Review Article
Austin J Nanomed Nanotechnol. 2014;2(4): 1023.
Development of Cuboidal Nanomedicine by Nanotechnology
Basavaraj K Nanjwade*1, Yallappamaharaj R Hundekar2, Meghana S Kamble3 and Teerapol Srichana4
1Department of Pharmaceutics, Omer Al-Mukhtar University, Libya
2Department of Pharmaceutics, KLE College of Pharmacy, India
3Department of Pharmaceutics, P. E. Society's Modern College of Pharmacy, India
4Department of Pharmaceutics, Prince of Songkla University, Thailand
*Corresponding author: Basavaraj K Nanjwade, Department of Pharmaceutics, Omer Al-Mukhtar University, Tobruk, Libya
Received: May 26, 2014; Accepted: May 28, 2014; Published: May 29, 2014
Abstract
When amphiphilic lipid systems are placed in aqueous environment that will formed self-assembled nanostructured dispersed particles which are expressed as "Cubosomes" whose size ranges from 10-500 nm in diameter and they appear like dots square shaped, slightly spherical in the form of bicontinuous cubic liquid crystalline phase. The discovery of cubosomes is unique story and spans in the field of food science, biological membranes, differential geometry and digestive process. Cubosomal drug delivery system is great potential in melanoma therapy owing to their potential advantage. Recently the research interesting is inducing on cubosomes due to their thermodynamically stable cubic structure, biodegrability of lipids, the ability of encapsulating hydrophobic, hydrophilic, amphiphilic substances is an excellent candidate for targeting and controlled release of drugs, proteins etc. The review article includes manufacturing techniques, characterisation, and applications of cuboidal drug delivery system.
Keywords: Cubosomes; Cubic phase; Bicontinuous; Biodegrability; Controlled release
Introduction
Cubic liquid crystals are physically looks transparent and isotropic phases that are stable in excess water and show a unique system for the production of pharmaceutical dosage forms. The liquid crystals of cubic phase are used in the controlled release of selected water and oil soluble molecules [1].
Cubosomes are bicontinuous cubic liquid crystalline materials are active ingredients because they give the unique structural ends to control release applications [2]. Cubosomes are vesicle derivatives [3], formed by the mixture of phospholipid (glycerol monooleate) and non-ionic surfactant (poloxamer 407- block copolymers of polyoxyethylene polyoxypropylene) in aqueous media by applying high energy dispersion such as sonication and homogenization, after the formation of cubosomes the dispersion is formulated into a product and then applied to a substrate of interest usually body tissue [4], due to the formation of vesicles the stability of system remains in the interested area (figure 1) [5].
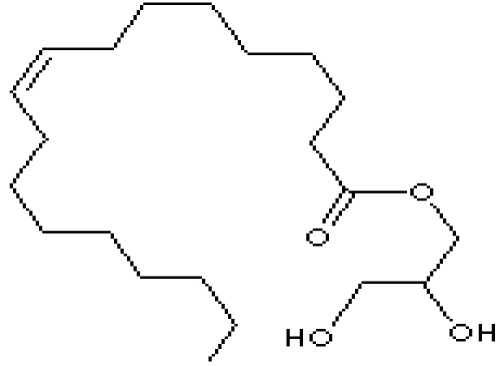
Figure 1: Glycerol monooleate.
Structurally cubosomes are lipid vesicles formed from amphiphilic building blocks [6], which mimics bio membranes or vesicular structure similar to liposomes that can be used for carrier potential of hydrophilic, lipophilic and amphiphilic drugs as compare to free drug directly to the particular site of action [7], thus allow drug targeting and the sustained or controlled release of conventional medicines [8]. Cubosomes can be administrated in many ways oral, Percutaneous, intravenous route [9].
Among the various colloid carriers or vesicular delivery system, liposome and cubosomes can encapsulate both hydrophilic and lipophilic drugs. The hydrophilic drug is encapsulated inside the vesicles whereas the lipophilic drug is partitioned between the hydrophilic domains. Liposomes are produced by the self-assembly of phospholipids in aqueous phase to form bilayer which may be spherical unilamellar or multilamellar vesicles. Liposome may undergo some problems like degradation by hydrolysis in aqueous solution, sedimentation and aggregation on storage and cannot sterilize for clinical use. Still, chemical instability like oxidation of phospholipids was not avoided. These pave the way to the discovery of non-ionic surfactant vesicles known as cubosomes [10].
