enables rational mechanism–based combination of PDT with a secondary mechanistically non–overlapping treatment. Through combinatorial approaches, the molecular responses elicited by PDT (or the other therapy) can sensitize the tumor to the second treatment modality [54,55]. In this context, multicompartmental nanoconstructs provide the opportunity to co–deliver multiple agents, such as PS and conventional or evolving drugs within a single construct (Figure 1),[23, 56]. These constructs are designed with appropriate mechanism–based combinatorial agents and spatiotemporal control release enabled by a combination of appropriate light switch and appropriate chemistries. Such combination treatments customized to deliver the nanoconstruct payloads to the right place at the right time show promise in early studies in pancreatic and ovarian cancer models [47, 50,57–59].
With the advent of sophisticated endoscopes and miniaturized light generating and delivery devices, most anatomical sites are accessible for PDT. For example, lung cancer treatments routinely use bronchoscopes incorporating optical fibers. Intra–operative and trans–cutaneous light delivery is also used as in the PDT of pancreatic cancer [60,61]. New approaches exploring nanoconstructs or cells themselves as sources of light are ongoing and have the potential of alleviating the problem of tissue penetration depth. It has been demonstrated that green fluorescent proteins in cells can be used as viable gain medium for optical amplification, creating a laser based on single live cell [62]. Up converting nanoparticles (i.e. NaYF4 nanocrystals co–doped with Yb3+ and Er3+) use longer wavelength light (i.e. deeper penetrating near–infrared light) to generate shorter (i.e. visible) wavelengths for the activation of PS payloads and the photodynamic destruction of cancers [63–67]. The use of penetrating X–rays to trigger nanoscintillators (i.e. LaF3: Tb3+, quantum dots, Tb2O3 covered by polysiloxane layer) for the excitation of the nanoconstruct coupled PS has been demonstrated [68,69]. This modality where X–rays–activate PS via nanoscintillators can be combined with radiotherapy for combination treatment of diseases that are endoscopically in accessible. Finally, the use of PS fluorescence to detect cancers, guide surgical resection of tumors, and to personalize PDT dose parameters is an ongoing and exciting area of research [2,70]. While several challenges remain, advances in optical technologies combined with actively targeted nanoconstructs containing ‘theranostic’ PS offer the potential for personalized photodynamic therapy and combinations for treatment of cancer and other diseases.
Acknowledgement
This work was supported by National Institutes of Health Grants P01CA084203, R01CA156177, and R01CA160998 (to T.H.).
References
- Hasan T, Ortel B, Solban N, Pogue B. Photodynamic therapy of cancer. Kufe DW, Bast RC Jr, Hait WN, Hong WK, Pollock RE, Weichselbaum RR, Holland JF, Frei E III, editors. In: Cancer Medicine 7th edn. Hamilton, Ontario: BC Decker, Inc. 2006; 537-548.
- Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010; 110: 2795-2838.
- Raabe, Oscar. Über die Wirkung fluoreszierender Stoffe auf Infusion. Z Biol. 1900; 39: 524-546.
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J. Photodynamic therapy. J Natl Cancer Inst. 1998; 90: 889-905.
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003; 3: 380-387.
- Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011; 61: 250-281.
- Roozeboom MH, Arits AH, Nelemans PJ, Kelleners-Smeets NW. Overall treatment success after treatment of primary superficial basal cell carcinoma: a systematic review and meta-analysis of randomized and nonrandomized trials. British journal of dermatology 2012; 167: 733-756.
- Pereira S. Photodynamic therapy for pancreatic and biliary tract cancer: the United Kingdom experience. J Natl Compr Canc Netw. 2012; 10 Suppl 2: S48-51.
- Gomer CJ. Induction of prosurvival molecules during treatment: rethinking therapy options for photodynamic therapy. Journal of the National Comprehensive Cancer Network. 2012; 10: S35-39.
- Savellano MD. Hasan T. Photochemical targeting of epidermal growth factor receptor: a mechanistic study. Clinical cancer research. 2005; 11: 1658-1668.
- Kosharskyy B, Solban N, Chang SK, Rizvi I, Chang Y, Hasan T. A mechanism-based combination therapy reduces local tumor growth and metastasis in an orthotopic model of prostate cancer. Cancer research. 2006; 66: 10953-10958.
