
Research Article
Austin J Nanomed Nanotechnol. 2016; 4(1): 1043.
Preparation and Evaluation of Methoxypolyethylene Glycol-Poly (DL-Lactic Acid) Nanoparticles Loaded with Glycyrrhetic Acid
Hiraku Onishi*, Ken-ichiro A, Sasatsu M, Ikeuchi Y and Machida Y
Department of Drug Delivery Research, Hoshi University, Japan
*Corresponding author: Hiraku Onishi, Department of Drug Delivery Research, Hoshi University, 2-4-41, Ebara, Shinagawa-ku, Tokyo 142-8501, Japan
Received: March 22, 2016; Accepted: May 30, 2016; Published: June 02, 2016
Abstract
Glycyrrhetic acid (GLA)-loaded methoxypolyethylene glycol-poly (DL-lactic acid) (PLA-MPEG) nanoparticles, named NP, were produced and evaluated in vitro and in vivo. NP were prepared by a simple O/W emulsification/evaporation method using 1% PVA as a surfactant. NP with the size of 300 – 400 nm and more than 3 % GLA content were obtained at the PLA-MPEG/GLA ratio of 6/1 (w/w). The NP were examined for in vitro release in PBS (pH 7.4). NP exhibited an initial burst of approximately 45 % and released 30 % slowly during 7 d. Furthermore, NP and GLA alone were examined for efficacy using mice with carbon tetrachloride-induced hepatitis. NP tended to show better and longer hepato-protective effect than GLA alone. NP are suggested as a potential delivery system of GLA for the liver injury.
Keywords: Glycyrrhetic acid; Methoxypolyethylene glycol-poly(DL-lactic acid); Nanoparticles; In vitro release; Hepato-protective effect
Introduction
Licorice root is a widely-used traditional medicine, and its major ingredient is glycyrrhizin (GLZ) [1,2]. GLZ exhibits various useful biological functions such as anti-allergy [3], anti-inflammation [4], anti-hepatitis [1,2,5], anti-virus [6,7] and interferon-γ inducing effect [1,8]. However, GLZ causes toxic side effects like edema and hypertension by its corticoid-like action called pseudo-aldosteronism [1]. Glycyrrhetic acid (GLA) is produced by intestinal bacteria after oral ingestion of GLZ [9]. GLA also has various biological functions containing anti-inflammatory functions [1,10,11]. In particular, GLA is more highly hepato-protective than GLZ [1,5]. As GLA also induces side effect like pseudo-aldosteronism, its use is often limited. Therefore, it is desirable to suppress its dosing amount and administration frequency.
Micro- and nanoparticulate dosage forms have been used as drug delivery techniques to improve the drug action. Since poly (DLlactic acid) (PLA), poly (DL-lactic acid-co-glycolic acid) (PLGA), methoxypolyethylene glycol (MPEG)-PLA block copolymer (PLAMPEG) and MPEG-PLGA block copolymer (PLGA-MPEG) are safe and easy to process, their microparticles and nanoparticles have been produced as drug delivery devices [12-15]. These microand nanoparticles can be used parenterally due to their highly safe properties. Previously, PLGA-based microparticles containing GLA (PLGA/GLA-M) were produced and examined for the hepato-protective function [16,17]. PLGA/GLA-M exhibited good localization to liver and prolonged release there, resulting in better hepato-protective effect than GLA alone.
However, PLGA/GLA-M needed to be washed with the mixture of methanol and phosphate-buffered saline (PBS) of pH 7.4 twice or more; because non-washed or water-washed PLGA/GLA-M showed a high initial rapid release of nearly 80 %. On PLA-MPEG and PLGAResearch MPEG, composed of hydrophobic and hydrophilic polymer chains, have been found to easily form nanoparticles in aqueous media. In addition, as those nanoparticles are highly safe and disperse well in aqueous media, they have been used as drug carriers for parenteral drug delivery systems. In this study, the development of PLA-MPEG nanoparticles containing GLA, called NP, has been attempted, and the obtained NP have been evaluated from the in vitro features and hepato-protective effectiveness in vivo using rats with carbon tetrachloride (CCl4)-induced hepatitis.
Materials and Methods
Materials
DL-Lactide was purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan). Stannous octoate (St-Oct), methoxypolyethylene glycol (MPEG; MW 2,000) and 18β-glycyrrhetic acid (GLA) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals were of reagent grade.