In several cases the conventional chemotherapy is not effective in the treatment of intracellular infection due to limited drug permeation into cells this can be overcome by the use of cubosomal drug delivery system [11], and also cubosomes reduces toxicity of encapsulated agent [12], dose related side effects, decreasing dosing frequency [13]. Like ethosomes cubosomal drug is administrated in semisolid form (gel or cream), producing high patient compliance rather than other vesicular drug delivery system [14]. With respect to liposomes cubosomes possesses a larger ratio between the bilayer area and the particle volume and a larger breaking resistance [15]. The actual motto of cubosomes is to control degradation of drugs and loss, prevention of harmful side effects and increase availability of drug at the disease site [16].
One of the reasons for preparing cubosomes is the high chemicophysical stability of surfactant than that of phospholipids which are used in the preparation of liposomes. Due to the presence of phospholipids are easily hydrolysed [17]. Manufacture of cubosomes on a large scale embodied difficulty because of their viscosity [18].
Cubosomes are useful over other drug delivery system due to their bioavailability improvement of poorly soluble drugs and enhances skin permeation, by this reduces the cost of therapy [19]. Surfactant used in cubosomes does not require special handling and storage condition [20].
Cubosomes have a transdermal effect of which has been reported by some researchers, this effect might be due to their structural organization similar to that of biomembranes [21], recently the structural organization of the stratum corneum can be investigates by the study of cryo-transmission electron microscopy. Although cubosome structure has been characterized by X-ray and NMR investigations [22].
As described above cubosomes are nanoparticles with size range of 10-500 nm in dimension, they look like dots square, slightly spherical shaped (figure 2). Each dot shows the presence of pore having aqueous cubic phases in lipid water system in X-ray scattering technique was first identified by Luzzati & Husson [23]. The cubic phase structure of cubosomes can be described by the concept of differential geometry and periodic minimal surfaces by analogy with soap films. Mathematically 'Schwarz' discovered three types (figure 3) of minimal surfaces in cubic phases based on their curvatures.
Figure 2: Square or spherical shaped cubosomes.
Figure 3: D-surface (Diamond surface), G-surface (Gyroid surface), P-surface (Primitive surface).
In the monoolein water system the D-surfaces formed at high water levels, the G-surface at lower levels and the P-surface is formed when caseins or amphiphilic block copolymer are added [24].
Overall, cubosomes have great potential in drug nanoformulations for melanoma therapy owing to their potential advantages, including high drug payloads due to high internal surface area and cubic crystalline structures, relatively simple preparation method, biodegradability of lipids, the ability of encapsulating hydrophobic, hydrophilic and amphiphilic substances, targeting and controlled release of bioactive agents like proteins and drugs [25].
The aim of this work is to study the performance of cubosomes as cutaneous as well as oral delivery systems for Non-Steroidal Anti- Inflammatory Drugs (NSAIDs) seem particularly interesting. The NSAID's shows their anti-inflammatory activity by obstructing the enzymes Cyclooxygenase (COX1 & COX2), which have to be proven an effective corticosteroidal alternative in the topical management of percutaneous inflammation [26]. Presently NSAIDs are widely used in treatment of allergic conjunctivitis, management of post-operative inflammation and control of pain after photo refractive keratectomy. Practically NSAIDs are irritating due to their acidic nature. By reducing the pH of their formulation also further increases their irritation potential [27].
The first part of the present study describes the production and characterization of NSAID's containing Monooleine (MO) dispersions, whereas the second part deals with in-vitro from different formulations using excised human skin membranes are Stratum Corneum Epidermis (SCE) mounted into Franz cells and an in-vivo investigation to describe the release of NSAID's after administration (table 1).
Researcher
Drug
Category
Associated disease
Engstrom et al.
2-amino-1-phenylpropanol HCl
Nitroglycerin
Oestriol
Antidepressant
Anti-anginal
Hormonal therapy
Mania, depression
Angina pectoris
Atropic vaginitis
Pruritus
Sadhale et al.
Cefazolin
Cefuroxime
Prilocaine
Antibiotics
Antibiotics
Local anaesthetic
Genito-urinary, respiratory tract infection
Meningitis, bone and soft tissue infection
In dentistry
Damani
Clindamycin phosphate
Antibiotics
Peritonitis, staphylococcal bone and joint infection
Engstrom et al.
Clomethiazole
Psychotropic
Insomnia
Engstrom et al.
Clotrimazole
Antifungal
Vagina, mouth, and skin infection
Engstrom et.al
Gramicidin
Insulin
Topical steroid
Hypo/hyper glycaemics
Corticosteroid sensitive dermatoses
Diabetes mellitus
Nielsen et al.
