
Research Article
Austin J Nanomed Nanotechnol. 2016; 4(1): 1044.
Distribution and Estimation of Imatinib Loaded Pegylated PPI Dendrimeron BDF1 Hybrid Mice-Bearing K-563 Acute Lymphoblastic Leukemia
Karthikeyan R¹*, Sai Koushik O¹ and Vijayraj Kumar P²
¹Department of Biotechnology, Acharya Nagarjuna University, India
²Department of Pharmaceutical Sciences, Vignan Pharmacy College, India
3Faulty of Pharmaceutical Sciences, UCSI University, Malaysia
*Corresponding author: Ramadoss Karthikeyan, Department of Biotechnology, Acharya Nagarjuna University, Nagarjuna Nagar, Guntur-522510, India
Received: May 25, 2016; Accepted: June 30, 2016; Published: July 05, 2016
Abstract
The Pharmacokinetics (PK) and Bio Distribution (BD) of Imatinib loaded PEGylated PPI dendrimer formulations with different drug release rates were studied on BDF1 hybrid mice-bearing k-563 acute lymphoblastic leukemia. Imatinib is an efficacious anticancer drug with a spectrum of potential antitumor applications limited by poor bio distribution at therapeutic concentrations, so Imatinib loaded PEGylated PPI dendrimer was assessed for pharmacokinetic distribution profile of PEGylated PPI dendrimer holding Imatinib. Its single dose (6.25 mg kg-1) was administered i.v. to male micebearing k-563 acute lymphoblastic leukemia. Imatinib concentration was measured in spleen, liver, kidney and lung using a HPLC assay. These findings encourage the development of novel Imatinib formulations to treat other cancers.Estimated the bio-distribution of Imatinib in different vital organ was carriedout by HPLC method and the study was promising the distribution of drug candidate.
Keywords: PEGylated dendrimers; Imatinib; Bio-distribution; Chromatographic method
Introduction
An effective anticancer drug, currently approved by the FDA is Imatinib used inthe treatment of gastrointestinal stromal tumours (GIST), post-surgical removal of GIST and chronic myelogenous leukaemia. Imatinib competitively binds to the ATP binding site in the Abl domain of the BCR-ABL and it is a sensibly designed inhibitor of signal transduction. Due to its ability to inhibit the tyrosine kinase c-Kit it is effective in GIST [1-3]. For a large spectrum of antitumor applicationsImatinib is employed due to its high target selectivity. Imatinib has shown to inhibit small-cell lung cancer proliferation and to decrease PDGFR-β phosphorylation in nonsmall- celllung cancer. To a-1-acid glycoprotein it has high binding affinity which significantly affects its pharmacokinetics. Because of the protein binding, P450 mediated metabolism and P-glycoprotein affinity features, Imatinib presents drug-drug interactions with a variety of drugs [4-6]. These interactions affect the systemic exposure due to changes in bioavailability, clearance and plasma free fraction and may need dose monitoring. Overall, these studies point out the potential that Imatinib presents but also its limitations: firstly, to penetrate effectively into tissues secondly, to reach sufficient exposure and residence time in the tissue; and third, the complex drug-drug interactions. In order to explore the pharmacokinetics and tissue distribution, Imatinib was formulated in PEGylated PPI dendrimers, which have been shown to alter the pharmacokinetics and toxicology of drugs. Therefore, the current study aims to assess the distribution profile and tissue distribution of PEGylated PPI dendrimers loaded Imatinib in a dendrimeric formulation after administration to on BDF1 hybrid mice-bearing k-563 acute lymphoblastic leukemia [7-9].
Materials and Methods
Materials
PEG4000, Reney Nickel was obtained from Sigma, Germany, Raney Nickel was procured from Merck pharmaceuticals private Ltd., Mumbai, India, Triethylamine, dioxan, ethylene diamine, N, N dicyclohexylcarbodiimide (DCC), Cellulose dialysis bag MWCO 12- 14 Kda, Himediaprivate Ltd., India, 4 dimethyl amino pyridine SDfine chemicals private Ltd., Mumbai, India, Imatinib was gifted from Shasunpharmaceuticalsprivate Ltd, Chennai, India.