- Kessel D, Reiners JJ Jr. Enhanced Efficacy of Photodynamic Therapy via a Sequential Targeting Protocol. Photochem Photobiol. 2014.
- Ferrario A, Gomer CJ. Targeting the tumor microenvironment using photodynamic therapy combined with inhibitors of cyclooxygenase-2 or vascular endothelial growth factor. Methods in molecular biology. 2010; 635: 121-132.
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005; 5: 161-171.
- Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008; 60: 1627-1637.
- Huang YY, Sharma SK, Dai T, Chung H, Yaroslavsky A, Garcia-Diaz MJC, et al. Can nanotechnology potentiate photodynamic therapy? Nanotechnology Reviews. 2012; 1: 111-146.
- Chen B, Pogue BW, Hasan T. Liposomal delivery of photosensitising agents. Expert Opin Drug Deliv. 2005; 2: 477-487.
- Jin CS, Zheng G. Liposomal nanostructures for photosensitizer delivery. Lasers Surg Med. 2011; 43: 734-748.
- Reddi E, Zhou C, Biolo R, Menegaldo E, Jori G. Liposome- or LDL-administered Zn (II)-phthalocyanine as a photodynamic agent for tumours. I. Pharmacokinetic properties and phototherapeutic efficiency. Br J Cancer. 1990; 61: 407-411.
- Ricchelli F, Biolo R, Reddi E, Tognon G, Jori G. Liposomes as Carriers of Hydrophobic Photosensitizers in Vivo: Increased Selectivity of Tumor Targeting. Proc. of SPIE. 1987; 0847.
- Jori G, Tomio L, Reddi E, Rossi E, Corti L, Zorat PL, Calzavara F . Preferential delivery of liposome-incorporated porphyrins to neoplastic cells in tumour-bearing rats. Br J Cancer. 1983; 48: 307-309.
- Guidelines for using verteporfin (visudyne) in photodynamic therapy to treat choroidal neovascularization due to age-related macular degeneration and other causes. Retina. 2002; 22: 6-18.
- Peng CL, Lai PS, Lin FH, Yueh-Hsiu Wu S, Shieh MJ. Dual chemotherapy and photodynamic therapy in an HT-29 human colon cancer xenograft model using SN-38-loaded chlorin-core star block copolymer micelles. Biomaterials. 2009; 30: 3614-3625.
- Yin R, Wang M, Huang YY, Huang HC, Avci P, Chiang LY, et al. Photodynamic therapy with decacationic [60] fullerene monoadducts: Effect of a light absorbing electron-donor antenna and micellar formulation. Nanomedicine. 2014; 10: 795-808.
- Van Nostrum CF. Polymeric micelles to deliver photosensitizers for photodynamic therapy. Adv Drug Deliv Rev. 2004; 56: 9-16.
- Weersink RA, Bogaards A, Gertner M, Davidson SR, Zhang K, Netchev G, et al. Techniques for delivery and monitoring of TOOKAD (WST09)-mediated photodynamic therapy of the prostate: clinical experience and practicalities. Journal of photochemistry and photobiology. B, Biology. 2005; 79: 211-222.
- Lepor H. Vascular Targeted Photodynamic Therapy for Localized Prostate Cancer. Rev Urol. 2008; 10: 254-261.
- Stephanopoulos N, Tong GJ, Hsiao SC, Francis MB. Dual-surface modified virus capsids for targeted delivery of photodynamic agents to cancer cells. ACS Nano. 2010; 4: 6014-6020.
- Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nature materials. 2011; 10: 324-332.
- Jin CS, Lovell JF, Zheng G. One minute, sub-one-watt photothermal tumor ablation using porphysomes, intrinsic multifunctional nanovesicles. Journal of visualized experiments. 2013; e50536.
- Khaing Oo MK, Yang Y, Hu Y, Gomez M, Du H, Wang H. Gold nanoparticle-enhanced and size-dependent generation of reactive oxygen species from protoporphyrin IX. ACS nano. 2012; 6: 1939-1947.
- Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013; 12: 958-962.
