
Special Article - Nanocarriers
Austin J Nanomed Nanotechnol. 2019; 7(1): 1053.
Nanomedicine: A New Therapeutic Approach in Liver Diseases
Sharma H, Flora SJS, Panghal A and Naqvi S*
Department of Pharmacology & Toxicology, National Institute of Pharmaceutical Education and Research Raebareli, India
*Corresponding author: Saba Naqvi, Department of Pharmacology & Toxicology, National Institute of Pharmaceutical Education and Research, Raebareli, New Transit Campus, Bijnor Road, Sarojini Nagar, NIPER-R/ Communication/068, Near CRPF Base Camp, Lucknow (UP), 226002, India
Received: April 23, 2019; Accepted: May 20, 2019; Published: May 27, 2019
Abstract
Liver fibrosis is known as a reversible wound healing process. It is defined as the collection of Extracellular Matrix (ECM) in the liver followed by liver injury where injured tissue displaced by the scar tissue. An advanced form of the liver fibrosis is known as liver cirrhosis. The cause for liver injury is heavy alcohol consumption, hepatitis B & C virus, and nonalcoholic fatty liver disease, etc. Liver cirrhosis, portal hypertension, ascites, and liver failure are some of the symptoms occurred due to liver fibrosis. Worldwide, liver disease is the 14th most common cause of death. Mortality due to liver diseases is approximately 1.03 million per year worldwide. Liver diseases became the 12th leading cause of death up to 2020. Nanotechnology is the emerging technology in the field of medicine, which opens the new avenues for the treatment of liver diseases. The present review explains about etiology, pathogenesis, symptoms, conventional approaches, current therapeutic strategies, and the role of nanotechnology in the liver fibrosis treatment. Conventional approaches provide only symptomatic treatment, it is not enough and effective to deliver the desired quantity of therapeutic agent in the liver. Nanotechnology is the current therapeutic approach, which involves various nanoparticles systems such as inorganic, liposomal, polymeric, albumin and nanomicelles, etc. These nanoparticles have numerous advantages over conventional treatment in the delivering of a therapeutic agent at specific sites. Various small drug moieties encapsulated in nanocarriers with modified surface chemistry used for the successful targeted delivery into the liver. Inorganic and liposomal nanoparticles have been widely studied for liver fibrosis treatment. Currently, nanomedicine generates much attention towards liver-associated diseases due to its high therapeutic efficacy and less adverse effect; hence it may provide great scope in the future for the treatment of liver disease.
Keywords: Liver fibrosis; liver cell receptors; Hepatic stellate cells; Nanotechnology; Inorganic nanoparticles; liposomes; micelles
Introduction
Fibrosis is the formation of scar tissue in the liver cells; it occurs when the liver tries to repair and displaces injured tissue by scar tissue [1]. Fibrosis itself does not cause any symptoms but cirrhosis is an advanced form of fibrosis leads to a variety of symptoms like portal hypertension and liver failure. Liver transplantation is the last therapeutic strategies adopted by the clinician when all injured tissues displaced by scar tissues [2]. There are various causes of liver fibrosis, but the major causes are heavy alcohol consumption, hepatitis B & C virus and nonalcoholic fatty liver disease [3].
Globally, the liver disease appeared as a major cause of global health burden. Worldwide, liver disease is the 14th most common cause of death. Liver diseases result in morbidity and mortality of 1.03 million per year worldwide, according to the global burden of disease 2010 study [4]. In India, the prevalence of liver disease was found to be around 9%-32% of the general population. A rural area is more prevalent for liver disease in India due to less awareness of health education and lack of medical facilities. Recently, WHO published its data in May 2014 that, in India, death due to liver disease reached 216,865 or 2.44% of total deaths [5]. It is projected that a rise in the deaths from the liver disease became it the 12th leading cause of death in 2020 [6].
Till date, there is no cure for liver fibrosis has been reported yet. There is only symptomatic treatment available for liver diseases which are not much effective for delivering a sufficient amount of therapeutic agent into the liver, furthermore, conventional approaches lead to various adverse and toxic side effects. Nanotechnology opens new opportunities for drug delivery in the treatment of liver disease. Advancement in nanotechnology develops a carrier system which can help in delivering a drug/peptide/gene to a targeted specific organ. Targeted delivery into the liver is gaining further interest in nanomedicine towards liver-associated diseases. There are various types of Nanoparticles (NP) like inorganic nanoparticles, liposomal, polymeric, albumin and other surface modified nanoparticles currently investigated for the treatment of liver diseases [7].
In current years, approaches based on nanomedicine have been investigated for liver disease treatment. Hence, keeping in mind the current scenario of liver-associated problems, the present review article describes the different types of nanoparticles and various nanoformulations, which are used for the treatment of liver diseases.
Etiology of Liver Fibrosis
Liver fibrosis has numerous possible causes; more than one cause can be even present in a single person. Overall, 57% of fibrosis is diagnostic for either hepatitis C (27%) or hepatitis B (30%). Another major cause is alcohol consumption, which accounts for at least 20% of the cases [8].
Virus
Infection by chronic hepatitis C virus and hepatitis B virus causes liver cell injury and inflammation in the liver, this inflammation and damage can lead to fibrosis (Figure 1). Among all the viral infected persons, 85% of persons infected with chronic hepatitis C [8] and 15% of persons infected with chronic hepatitis B virus will develop chronic hepatitis and lead to fibrosis. A viral infection is reported as the most common reasons for a liver transplant [9].
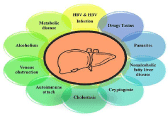
Figure 1: Etiology of liver fibrosis.
Parasitic
Developing countries are more prevalent in parasitic liver fibrosis. Out of many parasite groups, schistosomiasis group of the parasite is responsible for causing liver fibrosis. More than two hundred and twenty million people are suffering from this infection in Asia, Africa, and America [10].