Indomethacin
Isosorbidemononitrate
Lidocaine hydrochloride
NSAIDs
Antianginal
Aural Preparation
Gout, rheumatoid arthritis
Angina pectoris
Fungal infection of external ear
Boyd
Diazepam
Rifampicin
Griseofulvin
Propofol
Sedative-hypnotic
Bactericidal antibiotic
Antifungal
Hypnotic
Anxiety, insomnia, seizures
Tuberculosis
Fungal infection of skin
Procedural sedation, to induce and maintain Gemalanesthesia
Table 1: The following different types of drugs incorporated in cubosomes for sustained drug delivery [28].
Properties of cubosomes
- Cubosome dispersions have much lower viscosity.
- Cubosomes are discrete, sub-micron, nanostructured particles of bicontinuous cubic liquid crystalline phase.
- Cubosomes is perhaps the most intriguing [29].
- Cubic liquid crystals are transparent and isotropic phases that are physically stable in excess water.
- Due to small pore size of cubosomes are attractive for controlled release.
- It's having an ability of solubilizing hydrophobic, hydrophilic and amphiphilic molecules and its biodegrability by simple enzyme [30].
Advantages of cubosomes
Cuboidal systems have many advantages for drug delivery which are as following,
- They have ability to encapsulate both hydrophilic and hydrophobic also amphiphilic drugs.
- They have a sustained- release drug delivery characteristics.
- Cubosomes have biocompatibility and bioadhesivity properties.
- Bicontinuous cubic liquid crystalline phase of Cubosomes even stable in excess water [31].
- Cubosomes are excellent solubilizers, compared with conventional lipid or non-lipid carriers.
- They show high drug carrier capacity for a range of sparingly water-soluble drugs.
- These are an excellent vehicle to protect the sensitive drug from enzymatic degradation and in-vivo degradation, such as peptides and proteins.
- The cuboidal system enhances the bioavailability range twenty to more than one hundred times of water-soluble peptides [32].
Disadvantages of cubosomes
- Cubosomes may lead to low drug loading efficiency and drug leakage in preparation, preservation and transport in vivo, thus the major problem of their stability acts as a barrier and thus limiting their use [33].
Advantages of cubosomes (phospholipids based carrier system) in comparison to other delivery systems
- These systems show enhanced permeation of drug through skin for Percutaneous and dermal delivery.
- These are platform for the delivery of large and diverse group of drugs (peptides, protein molecules).
- Their composition is safe and the components are approved for pharmaceutical and cosmetic use.
- Low risk profile- the toxicological profiles of the phospholipids are well documented in the scientific literature.
- High market attractiveness for products with proprietary technology. Relatively simple to manufacture with no complicated technical investments required for production of Ethosomes.
- The vesicular system is passive and non-invasive, it is available for immediate commercialization (table 2) [34].
Sl.No
Cubosomes
Liposomes
Cubosomes are formation of bicontinuous cubic liquid crystalline phase by hydrating mixture of monoolein and poloxamer 407.
Liposomes are formations of vesicles by hydrating mixture of cholesterol and phospholipids.
Are artificial, colloidal and spherical vesicles of 0.05-5.0 μm diameter,
Are appear like dots square shaped, slightly spherical of 10-500nm in diameter
In cubosomes active chemical constituent molecules are anchored through chemical
bonds to the polar head of the phospholipids
In liposomes, the active principle is dissolved in the medium of the cavity or in the layers of the membrane. No chemical bonds are formed.
In cubosomes, polymer and the
individual drug compound form a1:1 or 2:1 complex depending on the substance.
In liposomes, hundreds and thousands of
phosphatidylcholine molecules surround the water soluble molecule.
Table 2: Difference between Cubosomes and Liposomes.
Materials used for preparing cubosomes
NSAID's as a drug, glyceryl monooleate (monoolein), Poloxamer 407, Glycerol, Carbopol 934P, Alcohol (ethanol), Stabilizing agent (Polyvinyl alcohol), Bidistilled water, etc.