- Hamblin MR, Miller JL, Rizvi I, Ortel B, Maytin EV, Hasan T. Pegylation of a chlorin(e6) polymer conjugate increases tumor targeting of photosensitizer. Cancer Res. 2001; 61: 7155-7162.
- Senior J, Delgado C, Fisher D, Tilcock C, Gregoriadis G. Influence of surface hydrophilicity of liposomes on their interaction with plasma protein and clearance from the circulation: studies with poly (ethylene glycol)-coated vesicles. Biochimica biophysica acta. 1991; 1062: 77-82.
- Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001; 46: 149-168.
- Kessel D. Delivery of photosensitizing agents. Adv Drug Deliv Rev. 2004; 56: 7-8.
- del Carmen MG, Rizvi I, Chang Y, Moor AC, Oliva E, Sherwood M, et al. Synergism of epidermal growth factor receptor-targeted immunotherapy with photodynamic treatment of ovarian cancer in vivo. Journal of the National Cancer Institute 2005; 97: 1516-1524.
- Anand S, Wilson C, Hasan T, Maytin EV. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer research. 2011; 71: 6040-6050.
- Sinha AK, Anand S, Ortel BJ, Chang Y, Mai Z, Hasan T, et al. Methotrexate used in combination with aminolaevulinic acid for photodynamic killing of prostate cancer cells. British journal of cancer. 2006; 95: 485-495.
- Edmonds C, Hagan S, Gallagher-Colombo SM, Busch TM, Cengel KA. Photodynamic therapy activated signaling from epidermal growth factor receptor and STAT3: Targeting survival pathways to increase PDT efficacy in ovarian and lung cancer. Cancer biology & therapy. 2012; 13: 1463-1470.
- Mew D, Wat CK, Towers GH, Levy JG. Photoimmunotherapy: treatment of animal tumors with tumor-specific monoclonal antibody-hematoporphyrin conjugates. Journal of immunology (Baltimore, Md. 1950). 1983; 130: 1473-1477.
- Schmidt-Erfurth U, Hasan T, Schomacker K, Flotte T, Birngruber R. In vivo uptake of liposomal benzoporphyrin derivative and photothrombosis in experimental corneal neovascularization. Lasers in surgery and medicine. 1995; 17: 178-188.
- Goff BA, Bamberg M, Hasan T. Photoimmunotherapy of human ovarian carcinoma cells ex vivo. Cancer Res. 1991; 51: 4762-4767.
- Duska LR, Hamblin MR, Miller JL, Hasan T. Combination photoimmunotherapy and cisplatin: effects on human ovarian cancer ex vivo. Journal of the National Cancer Institute. 1999; 91: 1557-63.
- Molpus KL, Hamblin MR, Rizvi I, Hasan T. Intraperitoneal photoimmunotherapy of ovarian carcinoma xenografts in nude mice using charged photoimmunoconjugates. Gynecologic oncology. 2000; 76: 397-404.
- Soukos NS, Hamblin MR, Keel S, Fabian RL, Deutsch TF, Hasan T. Epidermal growth factor receptor-targeted immunophotodiagnosis and photoimmunotherapy of oral precancer in vivo. Cancer Res. 2001; 61: 4490-4496.
- Rizvi I, Dinh TA, Yu W, Chang Y, Sherwood ME, Hasan T. Photoimmunotherapy and irradiance modulation reduce chemotherapy cycles and toxicity in a murine model for ovarian carcinomatosis: perspective and results. Israel journal of chemistry. 2012; 52: 776-787.
- van Dongen GA, Visser GW, Vrouenraets MB. Photosensitizer-antibody conjugates for detection and therapy of cancer. Adv Drug Deliv Rev. 2004; 56: 31-52.
- Makoto Mitsunaga, Mikako Ogawa, Nobuyuki Kosaka, Lauren T Rosenblum, Peter L Choyke, Hisataka Kobayashi. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nature medicine. 2011; 17: 1685-1691.
- Spring BQ, Abu-Yousif AO, Palanisami A, Rizvi I, Zheng X, Mai Z. et al. Selective treatment and monitoring of disseminated cancer micro metastases in vivo using dual-function, activatable immunoconjugates. Proc Natl Acad Sci USA. 2014; 111: e933-942.