Alcohol
Chronic intake of alcohol develops liver fibrosis in 10-20% of populations. Alcohol after entering in the body converted into acetaldehyde which is the reactive metabolite of alcohol, this metabolite blocks the normal metabolism of protein, fats, and carbohydrates. Presence of acetaldehyde accumulates other reactive product, which causes liver cell injury and inflammation and finally leads to liver fibrosis. Alcoholic liver fibrosis is found to be more prevalent in western countries mainly three times of hepatitis C due to the chronic intake of alcohol [11].
Autoimmune
Primary Biliary Hepatic Fibrosis (PBHF) and secondary biliary fibrosis both are an autoimmune disorder. It affects both males and females, but females of age 40-60 are more susceptible to this disease. In this disease antibodies produced against normal liver cells, causes inflammation, scar tissue formation, and fibrosis. Retention of bile acid in the liver takes place in Primary Biliary Hepatic Fibrosis (PBHF) while blocking of bile duct occurs in secondary biliary fibrosis. After blocking there is a gathering of serum globulins, mainly gamma globulins causing peri-portal inflammation and progressive fibrosis [12,13].
Metabolic diseases
Two metabolic diseases i.e. Wilson’s disease and hemochromatosis both cause liver fibrosis. Wilson’s disease is a genetic autosomal recessive disorder, which occurs due to the decrease in serum ceruloplasmin level and disturbance in copper metabolism in the liver [14], whereas hemochromatosis is a hereditary disorder, in which transfer of a gene from one generation to another generation takes place. In hemochromatosis iron does not metabolize properly, iron content increases inside the body and synthesis of hemoglobin get increased. The high concentration of hemoglobin deposits in the liver, which can lead to the generation of inflammatory signals and cause liver fibrosis [15].
Toxicants
Toxic substances such as carbon-tetrachloride, toluene, xylene, dimethylformamide, trichloroethylene, and thioacetamide are reported as well-known toxicants which cause liver cell injury. This toxic substance after entering in the body activates and releases various inflammatory mediators, which accumulates extracellular matrix in the liver and causes liver fibrosis [16].
Drugs
Liver cells can also be injured by the use of high concentration of some drugs like Isoniazid, this oxidizing, methotrexate, amiodarone, pyrimidine, tetracycline, and acetaminophen. Upon high concentration, these drugs can cause toxicity in the liver, which leads to cell injury, inflammation which activates various inflammatory mediators and liver fibrosis [17].
Nonalcoholic Fatty Liver Disease (NAFLD)
This disease seems similar in its signs to those, which occur by alcoholic liver disease, but in this case, patients do not have any alcohol history. It is mainly Non-Alcoholic Steatohepatitis (NASH) where fat deposits in the liver and cause liver scar tissue. The main cause of non-alcoholic steatohepatitis is obesity (40%), diabetes, coronary artery disorder, malnutrition, and steroids [18].
Liver Fibrosis Pathogenesis and Symptoms
Injury to liver cells causes scarring of tissue and cells which leads to fibrosis. In liver fibrosis Extracellular Matrix (ECM) proteins get the deposit (Figure 2) [1]. After an injury to the liver, injured tissues were replaced by the scar tissue. The mechanism by which this whole process takes place is the activation of quiescent Hepatic Stellate Cells (HSC) with the increase in different proteins such as,a-Smooth Muscle Actin (a-SMA), elastin, glycoproteins, proteoglycans and collagen [19].
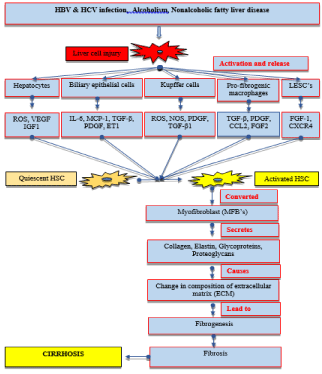
Figure 2: Schematic diagram of the pathogenesis of liver fibrosis.
Abbreviations: HBV & HCV: Hepatitis B Virus & Hepatitis C Virus; LESC’s:
Liver Sinusoidal Endothelial Cells; ROS: Reactive Oxygen Species; VEGF:
Vascular Endothelial Growth Factor; IGF1: Insulin Growth Factor1; IL6:
Interleukin-6; PDG F: Platelets Derived Growth Factor Receptor; FGF1:
Fibroblast growth factor; TGFβ: Transforming Growth Factor-β; NOS: Nitric
oxide synthetase; ET1: Endothelin-1; HSC’s: Hepatic Stellate Cells; MFB’s:
Myofibroblast.
There are numerous possible causes, even more than one cause will act simultaneously in the pathogenesis of disease at a time. Major etiological causes are hepatitis B, C virus and alcohol consumption. Other than major causes there is various other cause such as parasites, cholestasis, cryptogenic, metabolic disease, autoimmune, nonalcoholic fatty liver disease, drugs, and toxins [3]. All the etiological factor leads to liver cell injury and inflammation. Subsequently, there is activation of different inflammatory cells in the liver, those cells are hepatocytes, biliary epithelial cells, kupffer cells, pro-fibrogenic macrophages and Liver Sinusoidal Endothelial Cells (LSEC’s). Activated inflammatory cells release various inflammatory mediators, such as cytokines (interleukin, IFN, Transforming Growth Factor (TGF), Tumor Necrosis Factor (TNF), etc.), Reactive Oxygen Species (ROS), Nitric Oxide Synthetase (NOS), etc. all these factors are known as profibrogenic factors. Inflammatory mediators mainly cytokines are the key components in quiescent hepatic stellate cells activation, whereas reactive oxygen species responsible for the apoptosis and necrosis of hepatocytes.