Techniques used for production of Cubosomes
The following techniques are used to produce cuboidal and nanocuboidal drug delivery systems,
Top down technique
Bottom up technique
Heat treatment
Spray drying
Top down technique approach
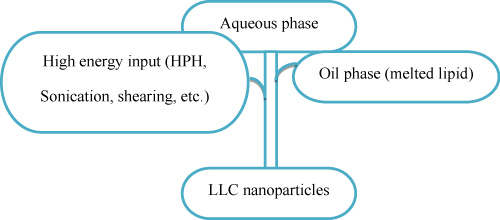
The viscous bulk cubic phase is prepared by mixing lipids with stabilizers, then the resultant mixture is dispersed into aqueous solution by the input of high energy (such as High-Pressure Homogenization [HPH], sonication or shearing) to form Lyotropic Liquid Crystal (LLC) nanoparticles (figure 4). HPH is the mostly used technique in the preparation of LLC nanoparticles. The cubosomes prepared through top-down approach are always observed to coexist with vesicles (dispersed nanoparticles of lamellar liquid crystalline phase) or vesicle-like structures [35].
Figure 4: Preparation of cubosomes by top down approach.Bottom up technique approach
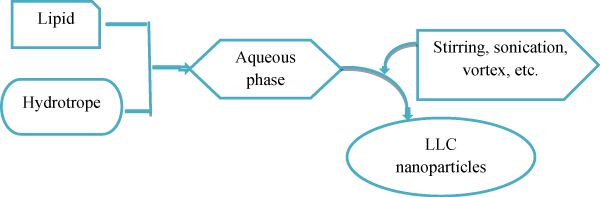
In the bottom-up approach the hydrotrope is dissolve in water-insoluble lipids to create liquid precursors and prevent the formation of liquid crystals at high concentration and needs less energy input (Almgren et al). Discuss the formation of cubosomes by dispersing inverse micellar phase droplets in water at 80 °C and allow them to slowly cool, gradually droplets get crystallizes to cubosomes (figure 5). The cubosomes are spontaneously formed by emulsification. This bottom-up approach cannot effectively avoid forming vesicles through cryo-TEM, many vesicles and vesicle-like structures were also observed to coexist with cubosomes [36].
Figure 5: Preparation of cubosomes by bottom up approach.Figure 5: Preparation of cubosomes by bottom up approach.Heat treatment approach
This technique is not an integrated process for the manufacture of cubosomes because it only promotes the transformation from non-cubic vesicles to well-ordered cubic particles comprising a homogenization and heat-treatment step as a result decrease in the small particle size fraction that corresponded to vesicles and form more cubic phases with narrow particle distribution and good colloidal stability (figure 6) [37].
Figure 6: Preparation of cubosomes by bottom up approach.Figure 6: Preparation of cubosomes by bottom up approach.Spray drying approach
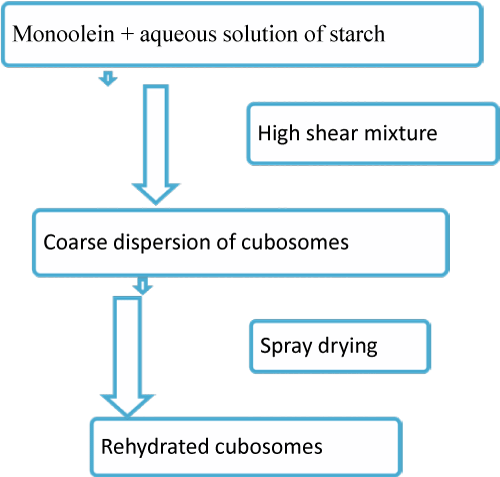
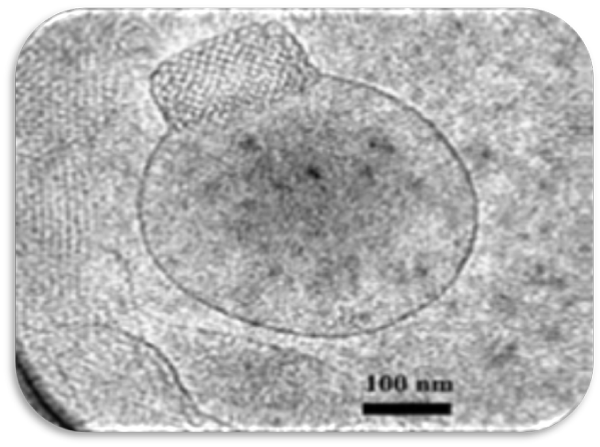
Because of the less flexibility of liquid precursor for formation of cubosomes (Spicer et al) developed dry powder precursor for cubosomes preparation. They utilized spray drying technique for preparation of starch encapsulated monoolein precursor and dextran encapsulated monoolein precursor. High proportion of polymer (75% w/w for starch and 60%w/w for dextran) for encapsulation decreased the amount of loading of active materials so system was limited for potent medicament, vitamins, flavours, or scents (figure 7 and 8) [38].