- Thompson DH, Gerasimov OV, Wheeler JJ, Rui Y, Anderson VC. Triggerable plasmalogen liposomes: improvement of system efficiency. Biochimica et biophysica acta. 1996; 1279: 25-34.
- Qualls MM, Thompson DH. Chloroaluminum phthalocyanine tetrasulfonate delivered via acid-labile diplasmenylcholine-folate liposomes: intracellular localization and synergistic phototoxicity. International journal of cancer. Journal international du cancer. 2001; 93: 384-392.
- Leung SJ, Romanowski M. Light-activated content release from liposomes. Theranostics. 2012; 2: 1020-1036.
- Hasan T. Using Cellular Mechanisms to Develop Effective Combinations of Photodynamic Therapy and Targeted Therapies. Journal of the National Comprehensive Cancer Network. 2012; 10: S23-S26.
- Gomer CJ, Ferrario A, Luna M, Rucker N, Wong S . Photodynamic therapy: combined modality approaches targeting the tumor microenvironment. Lasers Surg Med. 2006; 38: 516-521.
- Rahmanzadeh R, Rai P, Celli JP, Rizvi I, Baron-Lühr B, Gerdes J, Hasan T . Ki-67 as a molecular target for therapy in an in vitro three-dimensional model for ovarian cancer. Cancer Res. 2010; 70: 9234-9242.
- Mir Y, Elrington SA, Hasan T. A new nanoconstruct for epidermal growth factor receptor-targeted photo-immunotherapy of ovarian cancer. Nanomedicine: nanotechnology, biology, and medicine. 2013; 9: 1114-1122.
- Spring QB, Palanisami A, Zheng LK, Blatt EA, Sears RB, Hasan T. Efficient measurement of total tumor microvascularity ex vivo using a mathematical model to optimize volume subsampling. Journal of Biomedical Optics. 2013; 18: 096015.
- Abu-Yousif AO, Moor AC, Zheng X, Savellano MD, Yu W, Selbo PK, et al. Epidermal growth factor receptor-targeted photosensitizer selectively inhibits EGFR signaling and induces targeted phototoxicity in ovarian cancer cells. Cancer letters. 2012; 321: 120-127.
- M T Huggett, M Jermyn, A Gillams, R Illing, S Mosse, M Novelli, et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. British journal of cancer. 2014; 110: 1698-1704.
- Bown SG, Rogowska AZ, Whitelaw DE, Lees WR, Lovat LB, Ripley P, et al. Photodynamic therapy for cancer of the pancreas. Gut. 2002; 50: 549-557.
- Malte C. Gather, Seok Hyun Yun. Single-cell biological lasers. Nature Photonics. 2011; 5: 406-410.
- Menyuk N, Dwight K, Pierce JW. NaYF4: Yb, Er - an efficient upconversion phosphor. Appl. Phys. Lett. 1972; 21: 159-161.
- Chatterjee DK, Yong Z. Upconverting nanoparticles as nanotransducers for photodynamic therapy in cancer cells. Nanomedicine (Lond). 2008; 3: 73-82.
- Ungun B, Prud'homme RK, Budijon SJ, Shan J, Lim SF, Ju Y, Austin R . Nanofabricated upconversion nanoparticles for photodynamic therapy. Opt Express. 2009; 17: 80-86.
- Idris NM, Gnanasammandhan MK, Zhang J, Ho PC, Mahendran R, Zhang Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nature medicine. 2012; 18: 1580-1585.
- Wang C, Tao H, Cheng L, Liu Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials. 2011; 32: 6145-6154.
- Juzenas P, Chen W, Sun YP, Coelho MA, Generalov R, Generalova N, et al. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev. 2008; 60: 1600-1614.
- Anne-Laure Bulin, Charles Truillett, Rima Chouikrat, Francois Lux, Celine Frochot, David Amans, et al. X-ray-Induced Singlet Oxygen Activation with Nanoscintillator-Coupled Porphyrins. The Journal of Physical Chemistry C. 2013; 117: 21583-21589.
- Glidden MD, Celli JP, Massodi I, Rizvi I, Pogue BW, Hasan T. Image-Based Quantification of Benzoporphyrin Derivative Uptake, Localization, and Photobleaching in 3D Tumor Models, for Optimization of PDT Parameters. Theranostics. 2012; 2: 827-839.