Synthesis of 5.0 G PPI Dendrimers
Double Michael addiction reaction occurs between acrylonitrile and aqueous solution of ethylenediamine which leads to the half generation EDA-dendrimer-(CN) 4n was synthesized. Next to the exothermic initial phase, the mixture was heated for 1 h at 80°C to complete the addition reaction. By vacuum distillation excess of acrylonitrile was removed. Later, use of Reney Nickel as catalyst, the hydrogenation in methanolfor 1 h at 70°C and 40 atm hydrogen pressures the EDA-dendrimer-(NH2) 4n of full generation was synthesized. Then the reaction mixture was cooled and filtered. Under reduced pressure the solvent was evaporated [7]. The product was then dried under vacuum. By repetition of all the above steps consecutively, EDA-PPI dendrimers up to 5.0G were prepared with acrylonitrile in increasing quantity. The scheme of the synthesis is shown in Figure 1.
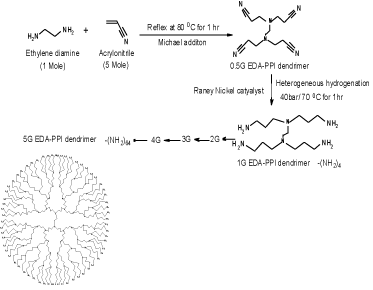
Figure 1: Schematic diagram for synthesis of PPI-5G dendrimer.
Synthesis of PEGylated 5.0G PPI dendrimers
To a solution of PEG 4000 (0.32 mmol) in DMSO (10 ml), N, N dicyclohexyl carbodiimide (DCC) (0.32 mmol) in DMSO (10 ml) and 5G EDA-PPI dendrimer (0.01 mmol) in dimethyl sulfoxide (DMSO) (10 ml) were added together. At room temperature the resultant solution was stirred for 5 days. By addition of water the product was precipitated, dialyzed and filtered by MWCO 12-14 Kda, Himedia, India. It was done against double distilled water for 24 h to remove free PEG 4000, DCC and partially PEGylated dendrimers. Later the lyophilization was done by Hetero dry winner, Germany. The synthesis was shown in Figure 2.
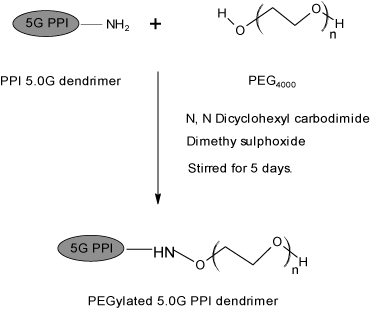
Figure 2: Synthesis of PEGylated PPI 5.0G dendrimers.
Drug Loading in PEGylated dendrimers
PEGylated-PPI dendrimers of known molar concentrations (1:0.5, 1:1, 1:2) were dissolved separately in methanol. Next they were mixed with methanolic solution of Imatinib. Using teflon beads the mixed solutions were incubated with slow magnetic stirring at 50 rpm for 24 h. These solutions were twice dialyzed in cellulose dialysis bag of MWCO 1000 Da Sigma, Germany. It was done against double distilled water under sink conditions for 10 min to remove free drug from the formulations.Later it was estimated spectrophotometrically at λ max 254 nm by using UV-1601, Shimadzu, Japan to determine the amount of drug loaded within the system indirectly. The dialyzed formulations were lyophilized and used for further characterization.
Bio distribution study
The in vivo studies were performed in male hybrid BDF1 mice.
The animals were divided into five groups containing each of six animals. Group- I received plain Imatinib, group-II received Imatinib loaded PEGylated dendrimers. The Antileukemic activity was studied on K-563 acute lymphoblastic leukemia cell lines respectively, with transplantation dose of 1x105 tumor cells/mouse, on day 0, intraperitoneally (i. p.). Imatinib and drug loaded PEGylated polypropyleneimine dendrimer were introduced intraperitoneally, once a day, on day 1, day 4 and day 8 after the tumor transplant.
Bio distribution studies of Imatinib loaded PEGylated dendrimer and plain Imatinib were performed in BDF1 hybrid mice-bearing K-563 acute lymphoblastic leukemia. Imatinib levels in the blood and various organs were estimated by HPLC method [8,9]. Separation was carried out on reversed-phase C18 column (250 4.6 mm, 5) and the column effluent was monitored by UV detector at 254 nm. The mobile phase of acetonitrile – phosphate buffer (pH 3.2; 25.0 mM) (24:76%, v/v) at a flow rate of 1.0 mL min-1. The separation was carried out at room temperature on Hypersil C18 Phenyl (250×4.6 mm, 5μ particle size).
Statistical analysis
The results are expressed as mean±standard deviation (S.D.) (n=6) and statistical analysis was performed by Graph Pad In Stat version 3.00.