Hepatic stellate cells are present as an inactive form in liver cells. After an injury to liver different inflammatory mediators, activates the inactive form of HSC into an active form. Activation of HSC is the main key step in the generation of liver fibrosis. Activated hepatic stellate cells differentiated into myofibroblast-like cells having fibrogenic and proinflammatory properties [20,21]. Myofibroblastlike cells and hepatic stellate cells after activation migrates at tissue injury sites, where they secrete a large amount of extracellular matrix along with various proteins, such as collagen, elastin, proteoglycans, and glycoproteins. All these factors ultimately change the composition of the extracellular matrix and regulate its degradation. Change in the composition of extracellular matrix leads to fibrogenesis, which is the normal wound healing response. But during chronic liver injury, this normal healing response gets deregulated and normal parenchymal cell removed by the scar tissue leads to liver fibrosis.
Different symptoms are occurred due to liver fibrosis (Figure 3). Cirrhosis is one of the symptoms of a fibrotic liver, it is known as the advanced form of liver fibrosis, where most of the liver tissue become scared. It is the irreversible process, causes many complications like swelling in lower legs, yellowing of skin, tiredness, weakness, and itching [22]. Hepatocytes are the main cell which is responsible for the metabolism of ammonia or waste product in the liver and portal vein help in excretion of waste material from liver into intestine, but due to liver injury or fibrosis hepatocyte gets degraded and portal vein injured, due to this accumulation of ammonia occur inside the liver which leads to hepatic encephalopathy. Hepatic encephalopathy causes movement problems, alteration in sleep, foul breath, respiratory acidosis, mood change and coma in higher stages [23].
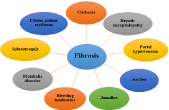
Figure 3: Schematic overview of symptoms in liver fibrosis.
Fibrosis also causes portal hypertension, increased blood pressure in the portal vein due to hepatic stellate cells and hepatocyte activation, which increases endogenous vasodilator amount in the system [24]. Vasodilator dilates the portal vein and blood pressure rises. Nitric oxide also has vasodilator properties, it is synthesized during liver injury and causes hypertension. In the case of ascites, there is fluid accumulation in the peritoneal cavity due to liver fibrosis and have some symptoms like shortness of breath, weight gain, and spontaneous bacterial peritonitis [25]. After fibrosis, there is a decrease in the metabolism of carbohydrates, fats and other product causes metabolic disorders such as hypoglycemia, hypercholesterolemia, etc. Bleeding tendencies elevated due to dropping in vitamin K absorption in the liver. Some other symptoms are splenomegaly [26] and jaundice etc.
Role of Epithelial-Mesenchymal Transition (EMT) and Endothelial-Mesenchymal Transition (EndMT) in Liver Fibrosis
The Epithelial-Mesenchymal Transition (EMT) is a mechanism for differentiating the cells found in complex tissues. Transition causes a decrease in polarity of epithelial cell and reduces adhesion of cells with each other’s, and get invasive properties to become mesenchymal cells [27]. EMT was first observed in embryogenesis and was crucial for the development of tissues and organs. It plays critical roles in wound healing, and organ fibrosis [28]. EMT are further classified as three types Type 1, Type 2 and Type 3 [29]. Type 1 EMT contributed in embryogenesis and organ development, whereas type 2 involved in the healing of wound, tissue regeneration and in organ fibrosis and type 3 EMT contributes in cancer cells to generate metastasis [30]. It has been reported that hepatocytes and cholangiocytes are two cells of the liver which contributes to liver fibrosis via EMT formation. Main signaling pathway involved in EMT has been explored by scientists, one of them is Hedgehog (Hh) signaling pathway. It has a role in organogenesis and tissue remodeling. It has been studied that Hh signaling was activated to guide remodeling of the biliary epithelia and stroma after cholestatic injury induced by bile duct ligation. Transforming Growth Factor (TGF) signaling pathway is another pathway involved in EMT. This pathway stimulates collagen and ECM production in HSCs during hepatic fibrogenesis. Extracellular Signal-Regulated Kinases (ERKs), namely the classical Mitogen-Activated Protein (MAP) kinases, are serine/threonine kinases that play crucial roles in the modulation of cell growth and differentiation. Evidence suggests that ERK signaling contributes to repression of EMT [28].
Endothelial-Mesenchymal Transition (EndMT), is another type of cellular transition, appeared as a possible mechanism in pathological fibrosis. In this transition, endothelial cells lose their specific endothelial cell markers, such as Vascular Endothelial cadherin (VEcadherin), and get a mesenchymal phenotype starting expression of mesenchymal cell products such as a-SMA and type I collagen. These cells have motile property due to which they are capable of migrating into surrounding tissues. TGF-β and Smad signaling pathways are the main pathways involved in EndMT transition during fibrosis [31].
Liver Cells and Different Types of Receptors
Liver is basically made up of two types of cells mainly parenchymal and non-parenchymal cells. Parenchymal cells contain hepatocytes whereas non- parenchymal cells lining the wall of the liver contain kupffer cells, liver sinusoidal endothelial cells, and hepatic stellate cells. These four cells are involved in the pathogenesis of liver fibrosis. Also, these cells contain different types of the receptor (Table 1) which are triggered by acute or chronic injury and increases the release of various profibrogenic molecules, induces fibrogenesis [3].
Cells
Receptor
Reference
Hepatocytes
Asialoglycoprotein Receptor (ASGP-R)
[32]
Glycyrrhizin/Glycyrrhetinic acid receptor
[33]
Scavenger receptor
[34]
IL-6 receptor
[33]
IgA receptor
[35]
Hepatic Stellate Cells (HSC)
Manose-6-phosphate Receptor(M6P-R)
[36]
PDGF-β receptor
Retinol Binding receptor
[37]
Integrin
[38]
Type VI collagen receptor
[39]
Kupffer Cell (KC)
Mannose receptor
[40]
Galactose receptor
[41]
Scavenger Receptor
[34]
Liver Sinusoidal Endothelial Cells (LSEC’S)
CD105 receptor
[42]
HA receptor
[43,44]
Table 1: Liver cells and different types of receptors.