Figure 7: Flow chart for formation of dry powder cubosomes.Figure 7: Flow chart for formation of dry powder cubosomes.Figure 8: Cubosomes prepared by dry powder precursors [38].Figure 8: Cubosomes prepared by dry powder precursors [38].General method of cuboidal preparation
Cubosomes are usually produced by combining monoolein and water at 40°C. The resultant cubic liquid crystalline gel is dispersed into particles via the application of mechanical or ultrasonic energy. High-pressure homogenizers are often employed to produce cubosomes. Finally, the cubosomes are stabilized against flocculation by polymer addition (figure 9) [39].
Figure 9: Flow chart of formation of Cubosomes.Figure 9: Flow chart of formation of Cubosomes.Evaluation parameters of cubosomes
The cuboidal dispersions can be evaluated by following parameters,
Thermal Analysis
Polarizing Light Microscopy
Cryo-Transmission Electron Microscopy
X-ray Diffraction Measurements
Drug Content of Dispersions
HPLC Procedure
In Vivo Studies
Tape Stripping
Statistical Analysis
In vitro drug release studies
Thermal analysis
In this study, DSC is used to evaluate the physical status of the drug within the cubosomes. Both ingredients of the cubosomes seem to melt together in temperature of around 37°C to 56°C which may results of plasticizing of Glyceryl Monooleate (GMO). The thermal events related to the drug's melting point are different from those of the native drug (no sharp drug melting peak at around 200°C). The thermal events observed between 200°C and 300°C may be related to glyceryl monooleate degradation process [40].
Polarizing light microscopy
Samples were viewed between crossed polarizers and a λ-sheet in a Zeiss III light microscope (Zeiss, Oberkochen, Germany) [41].
Cryo-transmission electron microscopy
A small amount of prepared sample at ambient conditions is placed on a pure thin bar 600-mesh TEM grid. The solution blotted with filter paper to form a thin film. Then the sample is vitrified by immersing into liquid ethane near its freezing point. This is transferred to a transmission electron microscope for imaging using a cryoholder with temperature maintains 175°C. Images are recorded digitally by a charge coupled device camera using an image processing system [42].
X-ray diffraction measurements (XRD)
The XRD is carried out using a Philips PW 1830 X-ray generator. Diffraction patterns were recorded by using an INEL Curved Position Sensitive 120 detector. Diffraction data are collected at 25°C controlling the temperature.
Little structural information was derived from X-ray diffraction patterns: once the symmetry of the lipid phases was found, the unit cell dimension was calculated by using Bragg's law as follows:
a = (h2 + k2 + l2 )1/2
Where shkl= 2sin ϑ/λ with 2 ϑ the scattering angle and ϑ (0.154 nm) the wavelength (2, 16) [43].
Drug content of dispersions
The drug content of dispersions evaluate by diluting the filtered dispersion sample in methanol (1:9 v/v) and analysed for drug content by High Performance Liquid Chromatography (HPLC) with the respective procedure. For Sedimentation Field Flow Fractionation (SdFFF) and stability studies, the amount of drug detected by HPLC after filtration was taken as reference of the total amount of drug [44].
HPLC procedure
amples were assayed by using a validated HPTLC densitometric method. Developed plates were stained using a solution containing mobile phase cupric sulfate (pentahydrate): phosphoric acid: water and quantified densitometrically using a UV light source set respected wavelength [45].
In vivo studies
In vivo experiments were performed on ten volunteers for evaluation of anti-inflammatory activity. In Vivo Anti-inflammatory Activity Ultraviolet-B-induced skin erythema was monitored byusing a reflectance visible spectrophotometer X-Rite model968 (X-Rite Inc. Grandville, MI, USA), calibrated and controlled. The reflectance spectra were obtained over the required wave length and 2-standard observer. From the spectral data obtained, the Erythema Index (EI) was calculated using Eq. (1) [46].
E.I=100[log 1/R560 + (log 1/R540 +log 1/R580) -2(log 1/R510 +log 1/ R610)] - (1)
Skin erythema is induced by UVB irradiation using a UVM- 57 ultraviolet lamp (UVP, San Gabriel, CA, USA) whose specific parameters are reported elsewhere. An irradiation dose is preliminarily determined twice the value of Minimal Erythemal Dose (MED) throughout the study [47].
Tape stripping
In this parameter the 200 mg of each hydrogel formulations is applied on defined cutaneous sites of forearm (three sites of application for each formulation induplicate). Spread the preparations uniformly by solid glass rod at the site and are then kept for 6h. After this period, the residual formulations are removed by wiping with cotton balls. Twenty individual 2 cm squares of adhesive tape are utilized on the application sites. Weigh each tape removed with stratum corneum cells then determination of stratum corneum removed by difference of weights; quantify the drug content in the tapes by some analytical technique [48].