Animals
Six-week old male ddY mice were purchased from Tokyo Laboratory Animal Science Co. Ltd. (Tokyo, Japan). They were housed with the breeding diet MF produced by Oriental Yeast (Tokyo, Japan) and water under the room conditions of 23 ± 1 oC and 60 ± 5 % relative humidity. The animal experimental protocol was approved Animal Research Committee of Hoshi University and the experiments were performed according to the Guideline Principles of Animal Care and Use of Hoshi University.
Preparation of nanoparticles
Methoxypolyethylene glycol-poly(DL-lactic acid) block copolymer (PLA-MPEG) was synthesized by referring to the methods by Gref et al. [14] and Bazile et al. [15], namely, a ring opening polymerization using St-Oct as a catalytic agent and subsequent purification by repeatedly putting the polymer-containing dichloromethane solution into water. The chemical structure of the product was examined using AV-400M digital NMR (Bruker BioSpin K.K., Yokohama, Japan). The polymerization degree, substitution degree of MPEG and molecular weight of PLA-MPEG were examined in the same manner as had been reported before [18,19]; namely, they were calculated by comparing the integrated intensity of each proton signal with that of the proton signal of the PLA terminal methine [18,19].
PLA-MPEG nanoparticles loaded with GLA, named NP, were produced as follows. PLA-MPEG (30 mg) and GLA (2.5, 5 and 10 mg) were dissolved in 1 mL of dichloromethane, and the solution was put into 0.01% HCl aqueous solution containing 1% (w/v) PVA. The mixture was stirred with a vortex mixer for 30 s, and sonicated at 28 Hz (100 W) for 30 min. The resultant emulsion was stirred with a magnetic stirrer at 25 oC for 2 h. The obtained suspension was condensed by evaporation in vacuo, and underwent gel-filtration using a Sephadex G50 column using water as elution solvent. The high molecular weight fractions were obtained as aqueous suspension of nanoparticles (NP).
Measurement of particle size and drug content
The particle size of NP was determined by measuring the dynamic light scattering of NP suspension with an ELS-800 apparatus (Otsuka Electronic Co., Ltd., Osaka, Japan). The drug content of NP was measured as follows. Acetonitrile (1.5 mL) and HPLC mobile (0.5 mL) were added to 0.1 mL of NP aqueous suspension, and the resultant solution was analyzed by HPLC on the concentration of GLA.
The NP amount in their aqueous suspension was determined as follows. After the NP aqueous suspension (3 mL) was freeze-dried, the residue and p-hydroxybenzoic acid (1 mg) were mixed in CDCl3, and the 1H-NMR spectrum of the resultant solution was measured. The integrated intensity of the proton signal of the PLA terminal methine was compared with that of the proton signal of p-hydroxybenzoic acid, leading to the amount of PLA-MPEG in the NP suspension. The GLA content was calculated from the ratio of (GLA amount)/ (NP amount).
Release of GLA from nanoparticles in PBS
The NP aqueous suspension was diluted 10 times with PBS of pH 7.4. The resultant suspension was divided into test tubes with equal volume of 2 mL. Each sample was incubated at 37oC. At 3 h, 7 h, 1 d, 2 d, 4 d and 7 d after the start of the incubation, three tubes were taken and centrifuged at 40,000 rpm for 20 min. After the supernatant was mixed with the same volume of HPLC mobile phase, the resultant solution was examined by HPLC for the concentration GLA, which gave the release amount of GLA.
The release profiles were further examined in the presence of rat plasma. Namely, the NP aqueous suspension was diluted 10 times with the mixture of murine fresh plasma and PBS (pH 7.4), and incubated in a manner similar to that stated above. At 2 h, 7 h and 24 h, the NP was precipitated similarly, and the supernatant was taken. Dichloromethane (5 mL) and 0.1 M acetate buffer of pH 5 (1 mL) were added to the supernatant (0.1 mL), and the mixture was shaken vigorously and centrifuged. The organic phase (4 mL) was taken and evaporated, and the resultant residue was dissolved in methanol (200 μL). The resultant solution was analyzed by HPLC, in which the data were corrected by the extraction ratio [16].