Therapeutic Strategies Involved in Liver Fibrosis
Standard treatment for liver fibrosis is still not yet available, despite having many efforts by scientists and clinicians. Various therapeutic strategies investigated to prevent the advancement of the disease. Most of the studies do not have translational potential from bench to bedside this may be ascribed as humans are probably not as much as sensitive to hepatic antifibrotic treatments as animals [45]. However, various preclinical studies in animal data showed good results. Serial liver biopsies with long-term follow up is carried out in human studies, this may result in the high therapeutic output. The effective antifibrotic treatment is those, which are target specific and not cause any harmful effect after administration for a prolonged period of time [2]. There are several strategies explored by the researcher for better treatment of liver fibrosis. Some of them are elucidated below:
• Eliminating the primary cause of injury, for example, reduce alcohol intake in alcoholic liver diseases, various etiological factors.
• Inhibit Hepatic Stellate Cells (HSCs) activation.
• Inhibition of inflammation or hepatocyte injury.
• Inhibition of scar tissue formation increases matrix degradation and stimulating hepatic stellate cells apoptosis.
• Another important strategy in the treatment of liver fibrosis is the blocking of Renin angiotensin aldosterone system, which used in liver fibrosis patients with renal and cardiac failure.
• Inhibition of extracellular and intracellular signaling pathways which is responsible for activation of HSCs [46].
Conventional Treatment
Self-care
Intake of sodium should be lower in the case of ascites. Sodium intake should not be more than 2000mg per day in those patients, because more than this concentration salt increases water retention in the body accumulates fluids in the peritoneal cavity. Reduce alcohol intake, daily intake of alcohol should be reduced in alcoholic liver disease.
Diuretics
Loop diuretics, thiazides diuretics mainly used in symptomatic treatment, always used in combination with spironolactone to prevent hypokalemia (decreasing potassium level). Diuretics increase urine production and reduce salt and water intake. Due to this effect, they are useful in the treatment of cirrhotic ascites. Ascites caused by the accumulation of fluid within the peritoneal cavity owing to multiple circulatory, vascular, functional, biochemical, and neurohormonal abnormalities. Diuretics help in removing fluid from the body and prevent ascites.
Antihypertensive agents
Various antihypertensive agents like Angiotensin II (AT-II) receptor blocker, Angiotensin-Converting Enzyme (ACE) inhibitor,a- blocker, β- blocker, dilators, etc. mainly used to treat portal hypertension occur due to liver fibrosis.
Beta-blockers
Beta blockers are widely used in symptomatic treatment. Mainly nonselective beta-blockers are used to treat symptoms like high portal pressures, spontaneous bacterial peritonitis [47] and ascites [48]. Sometimes, nonselective beta-blockers are useful in the prophylaxis treatment of variceal hemorrhage [49].
Statins
Lovastatin, Simvastatin, Atorvastatin, etc. are the inhibitor of 3-Hydroxy-3-methylglutaryl coenzyme-A reductase enzyme, this enzyme performs the rate-limiting step in cholesterol synthesis and increases fat deposition in the liver. Statins are used safely in the treatment of nonalcoholic fatty liver disease [50].
Antibiotics
Many antibiotics are used in the treatment of various symptoms of liver fibrosis. Antibiotics like norfloxacin, ciprofloxacin, trimethoprim, and sulfamethoxazole used in the treatment of Spontaneous Bacterial Peritonitis (SBP), whereas antibiotics like rifaximin used in the treatment of hepatic encephalopathy. Rifaximin is a broad spectrum antibiotic active against both gram-positive and gram-negative aerobes and anaerobes.
Small molecule drugs
Small molecule drugs have a molecular weight less than 1000 Daltons. They mainly administered intravenously or orally. Due to their small size, they enter the target cells by crossing the cell membrane, also they are able to penetrate the blood-brain barrier [51,52].
Nuclear receptors
Hepatic stellate cells upon activation express various groups of nuclear receptors (Farnesoid X receptor (FXR) and peroxisome Proliferator-Activated Receptor ϒ (PPARϒ)) which act as transcription factors and play an important role in HSC regulation [53]. PPARϒ receptors are highly expressed in the quiescent HSCs and upon HSCs activation its expression diminishes [54]. Treatment with PPARϒ agonists restored PPARϒ expression and reduce HSC activation [55], whereas, FXR receptor mostly present in the liver and small intestine. It regulates transcription of multiple genes which are involved in bile acids synthesis and transport and maintaining homeostasis of bile acids and cholesterol [56]. In HSCs, activation of FXR decreases the collagen production. FXR agonists may be usefu in decreasing collagen production [57].
Renin-Angiotensin-Aldosterone System (RAAS)
RAAS system is an important hormonal regulatory mechanism. It regulates the blood pressure and fluid homeostasis in the body. In RAAS the angiotensin I converted into Angiotensin II (Ang II) by the action of enzyme Angiotensin I Converting Enzyme (ACE) [58]. Ang II directly binds to the Ang II Type 1 Receptor (AT1-R) and induces HSC activation, it also increased TGFβ, TIMP1 expression, and collagen deposition [59]. This interaction with AT1-R has an important role in liver fibrosis. Use of ACE inhibitors (ACEi) and AT1-R Blockers (ARBs) may be a good therapeutic option for the reatment of liver fibrosis [46].