Statistical analysis
This statistical analysis of in vitro data was performed using Student's t test. Statistical differences of in vivo data are determined using repeated-measures analysis of variance (ANOVA) followed by the BonferroniYDunn post hocpairwise comparison procedure. A probability of p<0.05 is considered significant in this study [49].
In vitro drug release studies
In vitro release of drug from cubosomes is evaluated using a dynamic dialysis method. The samples of various formulations were placed in dialysis bags (cellulose membrane), then immersed in 500 mL of simulated tear at 37±1 °C, Maintained the required paddle rotation speed. At predetermined time intervals, a 5 mL sample was withdrawn and immediately replaced with an equal volume of tear. Then the concentration of released drug is measured [50].
Stability studies on storage
The monoolein dispersions stored at room temperature leads to change with time. After preparation the dispersions are clear and transparent which will turn turbid with storage time. A number of macroscopically visible particles with sizes in the micrometer to millimeter range were formed during storage within days or weeks after preparation making these systems unsuitable for intravenous drug delivery.
At refrigerator temperature the storage of homogenized dispersions results in the formation of white, semi-solid, ointment-like gels. This phenomenon is correlated with the crystallization of the colloidally dispersed monoolein. This type of behavior is observed in monoolein/water bulk systems due to high tendency for super cooling at sub-ambient temperatures. But the crystallization in the ternary monoolein/poloxamer/water bulk system could not be observed due to addition of poloxamer which modifies the crystallization behaviour of monoolein [51].
Applications of Cuboidal drug delivery system
- Cubosomes are widely used in melanoma (cancer) therapy [52].
- The monoglyceride based cubosome dispersion can be used for topically, such as for percutaneous or mucosal applications.
- Due to microbiocidal properties of monoglycerieds, can be used to design intravaginal treatment of sexually transmitted diseases caused by viruses (e.g. HSV, HIV) or by bacteria (eg. Chlamydia trachomatis and neisseria genorrticae).
- The cubosomal technology is used to develop a synthetic vernix (complex mixture of lipid (fats), proteins and water) the chessy white substance that coats infants in late gestation to help premature infants who are born without it. It is formed late in gestation and has an integral role in normal skin development.
- Cubosome particles are used as oil water emulsion stabilizers and pollutant absorbents in cosmetics.
- More recent use is as skin care, hair care, cosmetics and antiperspirants [53].
Future perspective
As discussed in this article, cubosomes contains drugs can be delivered to a patient by transdermal, oral and intravenously. With the sequence of human genome, biotechnology companies are developing a peptide and protein based drugs. It is expected that in the next 10 to 20 years, cubosomes containing protein and peptide based drugs will constitute more than half of the new drugs introduced into the market and more than 80% of these protein drugs will be antibodies due to control release activity. These biopharmaceuticals (proteins, peptides, carbohydrates, oligo-nucleotides, and nucleic acids in the form of DNA) present drug delivery challenges because these are often large molecules that degrade rapidly in the blood stream. Moreover, they have a limited ability to cross cell membranes and generally cannot be delivered orally. Such molecules will be much more difficult to deliver via conventional routes and injections may be the only means of delivery (at least as of today). The routes of administration will be dictated by the drug, disease state, and desired site of action. Some sites are easy to reach such as the nose, the mouth, and the vagina. Others sites are more challenging to access, such as the brain.
Conclusion
A simple process, based on dispersion and homogenization of monooleine and poloxamer 407 in water leads to the formation of a complex heterogeneous system, suitable for the delivery of lipophilic, hydrophilic drugs through the skin and use in treatments of hair, and other body tissue. The data here reported indicate a prolonged anti-inflammatory activity exerted by NSAID's. The stratum corneum like structure attributed to cubosome by other authors suggests the hypothesis of a cubosome depot effect on the epidermis. Further specialized studies are required to confirm this fascinating hypothesis and to better investigate the role of vesicles and cubosomes in controlling the release of the drug.
References
- Shah JC, Sadhale Y, Chilukuri DM. Cubic phase gels as drug delivery systems. Adv Drug Deliv Rev. 2001; 47: 229-250.
- Andersson S, Jacob M. Structure of Cubosomes-A Closed Lipid Bilayer Aggregate. Zeitschrift fier Kristallographie. 1995; 210: 315-318.