Therapeutic effect in mice with carbon tetrachlorideinduced hepatitis
The in vivo studies were conducted as shown in Figure 1, in which therapeutic effects of NP and GLA alone were tested at single and twice administration schedules. First, hepatitis model mice were produced by i.p. injection of a 25 % (v/v) carbon tetrachloride (CCl4) solution in olive oil at 4 mL/kg to each mouse at 0 and 48 h. NP suspension or GLA suspension in 2 % (w/w) Tween 20 aqueous solution, called GLA alone, was administered intravenously via tail vein at 5 mg/10 mL/kg at 3 h after the first CCl4 injection (single administration), and at 3 and 27 h after the first CCl4 injection (twice administration). The blood sampling was performed just before and at 24, 48, 72 and 96 h after the first CCl4 injection. Just after each sampling, the plasma was obtained by centrifugation
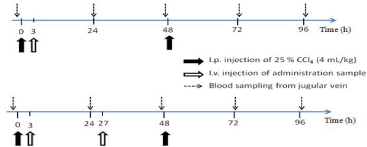
Figure 1: Animal experiment schedules for single (A) and twice (B)
administration to mice with CCl4-induced hepatitis.
The plasma levels of glutamic-oxaloacetic transaminase (GOT) were examined using a Transaminase CII Test Wako kit (Wako Pure Chemical Industries., Ltd., Osaka, Japan). The hepato-protective effects of NP and GLA alone were evaluated from the comparison of the plasma levels GOT.
HPLC assay
The drug content and release from NP were analyzed by HPLC at room temperature. A Shimadzu LC-6A and Shimadzu SPD-10 AV were used as a pump and absorbance detector, respectively. A C-R7A plus (Shimadzu) was used as a recorder. A μ-Bondasphere C18 (5 μm, 100Å; 3.9 mm I.D. x 150 mm length) column was applied as an analytical column, and a mixture of methanol, water, acetic acid and perchloric acid (87: 17: 0.5: 0.5, v/v) was used as the mobile phase, in which the absolute calibration method was used for the analysis.
Statistical analysis
Comparison of the data was performed using ANOVA followed by the Dunnett’s post hoc test. Significant difference was set as p < 0.05.
Results and Discussion
Preparation and NP characteristics
The obtained PLA-MPEG was MW 15,300, and the polymerization degree of DL-lactic acid was 184. The substitution degree of MPEG to PLA was calculated as 66 % (mol/mol). This PLA-MPEG was used for the preparation of NP. Formulations and particle characteristics are shown in Table 1. NP-A had lager particle size. Althourh NP-C showed high drug content, the reproducibilities of drug content and particle size were not good. NP-B exhibited the smallest size, mean size of 378 nm. Also, the drug content of NP-B was higher than 3 %. Further, the variations of drug content and particle size in NP-B were the least among the products. Thus, NP-B were chosen as the most adequate nanoparticles, and used as NP for the following studies.
Formulation
PEG-PLA (mg)
GLA (mg)
GLA content* (%, w/w)
Particle size* (nm)
NP-A
30
10
3.56 ± 2.04
541 ± 46
NP-B
30
5
3.16 ± 0.56
378 ± 19
NP-C
30
2.5
12.60 ± 5.91
464 ± 102
* The results are expressed as the mean ± S.D. (n=3).
Table 1: Formulation and particle characteristics of NP.
In vitro release of GLA from nanoparticles
The in vitro release from NP was performed using PBS (pH 7.4) at 37 oC under the conditions in which the GLA concentration corresponding to 100 % release was less than the solubility in PBS. The GLA release profile from NP was obtained as Figure 2. After the incubation for 1 d, approximately 50 % was released. Then, the GLA was released gradually; 61, 66 and 73 % of GLA were released on 2, 4 and 7 d.
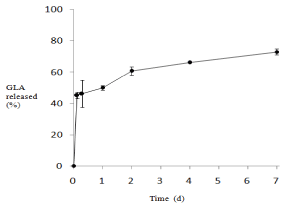
Figure 2: In vitro release profile of GLA from NP in PBS (pH 7.4) at 37 °C.
NP-B was used as NP.
The results are expressed as the mean ± S.D. (n=3).
For the present NP, the initial burst release was not high, less than 50 %. Therefore, the washing after preparation was considered to be unnecessary in the preparation of NP; on the other hand, the washing with methanol/PBS mixture (3/7, v/v) was needed for the previouslyreported PLGA microparticles loaded with GLA because of their large initial burst release (70 – 90 %) [16,17]. The release rate of NP in the slow release phase appeared to be faster than that in the previous GLA-loaded PLGA microparticles [16,17]. The present NP, prepared with PLA-MPEG, was considered to be superior in the release profile and ease of preparation.
In addition, effect of plasma to the release profile was examined in the presence of murine plasma at 45 % (v/v). The results are shown in Figure 3. Although the GLA release was accelerated to some extent in the early time, the difference in the release rate was not marked between the presence and absence of plasma. This was considered due to the good retention of GLA inside the nanoparticles and good stability of the nanoparticles. The delivery of GLA was expected to be controlled by the biodisposition of NP.