Endocannabinoid system
Endocannabinoid system has a role in liver diseases which includes viral hepatitis, NAFLD, and alcoholic liver disease. Cannabinoid receptors CB1 and CB2 are up-regulated in chronic liver diseases and several studies have convincingly demonstrated antagonism between CB1 and CB2, that is, CB1 increases while CB2 decreases liver damage [60,61]. CB1 antagonists and CB2 agonists may be useful therapeutic approaches for liver diseases [46]. Table 2 represents the various drugs which are under clinical trial used by different receptors.
Sr.No
Therapeutic target
Drugs
Mechanism
Therapeutic use
Clinical Phase trial
1
Nuclear receptor:
FXR receptor
Obeticholic acid
FXR receptor agonist
Primary biliary cirrhosis,
3
NASH fibrosis
Obeticholic acid +
ursodeoxycholic acid (URSO)
FXR receptor agonist
Primary biliary cirrhosis
2
PPARγ receptor
Pioglitazone
PPARγ receptor agonist
NASH
2
Pioglitazone + vitamin E
PPARγ receptor agonist
NAFLD with diabetes mellitus type 2 (T2DM)
4
2
RAS system:
Angiotensin II type 1 receptor
Losartan
Angiotensin II type 1 receptor Antagonist
Liver fibrosis with
4
Irbesartan
Chronic HCV infection
3
Angiotensin II type 1 receptor Antagonist
NASH
Candesartan
Alcoholic liver fibrosis
2
Angiotensin1 converting enzyme
Moexipril
Angiotensin1 converting enzyme inhibitor/antagonist
Primary biliary cirrhosis
2
3
Antioxidant
Glycyrrhizin
Antioxidant action, inhibit ROS generation
Chronic hepatitis C and
3
liver fibrosis
4
Tumor necrosis factor 𝛼 production
Pentoxifylline
TNF 𝛼 suppressing
Primary biliary cirrhosis
2
phosphodiesterase inhibitor
5
Inflammatory cytokines
Cenicriviroc
CCR2 and CCR5 antagonist
NASH
2
6
Protein kinase receptor
Sorafenib
Tyrosine kinase inhibitor
Liver cirrhosis with portal
hypertension
2
Liver cirrhosis
Erlotinib
EGFR TK inhibitor
2
Table 2: Summary of the registered clinical trials.
Inflammation and oxidative stress
One of the major causes of liver fibrosis is Inflammation. There are different types of inflammatory response generated during fibrosis which affects the normal function of HSCs, and use of anti-inflammatory drugs provides rationale therapeutic approach, for examples, Corticosteroids (e.g., prednisone, prednisolone, methylprednisone, and triamcinolone) are useful in the treatment of liver diseases or even used in preventing liver rejection after liver transplantation. These corticosteroids work by inhibiting the release of inflammatory cytokines or neutralize released cytokine by acting on their receptor and antagonize them. Liver injury by various agents produces/generate oxidative stress or Reactive Oxygen Species (ROS) in the liver which is one of the factors responsible for the activation of HSCs. Therefore, inhibition of oxidative stress or ROS inhibits inflammation resulting in amelioration of liver fibrogenesis. Antioxidants can attenuate ROS generation and therefore emerge as potential antifibrotic therapies [46].
Protein kinases/kinase receptors
Activation of HSCs during liver fibrosis up-regulated a number of receptor tyrosine kinases, that was, Epidermal Growth Factor (EGFR), Platelet-Derived Growth Factor Receptor (PDGFR), Fibroblast Growth Factor Receptor (FGFR), Vascular Endothelial Growth Factor Receptor (VEGFR). These receptors produce different inflammatory signals resulted in differentiation and proliferation of quiescent HSCs. Antagonism of these pathways via tyrosine kinase inhibitors prevents liver fibrosis [46,62].
Nanomedicines in Liver Disorders
Nanotechnology is a branch of science and technology, it deals with the study and application of small particle mainly those are less than 100nm. Nanoparticles are very small and spherical in shape showing very unique surface properties. Nanoparticles which are used in nanomedicine are synthesized using biocompatible materials, having compatibility with living tissue. These materials should be nontoxic, non-injurious and harmless without causing any immunological response in the biological system. Nanoparticles are successfully designed for the delivering of therapeutic agents in a specific manner to a particular site in the system. Nanoparticles deliver the drugs either passively or actively. For the passive delivery of the nanoparticles, physicochemical properties of drugs should be properly optimized, whereas for active delivery cell-specific homing devices are used which provide the specific targeting at disease site and reduce the other adverse effect. By use of these technologies, we overcome our major pharmacological and clinical problems like less solubility of drug, organ toxicity non-specific, and short half-life of the drug molecule in systemic circulation and resistance of drugs with conventional therapy. Nanomedicine is the branch of medicine that deals with the biomaterials having nano dimensions through applications in numerous medical problems or issues via different nanoparticles like inorganic, polymeric, liposomal, albumin and others. Nanomedicine based on nanoparticles approach has been investigated by the researcher for liver disease treatment [63,64].
Advantages of Using Nanoparticles Delivery Systems
Nanoformulations are the current thrust in the systemic delivery of therapeutic agents at a specific site in a precise manner. Henceforth, they have an advantage over the conventional drug treatment options such as 1) Release of the drug in a controlled fashion; 2) Nanoparticles form a barrier around the core substance and thus prevents inactivation of therapeutic agents before it reaches to the site of action. This barrier protects the nucleic acids or biomacromolecules inside their core from enzymatic degradation in extracellular spaces; 3) Useful for delivering both hydrophilic and hydrophobic drugs at same time via different surface chemistry; 4) Increases bioavailability; 5) Minimize the toxicity and other adverse effects of drug; 6) It can be administered by various route external, oral, ophthalmic, parenteral and dermal, etc.; 7) Target-specific drug delivery.