- Bouwstra JA, Honeywell-Nguyen PL. Vesicles as a Tool for T.D.D.S. Drug Discovery Today: Technologies, Drug Delivery from Nanotech. 2005; 2: 67-74.
- LUZZATI V, HUSSON F. The structure of the liquid-crystalline phasis of lipid-water systems. J Cell Biol. 1962; 12: 207-219.
- Goldberg EP, Editor. In Targeted Drugs, Wiley, NY. 1983; 2: 312.
- Gregoriadis G. Targeting of drugs. Nature. 1977; 265: 407-411.
- Hunter CA, Dolan TF, Coombs GH, Baillie AJ. Vesicular systems (niosomes and liposomes) for delivery of sodium stibogluconate in experimental murine visceral leishmaniasis. J Pharm Pharmacol. 1988; 40: 161-165.
- Goldberg EP, Editor. In Targeted Drugs, Wiley, NY. 1983; 2: 312.
- Hunt C, Tsang S. Int J. Pharm. 1981; 8: 101.
- Gilbert S, Christopher T. "Modern Pharmaceutics", Fourth Edition, Marcel Dekker Inc. 2002.
- Post G, Krisch R, Koestler T. In Gegoriadis G, Editor. Liposomes Tech, CRC Press Inc. 1983; 1: 29.
- Hunt C,Tsang S. Int J. Pharm, 1981; 8: 101.
- Goldberg EP, Editor. In Targeted Drugs, Wiley, NY. 1983; 2: 312.
- Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, Jain NK. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J Control Release. 2007; 123: 148-154.
- LUZZATI V, HUSSON F. The structure of the liquid-crystalline phasis of lipid-water systems. J Cell Biol. 1962; 12: 207-219.
- Gregoriadis G. Targeting of drugs. Nature. 1977; 265: 407-411.
- Uchegbu FI, Vyas PS. Nonionic Surfactant Based Vesicles in Drug Delivery. Int. J. Pharm. 1998; 172: 33-70.
- Boyd BJ. Characterisation of drug release from cubosomes using the pressure ultrafiltration method. Int J Pharm. 2003; 260: 239-247.
- Gregoriadis G. Targeting of drugs. Nature. 1977; 265: 407-411.
- Handjani-Vila RM, Ribier A, Rondot B, Vanlerberghie G. Dispersions of lamellar phases of non-ionic lipids in cosmetic products. Int J Cosmet Sci. 1979; 1: 303-314.
- Esposito E, Cortesi R, Drechsler M, Paccamiccio L, Mariani P, Contado C, et al. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm Res. 2005; 22: 2163-2173.
- Garg G, Saraf S, Saraf S. Cubosomes: an overview. Biol Pharm Bull. 2007; 30: 350-353.
- Wörle G, Siekmann B, Koch MH, Bunjes H. Transformation of vesicular into cubic nanoparticles by autoclaving of aqueous monoolein/poloxamer dispersions. Eur J Pharm Sci. 2006; 27: 44-53.
- Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Topical ocular delivery of NSAIDs. AAPS J. 2008; 10: 229-241.
- Madhurilatha T, Paruchuri S. Overview of Cubosomes: A Nano particle. International Journal of Research in Pharmacy and Chemistry. 2011; 1: 535-541.
- Schalnus R. Topical nonsteroidal anti-inflammatory therapy in ophthalmology. Ophthalmologica. 2003; 217: 89-98.
- Prashar D, Sharma D. Cubosomes: A Sustained Drug Delivery Carrier. Asian J. Res. Pharm. Sci. 2011; 1: 59-62.
- Spicer PT, Hayden KL. Novel Process for Producing Cubic Liquid Crystalline Nanoparticles (Cubosomes). Langmuir. 2001; 17: 5748-5756.
- Saurabh Bansal, Chandan Prasad Kashyap, Geeta Aggarwal. A comparative review on vesicular drug delivery system and stability issues. IJRPC. 2012; 2: 704-713.
- Ankur J, Chauhan JS, Budhwani AK. Cubosome: A novel approach for Nanotechnology. International Journal of Applied Biology and Pharmaceutical Technology. 2011; 2: 19-21.Spicer PT. Cubosome© Formation via Dilution - Kinetic Effects and Consumer Product Implications. American Chemical Society. 2003; 1-14.
- Spicer PT. Cubosome© Formation via Dilution - Kinetic Effects and Consumer Product Implications. American Chemical Society. 2003; 1-14.
- Spicer PT. Cubosome processing industrial nanoparticle technology a development. Chem. Eng. Res. Des. 2005; 83: 1283-1286.