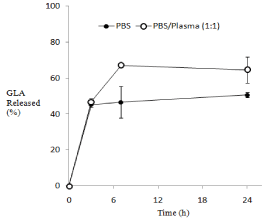
Figure 3: Comparison of In vitro release profiles of GLA from NP in PBSmurine
plasma mixture (1:1, v/v) at 37 °C.
NP-B was used as NP.
The results are expressed as the mean ± S.D. (n=3).
Hepato-protective effects in mice with CCl4-induced hepatitis
The efficacy of NP and GLA alone was examined using mice with CCl4-induced hepatitis. The injury was made longer by repeated i.p. injection on 0 and 48 h. The drug administration was conducted at a single (3 h) or twice (3 and 27 h) dosing. They GOT levels in the plasma at 24, 48, 72 and 96 h were measured and compared among the administration samples (saline, NP and GLA alone). The effect of the single administration is shown in Figure 4, in which the vertical axis was shown in the logarithmic scale. As to the time points, 24 and 72 h, as just 24 h had passed after CCl4 injection, they GOT levels were raised due to the liver injury. Since just 48 h had passed at the time pints, 48 and 96 h, the plasma GOT levels recovered to a considerable extent. NP and GLA alone tended to lower plasma GOT levels against the control at 24 h after the administration. Furthermore, NP had the tendency to suppress the GOT levels during the observation period, while GLA alone exhibited no decrease in the GOT levels. Overall, NP appeared to lower the plasma GOT activity than GLA alone. At each time, the tested groups were compared with the control by the Dunnett’s post hoc test after ANOVA. Only at 96 h, the GOT value of the NP group was significantly less than that of the control (p < 0.05). The results suggested NP should have higher and prolonged hepato-protective potential as compared with GLA alone. Although the detailed mechanism was not investigated, the release characteristics and biodisposition of NP are presumed to influence these in vivo results. The prolonged release might lead to the prolonged effects. Generally, although PLA-MPEG nanoparticles are less taken up by reticuloendothelial systems in liver and spleen than PLA nanoparticles, due to hydrophilic properties of PEG, PLAMPEG nanoparticles show the accumulation to liver and spleen to a certain extent [14,15]. This probably results in better efficacy of NP. In twice administration, both NP and GLA alone tended to decrease the GOT levels during the observation period Figure 5; GLA alone seemed to show the effect long because of its repeated administration. However, even in twice administration, the results at 96 h indicated that NP appeared to show the prolonged effect better than GLA alone, though the significant effect of NP was not obtained because of the large variance of control data at 96 h.
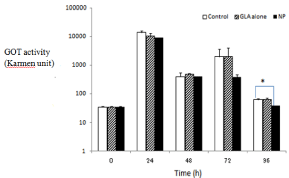
Figure 4: Hepato-protective effects in mice with CCl4-induced hepatitis at
single i.v. administration at 5 mg GLA eq. /kg. NP-B was used as NP.
The results are expressed as the mean + S.E. (n=3); Error bars show S.E.
*P < 0.05 (Dunnett’s post hoc test).
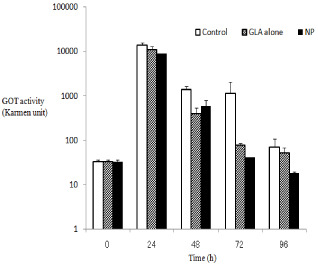
Figure 5: Hepato-protective effects in mice with CCl4-induced hepatitis at
twice i.v. administration at 5 mg GLA eq. /kg. NP-B was used as NP.
The results are expressed as the mean + S.E. (n=3); Error bars show S.E.
Overall, NP exhibited better suppression of the plasma GLP level.
The release profile and biodistribution of the NP were considered to be different from those of the previous PLGA microparticles. Since the NP have a hydrophilic shell of PEG, the rate and amount accumulated into the liver and spleen should be suppressed in the NP as compared with microparticles made of PLA or PLGA [14,15]. In the future, more detailed analyses using other approaches such as histological assessment of liver etc. might prove more clearly the efficacy or toxicity of the NP and the difference from GLA- loaded PLGA.
Conclusion
Novel nanoparticles loaded with GLA (NP) were prepared using an amphiphilic block copolymer, PLA-MPEG. NP could be obtained by simple O/W emulsification containing 1 % PVA. In the GLA/ PLA-MPEG ratio at 1/6 (w/w), NP, having the size of 300 – 400 nm and more than 3 % GLA content, could be produced. NP exhibited a prolonged release with a certain initial burst. In mice with CCl4- induced hepatitis, NP tended to show better and more prolonged hepato-protective effect than GLA alone in the single and twice dosing. NP are suggested as a potential delivery system of GLA for the liver injury. In order to evaluate the NP more clearly, further examinations such as histological studies might be required.