Nano Therapeutic Strategies for Liver Fibrosis
Nanoparticles for liver fibrosis are primarily developed using liposomes, polymers, albumins and inorganic molecule. Nanoparticles enhances transport of various drug molecules, nucleic acids, antibodies and different moieties for targeted drug delivery [65]. Different nanotherapeutics has been investigated by researchers for liver fibrosis treatment, in the current review article we categorize the nanoparticles according to their chemical structure and components present in them: 1) Inorganic nanoparticles; 2) Liposomal nanoparticles; 3) Polymeric nanoparticles; 4) Albumin nanoparticles and 5) other Nanoparticles like nano micelles (Figure 4).
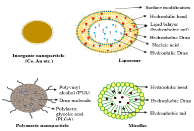
Figure 4: Schematic representation of different nanoparticles.
Inorganic nanoparticles
Inorganic nanoparticles are considered as an acceptable therapeutic choice, with its specific structure to take drugs for the treatment. They are nontoxic in nature, nanoparticles are made up of two layers one is the core membrane and the other is the outer surface. In inorganic nanoparticles, core is made up of metal or metal oxide and outer membrane is made up of an organic layer. Optical, electrical and magnetic properties are some of the unique properties of metalcore. The main advantage of these types of nanoparticles is that we can incorporate different types of drugs into them. Nowadays inorganic nanoparticles are extensively used for the treatment of liver fibrosis. Some nanoparticles-like Cerium Oxide (CeO2NPs) nanoparticles, gold (Au), silver and silica NPs [66] are commonly used in liver fibrosis treatment. Physical and chemical modification in the structure of NPs can be exploited for delivering drugs in liver disease treatment, for example, cisplatin, doxorubicin (DOX) and capecitabine [67].
There are different animal studies carried out for checking the effect of nanoparticles. According to one study, liver fibrosis in an animal model was induced by carbon tetrachloride (CCl4) and treatment was done with the cerium oxide (CeO2 NPs) nanoparticles. Systemic and hepatic effects of CeO2 NP were checked in rats after induction of liver fibrosis. All the function was checked properly after nanoparticles treatment. The effect of nanoparticles on liver fibrosis was confirmed by checking a decrease in inflammatory cytokines mRNA expression and oxidative stress mRNA expression. Further, from the histopathological study of the liver section, it has been observed that reduction in liver fibrosis was reported to a greater extent after giving nanoparticles treatment [68].
In another study, silymarin-coated gold nanoparticles are used in the treatment of liver fibrosis induced by carbon tetrachloride (CCl4). Gold nanoparticles work through downregulating hepatic stellate cells and kupffer cells. In animal model after treatment with silymarin - coated gold nanoparticles, there is a decrease in different inflammatory markers which are supposed to be the key markers for inducing fibrosis. A decrease in the level of alpha-smooth muscle actin (a-SMA) gives the exact result in decreasing fibrogenesis and liver fibrosis [69]. In another study, the silver/silica nanoparticles were used to target folic acid in case of radiation-induced liver cancer (Figure 5). Silver/silica nanoparticles were loaded with the indocyanine green. This nanoparticle released two molecules at a time, silver as well as indocyanine green which has the potential properties against folic acid, thus preventing liver cancer [70].
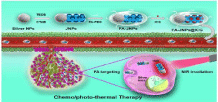
Figure 5: Folic acid targeted Janus nanoplatform with the NIR irradiationtriggered
release behavior of ICG and silver ions for synergistic liver cancer
chemo/photothermal therapy [70].
Liposomal nanoparticles
A liposome is the smallest spherical vesicle produced by the natural non-toxic phospholipid and cholesterol. It is a microscopic vesicle inside core is made up of the aqueous layer and this core is enclosed within another one or more phospholipid bilayer. Liposomes are used to deliver vaccines, enzymes, both hydrophilic and hydrophobic drugs at particular targeted sites. The main advantages of liposomal nanoparticles are its biodegradability, biocompatibility, low antigenicity, and their low toxic nature. Liposomes mainly prepared by thin film hydration, Dehydrated-Rehydrated Vesicles (DRV) freeze-thawing and detergent methods, etc. they are two types Small Unilamellar Vesicles (SUV) have 15-30 nm diameter and Large Unilamellar Vesicles (LUV) have 500-3000 nm diameter. Recently, liposomal nanoparticle-based drug therapy such as vitamin A-conjugated siRNA, dexamethasone loaded liposomes, INF-a loaded liposomes, etc. for liver fibrosis is reported under clinical trial, which shows its higher efficiency than other nanoparticles in clinical practice.
In the case of vitamin A-conjugated siRNA, it is a type of lipid nanoparticle mainly used for gene delivery. Currently, it is under clinical phase trial 1 for therapy of liver fibrosis. Delivery of these nanoparticles in the system occurs through targeting PLK-1 lipid particle or in the form of encapsulated liposomes. In this particular study, the researcher study effect of vitamin A-conjugated siRNA in CCL4 and bile duct ligated fibrosis in a mouse model. A study concludes that siRNA is delivered properly at a specific target in the hepatic stellate cell by conjugating with vitamin-A liposomes. They bind to the gp46 gene present in hepatic stellate cells and reduces collagen secretion and decreases liver fibrosis in the mouse model [46].
In another study dexamethasone are used, it is a corticosteroid medication and having anti-inflammatory activity. Therefore, dexamethasone loaded liposome is prepared to show antiinflammatory activity and used in the treatment of liver fibrosis. They target liver macrophages and decreases helper T cells in the liver through an immune reaction, finally helps in decreasing inflammatory mediator, inflammation, and fibrosis [71]. Some researcher study on PPAR-ϒ ligand-loaded Mannose-6-Phosphate (M6P)-Human Serum Albumin (HSA)-conjugated liposomes in CCL4 induced fibrosis mouse model and showed the therapeutic effect of these nanoparticles in liver fibrosis. These nanoparticles specifically target the mannose-6-phosphate receptor present in hepatic stellate cells. Inhibit the conversion of inactivated HSC in activated HSC and thus prevent further activation of fibro genetic pathway. These conjugated liposomes are useful in both in-vitro and in-vivo study for the treatment of liver fibrosis [72].