- Breimer DD, Speiser R. Topics in Pharmaceutical Sciences. Elsevier Science Publishers, New York, USA. 1985; 57: 291.
- Sindhumol PG, Thomas M, Mohanachandran PS. Phytosomes: A Novel dosage form for enhancement of bioavailability of botanicals and nutraceuticals". International Journal of Pharmacy and Pharmaceutical science. 2010; 2: 10-14.
- Spicer PT. Cubosome processing industrial nanoparticle technology a development. Chem. Eng. Res. Des. 2005; 83: 1283-1286.
- Spicer PT. Cubosome© Formation via Dilution - Kinetic Effects and Consumer Product Implications. American Chemical Society. 2003; 1-14.
- Barauskas J, Johnsson M, Joabsson F, Tiberg F. Cubic phase nanoparticles (Cubosome): principles for controlling size, structure, and stability. Langmuir. 2005; 21: 2569-2577.
- Patel PV, Patel JB, Dangar RD, Patel KS, Chauhan KN. Liquid Crystal Drug Delivery System. Int J Pharm and applied sci. 2010; 1: 118-123.
- Ankur J, Chauhan JS, Budhwani AK. Cubosome: A novel approach for Nanotechnology. International Journal of Applied Biology and Pharmaceutical Technology. 2011; 2: 19-21.
- Bei D, Zhang T, Murowchick JB, Youan BB. Formulation of dacarbazine-loaded cubosomes. Part III. Physicochemical characterization. AAPS PharmSciTech. 2010; 11: 1243-1249.
- Siekmann B, Bunjes H, Koch MHJ, Westesen K. Preparation and structural investigations of colloidal dispersions prepared from cubic monoglyceride-water phases. Int J Pharm. 2002; 244: 33-43.
- Sagalowicz L, Michel M, Adrian M, Leser ME, Frossard P, Rouvet M, et al. Crystallography of dispersed liquid crystalline phases studied by cryo-transmission electron microscopy. J Microscopy. February 2006; 221: 110-121.
- Murgia S, Falchi AM, Mano M, Lampis S, Angius R, Carnerup AM, et al. Nanoparticles from lipid-based liquid crystals: emulsifier influence on morphology and cytotoxicity. J Phys Chem B. 2010; 114: 3518-3525.
- Esposito E, Cortesi R, Drechsler M, Paccamiccio L, Mariani P, Contado C, et al. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm Res. 2005; 22: 2163-2173.
- Nguyen TH, Hanley T, Boyd BJ. Nanostructured liquid crystalline particles provide long duration sustained-release effect for a poorly water soluble drug after oral administration. J Controlled Release. 2011; 153: 180-186.
- Dawson JB, Barker DJ, Ellis DJ, Grassam E, Cotterill JA, Fisher GW, et al. A theoretical and experimental study of light absorption and scattering by in vivo skin. Phys Med Biol. 1980; 25: 695-709.
- Ricci M, Puglia C, Bonina F, Di Giovanni C, Giovagnoli S, Rossi C, et al. Evaluation of indomethacin percutaneous absorption from nanostructured lipid carriers (NLC): in vitro and in vivo studies. J Pharm Sci. 2005; 94: 1149-1159.
- Escobar-Chávez JJ, Merino-Sanjuán V, López-Cervantes M, Urban-Morlan Z, Pi&nTilde;ón-Segundo E, Quintanar-Guerrero D, et al. The tape-stripping technique as a method for drug quantification in skin. J Pharm Pharm Sci. 2008; 11: 104-130.
- Barauskas J, Johnsson M, Joabsson F, Tiberg F. Cubic phase nanoparticles (Cubosome): principles for controlling size, structure, and stability. Langmuir. 2005; 21: 2569-2577.
- Han S, Shen JQ, Gan Y, Geng HM, Zhang XX, Zhu CL, et al. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol Sin. 2010; 31: 990-998.
- Siekmann B, Bunjes H, Koch MH, Westesen K. Preparation and structural investigations of colloidal dispersions prepared from cubic monoglyceride-water phases. Int J Pharm. 2002; 244: 33-43.
- Madhurilatha T, Paruchuri S. Overview of Cubosomes: A Nano particle. International Journal of Research in Pharmacy and Chemistry. 2011; 1: 2231-2781.
- Patel PV, Patel JB, Dangar RD, Patel KS, Chauhan KN. Liquid Crystal Drug Delivery System. Int J Pharm and applied sci. 2010; 1(1): 118-123.