Results and Discussion
Synthesis and characterization PEGylated dendrimers
FTIR and NMR spectroscopy: PPI 5.0G dendrimers were synthesized with slight modification of the procedure reported by Kumar et al., 2006 using ethylene diamine as initiator core. Synthesis of 0.5G PPI was confirmed by IR peaks, mainly of nitrile at 2248 cm-1. All the nitrile terminal 0.5G PPI got converted to (NH2)4, which was confirmed by IR of PPI 1.0G that exhibited major peak at 3284.78 cm-1 for amine (N-H stretch). Likewise, IR peaks also confirmed the synthesis of PPI 5.0G dendrimers. The main peaks are of C-C bend (1115.21 cm-1); C-N stretch (1243.44 cm-1, 1374.50 cm-1); C-H bend (1477 cm-1); N-H deflection of amine (1665.40cm- 1) and primary amine at 3410 cm-1(N-H stretch), confirming that a mine terminals were converted from nitrile terminal groups of dendrimer. The results matched with the reported synthesis of PPI dendrimers. The synthesized dendrimers were PEGylated using DCC and PEG 4000. IR and NMR data proved the synthesis of PEGylated dendrimers. The IR spectrum of PEGylated PPI 5.0G dendrimer exhibited major peak of N-H stretch of amide at 3324.70 cm-1. An important IR peak at 1242.75 cm-1 of ether linkage (C-O) appears in the spectrum of PEGylated dendrimers. C- O stretch of amide group has been found near 1624.29cm-1. The important peak of C-N stretch of amide also appears at 2925.43 cm-1. NMR spectrum and shifts of PEGylated dendrimers as compared to that of simple dendrimers proved PEGylation. There was increase in integral value for the shift of secondary -CH2 groups on PEGylation. This is due to the increase in number of secondary -CH2 groups in PEG that are linked on PEGylation. Similarly, strong peak of ether linkage appears at 3.507 ppm due to the presence of ether linkages in PEG in high amount, remaining free amines –CH2-NH2 appears at 3.341-3.410 ppm. The characteristic peak of amide linkage appeared near 2.504 ppm and 2.496ppm for carbonyl -CH2C=O in NMR spectrum of PEGylated dendrimers. The link formed between PEG and dendrimer periphery containing NH2 was shown in Figure 3.
Figure 3: Represents the formation of ether linkage and removal of amide peak.
Drug loading in to the PEGylated dendrimers
The known molar concentrations (1:0.5, 1:1, 1:2) of PEGylated- PPI dendrimers and drug Imatinib was used to load the drug in to PEGylated dendrimer system for getting optimized formulation. Non-covalent interactions between Imatinib and PEGylated PPI 5.0G dendrimers, such as hydrophobic interaction and hydrogen bonding, contributed to the physical binding of drug molecules inside dendritic micelles and surface PEG layers. The percentage loading of both the drugs in PEGylated PPI 5.0G dendrimers was significantly increased in 1:1 ratio of dendrimer: drug for the formulation (p value 0.0001, extremely significant) compared to 1:0.5 and 1:2 molar concentration of the drug. PEGylation increases the Imatinib loading capacity of the PPI 5.0G dendrimers due to more interaction of drug and PEG at the peripheral portions of dendrimers. Imatinib entrapment in PEGylated dendrimers increased significantly due to more sealing of dendrimeric structure by PEG at the peripheral portions of dendrimers as coat, which prevented drug release by enhancing complexation probably by increasing steric hindrance over dendrimer periphery. Number of moles of both the drugs entrapped in 1 mol of PEGylated dendritic architecture was found to be in 1:1 ratio of dendrimers and drug is suitable as 92.08 ± 1.2 mol for Imatinib as compared to 12.42 ± 0.8 mol in 1:0.5 molar concentration and 51.1 ± 1.0 molar concentrations in 1:2 ratio. Here, the proportion of 1: 1 ratio of dendrimer and drug showed the maximum drug load because the periphery PEG chain may prevent the high load of drug in to the system and it may saturated. If the drug entrapment is more than the required quantity leads to toxic to the host, increase in size leads to internal pressure were by leakage of drug from the system may happen. So the study considered to take up only the 1:1 ratio molar concentration followed in the preparation Table 1. Showed the drug entrapment in to dendrimers in various concentrations.
S.No
Formulation code
Ratio of (dendrimer: drug ) In mol. Con
% of drug entrapped
1.
DLDI
1:0.5
12.42±0.8
2.
DLDI
1:1
92.08±1.2*
3.
DLDI`
1:2
51.01±1.0
Table 1: Drug entrapment efficiency of Imatinib loaded PE Gylated dendrimer.