INF-ϒ has a beneficiary role in the treatment of liver disease. The researcher suggested that Platelets Derived Growth Factor (PDGF) have some role in the activation of hepatic stellate cells in the liver. So in this particular study researcher induces fibrosis in a mouse model using Thioacetamide (TAA) and liposomes loaded with interferon (INF-ϒ) are checked for their effect on TAA-induced liver fibrosis. Liposomes loaded with interferon (INF-ϒ) act through targeting the PDGF receptor and inhibit these receptors, which further prevent activation of HSC and prevent liver fibrosis [37].
Polymeric nanoparticles
A polymeric nanoparticle is colloidal in nature and has particles size in the nano range. It is mainly used for the Targeted Drug Delivery System (TDDS) to target particular sites. Drug of choice is either adsorbed or encapsulated or conjugated on or within the polymer nanoparticles. These polymeric nanoparticles prepared by solvent evaporation, emulsification and salting out phenomenon. They are used for the delivery of different drugs, such as nucleic acid, sorafenib, and nitric oxide. These nanoparticles deliver drugs to different liver cells for therapy of liver fibrosis [73]. Tyrosine kinase is an enzyme which is responsible for the production of collagen in a liver. Sorafenib is the antifibrotic agent and it is tyrosine kinase enzyme inhibitor. In CCL4 induced fibrosis mouse model sorafenib is delivered using polymer conjugation using Poly (Ethylene Glycol)- b-poly (Lactic-co-Glycolic Acid) (PEG-PLGA) copolymers with PLGA. It delivers sorafenib in systemic circulation where it inhibits tyrosine kinase enzyme and decreases collagen production with shrinking of blood vessels and microvascular density, finally reduced liver fibrosis [74]. It was found that nitric oxide has some potential properties in the treatment of liver fibrosis so for this researcher uses a copolymer, poly (oligo ethylene glycol)-methyl ether-methacrylateblock- 2-vinyl-4,4-dimethyl-5-oxazolone which was coated with the vitamin-A nanoparticle. This combined form of nanoparticle was a good carrier for the nitric oxide in hepatic stellate cells. Nitric oxide in HSC act through decreasing collagen I level in the injured liver.
Albumin nanoparticles
Albumin is a protein which abundantly presents in the body and it is obtained either from humans or animals. Protein aggregates such as Human Serum Albumin (HSA) and Bovine Serum Albumin (BSA) obtained from human and animal uses in liver fibrosis therapy. Biodegradable nanoformulations are most preferable in nanomedicine due to their properties like non-toxic, low cost, nonimmunogenic nature; it is a vital factor in designing nanotechnologybased drug delivery protocol. In one study, a drug moiety Berberine was coupled with the bovine serum albumin to prepare nanoparticles. Berberine shows antiproliferative properties against CCL4 induced liver fibrosis in a mouse model both in-vivo and in-vitro. Berberine BSA nanoparticle target hepatic stellate cells in the liver and inhibit the activation of hepatic stellate cells thus prevent further fibrogenesis [75]. In another study, a mouse model was used to study liver fibrosis and it was induced using bile duct ligation. A corticosteroid medicine dexamethasone is injected into the mouse model in the form of nanoparticle which was coupled with the mannosylated albumin. These nanoparticles deliver drugs to a specific target kupffer cell in the liver and show their anti-inflammatory effect. In an invitro study, it inhibits Tumor Necrosis Factor (TNF) and in-vivo it decreases Reactive Oxygen Species (ROS) [71]. Another target found by the researcher was Transforming Growth Factor (TGFβ) which was targeted using MP6/IG II receptor. TGFβ is cytokines which get activated after the injury to the liver and induces liver fibrogenesis. Human serum albumin is altered with Mannose-6-Phosphate (M6P) nanoparticle, particularly used for targeting M6P/IG II receptors which inhibit TGFβ cytokines signals and decreases collagen production or liver inflammation in a rat model.
Many other drugs, such as cisplatin, chlorambucil, and doxorubicin are studied for their therapeutic potential in liver diseases by targeting particularly mannose-6-phosphate receptor. They are altered using human serum albumin nanoparticles for delivering the drug to targeted sites due to their antiproliferative and antifibrotic action, they are used in the treatment of liver fibrosis. Table 3 showing various nanoformulations which are used for liver fibrosis.
Sr.No
Nanoparticle system
Nanoparticle formulation
Targeting cells and Action
References
1
Inorganic nanoparticle
Cerium oxide nanoparticle (CeO2NPs)
Target HSC,
[76]
Action through: decreasing macrophage infiltration, inhibition of 𝛼-SMA and caspase-3, decreasing inflammatory cytokines
Gold nanoparticle
Inhibition of 𝛼-SMA by targeting HSC
[67]
2
Liposomal nanoparticle
Dexamethasone–liposome
Target liver Macrophages, and decreases T cells production
[71]
HSC, suppression of collagen secretion
Vitamin A-coupled liposomes
HSC, inhibition of collagen production
HSA-M6P-liposomes
HSC, decreases collagen synthesis and a-SMA levels
[46]
Cationic liposome microRNA
[77]
3
Polymeric nanoparticle
PEG-PLGA sorafenib
Tyrosine kinase inhibitor decreases collagen production and microvascular density
[74]
Targeting HSC, inhibition of collagen I
POEGMA-b-VDM-vitamin A nanoparticles
[69]
4
Albumin nanoparticle
Berberine-BSA nanoparticles
HSC, antiproliferative action
[75]
Dexamethasone-mannosylated albumin
M6P-HSA-DOX
Kupffer cells (TNF-𝛼), reduced intrahepatic ROS
[71]
HSC, antifibrotic effect
[36]
5
Other nanoparticle (Nano micelles)
HA micelles
HA-PLA-curcumin
HSC, inhibition of HSC activation
HSC, antioxidant and anti-inflammatory action
[44]
[43]
Table 3: Nanoformulation for liver fibrosis.