References
- Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000; 120: 849-862.
- Ploeger B, Mensinga T, Sips A, Seinen W, Meulenbelt J, DeJongh J. The pharmacokinetics of glycyrrhizic acid evaluated by physiologically based pharmacokinetic modeling. Drug Metab Rev. 2001; 33: 125-147.
- Xiong H, Xu Y, Tan G, Han Y, Tang Z, Xu W, Zeng F, Guo Q. Glycyrrhizin ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice and inhibits TNF-a-induced ICAM-1 expression via NF-?B/MAPK in HaCaT cells. Cell Physiol Biochem. 2015; 35: 1335-1346.
- Kao TC, Shyu MH, Yen GC. Glycyrrhizic acid and 18beta-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3beta signaling and glucocorticoid receptor activation. J Agric Food Chem. 2010; 58: 8623-8629.
- Higuchi T, Nishida K, Nagamura Y, Saito S, Ito M, Ishiguro I. Preventive effects of glycyrrhizin and its derivatives on liver injury in mice treated with carbon tetrachloride. Journal of Medical and Pharmaceutical Society for WAKAN-YAKU. 1992; 9: 59-65.
- Pompei R, Flore O, Marccialis MA, Pani A, Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature. 1979; 281: 689-690.
- Matsumoto Y, Matsuura T, Aoyagi H, Matsuda M, Hmwe SS, Date T, et al. Antiviral activity of glycyrrhizin against hepatitis C virus in vitro. PLoS One. 2013; 8: e68992.
- Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, Armanini D, et al. Antiviral effects of Glycyrrhiza species. Phytother Res. 2008; 22: 141-148.
- Takeda S, Ishthara K, Wakui Y, Amagaya S, Maruno M, Akao T, et al. Bioavailability study of glycyrrhetic acid after oral administration of glycyrrhizin in rats; relevance to the intestinal bacterial hydrolysis. J Pharm Pharmacol. 1996; 48: 902-905.
- Inoue H, Mori T, Shibata S, Koshihara Y. Modulation by glycyrrhetinic acid derivatives of TPA-induced mouse ear oedema. Br J Pharmacol. 1989; 96: 204-210.
- Inoue H, Inoue K, Takeuchi T, Nagata N, Shibata S. Inhibition of rat acute inflammatory paw oedema by dihemiphthalate of glycyrrhetinic acid derivatives: comparison with glycyrrhetinic acid. J Pharm Pharmacol. 1993; 45: 1067-1071.
- Ogawa Y, Okada H, Yamamoto M, Shimamoto T. In vivo release profiles of leuprolide acetate from microcapsules prepared with polylactic acids or copoly(lactic/glycolic) acids and in vivo degradation of these polymers. Chem Pharm Bull (Tokyo). 1988; 36: 2576-2581.
- Ogawa Y, Yamamoto M, Okada H, Yashiki T, Shimamoto T. A new technique to efficiently entrap leuprolide acetate into microcapsules of polylactic acid or copoly(lactic/glycolic) acid. Chem Pharm Bull (Tokyo). 1988; 36: 1095-1103.
- Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994; 263: 1600-1603.
- Bazile D, Prud’homme C, Bassoullet MT, Marlard M, Spenlehauer G, Veillard M. Stealth Me.PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharm Sci. 1995; 84: 493-498.
- Takahashi H, Onishi H, Machida Y. Glycyrrhetic acid-loaded microparticles: liver-specific delivery and therapeutic potential against carbon tetrachlorideinduced hepatitis. J Pharm Pharmacol. 2004; 56: 437-444.
- Onishi H, Takahashi H, Machida Y. Preparation and evaluation of glycyrrhetic acid-containing microparticles as an anti-hepatotoxic system. Drug Dev Res. 2006; 66: 189-199.
- Miura H, Onishi H, Sasatsu M, Machida Y. Antitumor characteristics of methoxypolyethylene glycol-poly(DL-lactic acid) nanoparticles containing camptothecin. J Control Release. 2004; 97: 101-113.
- Sasatsu M, Onishi H, Machida Y. Preparation of a PLA-PEG blocks copolymer using a PLA derivative with a formyl terminal group and its application to nanoparticulate formulation. Int J Pharm. 2005; 294: 233-245.