Bio distribution of Imatinib fromdendrimeric formulation
In order to understand the fate of drug loaded PEGylated PPI 5.0 G dendrimers in vivo, the bio distribution to various major organs was investigated. The amount of drug in the body depends upon its release, distribution, metabolism and excretion from the body. The bio distribution of Imatinib is generally more in bile and excretion is mainly through kidneys. But due to sustained drug delivery and long circulatory nature, PEGylated PPI 5.0G dendrimers made the drug molecules more available in blood than in bile for longer period of time. The amount of plain drugs in different metabolic and excretory organ was found to be higher at 4th hr as compared to PEGylated dendrimer formulation, which indicated slow release pattern from the PEGylated dendritic formulations.
The amount of drug in the case of plain Imatinib was found to be 254 ± 2.6 ng/ml in kidneys at 4th hr, which was higher as compared to PEGylated dendritic formulation (170± 5.9 ng/ml). The reversal of the position at the 8thhr indicated that most of the plain drug was eliminated out of the body. The presence of the higher amount of drug in the excretory organs at the 8thhr in the case of PEGylated dendritic formulations (Table 2), demonstrates slow release of drugs from them. Similar results were observed in the case of lungs also. PEGylation have reduced the hepatic and bile accumulation of the drugs. These results agreed with the reports that the PEGylation of drug carriers such as nanoparticles and other polymer could improve their bio distribution characteristics of drugs.
Concentration of Imatinib (ng/ml) at Different Time Interval(Mean±S.D)
Organ
System
4 hr
8 hr
Liver
A(Free Imatinib)
B(Imatinib Dendrimers)
124 ± 2.3
114 ± 1.6
98 ± 4.1
90 ± 2.7
Kidney
A(Free Imatinib)
B(Imatinib Dendrimers)
254 ± 2.6
123 ± 4.7
170 ± 5.9
90 ± 7.2
Lung
A(Free Imatinib)
B(Imatinib Dendrimers)
118 ± 5.2
109 ± 7.4
72 ± 4.3
70 ± 6.5
Spleen
A(Free Imatinib)
B(Imatinib Dendrimers)
126 ± 3.8
101 ± 7.1
89 ± 3.4
92 ± 3.9
Table 2: The Imatinib level attained at various time intervals in different organ.
Conclusion
The bio distribution of Imatinib also showed different tissue uptake patterns that may indicate the contribution of a variety of mechanisms that affect thetissue distribution and pharmacokinetics of Imatinib in a dendrimeric formulation. The result of these studies has signified the ability of the drug loaded PEGylated PPI 5.0G dendrimer to alter the pharmacokinetics and tissue distribution pattern of drug. It is very essential that any delivery system should not affect the distribution of candidate molecule at cause.
Acknowledgement
The authors are thankful to Dr. P. Balasubramaniyam, Executive Director, Shasun research centre, Chennai. India for synthesis of PPI dendrimer.
References
- Griffin MR. Epidemiology of nonsteroidal anti-inflammatory drug-associated gastrointestinal injury. Am J Med. 1998; 104: 23S-29S.
- Chi SC, Jun HW. Anti-inflammatory activity of ketoprofen gel on carrageenaninduced paw edema in rats. J Pharm Sci. 1990; 79: 974-977.
- Karthikeyan R, Kumar PV, Koushik OS. Dendrimeric Biocides - A Tool for Effective Antimicrobial Therapy. J Nanomed Nanotechnol. 2016; 7: 359.
- Lin SZ, Wouessidjewe D, Poelman MC, Duchone. In vivo evaluation of indomethacin/cyclodextrin complexes gastrointestinal tolerances and dermal anti-inflammatory activity. D. Int. J. Pharm. 1994; 106: 63-67.
- Chan TA. Nonsteroidal anti-inflammatory drugs, apoptosis, and colon-cancer chemoprevention. Lancet Oncol. 2002; 3: 166-174.
- Karthikeyan R, Kumar PV, Koushik OS. Pegylated PPI Dendrimer Cored with Ethylene Diamine for Prolonged Release of Prednisolone. J Nanomed Nanotechnol. 2016; 7: 362.
- Jacobs J, Goldstein AG, Kelly ME, Bloom BS. NSAID dosing schedule and compliance. Drug Intell Clin Pharm. 1988; 22: 727-728.
- Tomalia DA, Baker H, Dewald JR. Dendritic macromolecules: synthesis of starburst dendrimers. Macromolecules. 1986; 19: 2466–2468
- Kumar PV, Asthana A, Dutta T, Jain NK. Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. J Drug Target. 2006; 14: 546- 556.