Other nanoparticulate systems: nano micelles
Nano micelle is the form of a micelle formed at Critical Micelles Concentration (CMC). They are the current novel approach in nanomedicine. Nano micelles are nano-size range colloidal particle, it is made up of the hydrophobic core and hydrophilic shell but in reverse nano micelles core is made up of hydrophilic tail and shell made up of the hydrophobic head. Normal micelles are used for delivering hydrophobic drugs to increases their concentration in systemic circulation whereas reverse micelles are delivered hydrophilic drugs to a particular site [65].
Liver Sinusoidal Endothelial Cells (LSEC’S) and hepatic stellate cells are targeted by targeting Hyaluronic Acid (HA) receptor. Losartan is the Angiotensin Receptor Type 1 (AT1) receptor blocker conjugated with Hyaluronic Acid (HA) micelles used in therapy of advanced liver fibrosis in a C3H/HeN mouse model. During liver injury, CD44 receptor gets over expressed which activated HA receptor-mediated HSC activation. They deliver drugs to HSC and decreases the level of a-SMA and fibrogenesis [44]. There are various natural substances currently under study for liver fibrosis therapy.
Curcumin is among one of them, it exhibits antioxidant and antiinflammatory effects. Curcumin is a non-toxic natural compound easily available around the world but their low bioavailability limits their therapeutic potential. For this, curcumin nanoparticle was encapsulated using HA-polylactic acid micelles which deliver the drug to HSC and have potential to minimize the toxic effect of TAA upon HSC in a TAA-induced liver fibrosis mouse model [43].
Role of Kupffer Cells in Nanoparticles Drug Delivery
Kupffer cells are one of the macrophages present in the liver which were used for homeostasis of tissues. Macrophages have high phagocytic activity, due to which they easily respond to danger signal and maintain immune homeostasis. In a healthy person, kupffer cells function as an immune protector that activates other cells upon pathogenic threats. Phagocytosis phenomena occur in kupffer cell when a nano or other delivery system enters into the liver. Macrophages recognize the nanoparticles as opsonins via interaction with receptors present on kupffer cells [78]. There are many factors which are helpful for understating the role of kupffer cells in nanoparticles drug delivery. The contact between the kupffer cells and drug delivery system depend upon the size and radius of nanoparticles. Mainly, a diameter of 1-3 μm is sufficient for interaction with cells. If size and radius of nanoparticles are very small, in that case, they are not easily entered into the cell membrane but they enter into cells via either pinocytosis or endocytosis whereas large size particles does not get contact with the cell membrane [79]. Shape of nanomaterials also plays an important role in drug delivery. It has great impact on the interaction of the delivery system and macrophages. Large particles, elongated shapes stimulate interaction between them and smaller particle shape influences the speed of internalization into cells. Some studies have shown that, in comparison to their spherical shape, nonspherical particles are distributed more in different tissue by hydrodynamic forces in the bloodstream [80]. Also, there are other parameters that influences the interaction and distribution of nanoparticles. The flexibility of the smaller hydrophilic delivery system was found to affect in vitro kinetics and internalization pathways in macrophages [81] and other nonphagocytic cells [82]. In vivo, the flexibility and deformability of delivery systems have great impact on their tissue distribution and retention, for example, RBC. These properties influence interaction in a complex manner. Positive charges on the surface of the delivery system have a deleterious effect on circulation time, whereas different findings are reported regarding the impact of negative charge. It have been studied that effects of particle size and surface charge on cellular uptake/bio distribution and concluded that in vivo bio distribution of Nanoparticles (NPs) with slight negative charges and particle size of 150nm tended to accumulate in tumor more efficiently, while it was reported that negatively charged liposomes have a shorter half-life in the blood [83]. Thus, macrophages have major role in fibrosis due to their unspecific nanoparticle uptake and also for the recognition of associated antigens. Therefore, macrophages represent as an attractive targets for nanomedicine in liver disease, but it should also be considered as particle scavenging cells in nanoparticle mediated drug delivery.
Future Directions
Scientists explore numerous therapeutic agents for the treatment of disease related to the liver, but the translational potential of these agents from bench to bedside is still very poor. From the current review, we understood the pathogenesis of liver fibrosis, conventional and nanomedicine treatment approaches. In the future, there is a need to identify the molecular mechanism, genetic modification and different molecular pathways responsible for causing liver fibrosis to investigate new therapeutic targets. Recently, the field of nanomedicine developed as an encouraging area of research for liver diseases treatment. Biodegradable, multifunctional nanocarriers, conjugated with a number of drug molecules, having efficacy and safety properties will provide infinite opportunities in targeted delivery for treatment of liver disease, this should be one of the important strategies for future in nano theranostic approach. The urge for developing simple and harmless noninvasive indicators for liver fibrosis is the main objective in clinical hepatology that will simplify the design of clinical trials. Comprehensive epidemiological genetic studies data are markedly required for an epidemiological survey, which are very useful for identifying high-risk patients with the liver fibrosis and cirrhosis. Mechanism and effect of stem cell therapy in liver fibrosis treatment is not clearly understood yet, therefore, a systematic study on stem cell therapy should be required for a better understanding of its delivery and therapeutic effect. It may provide the potential approach for liver fibrosis regeneration therapy in the future.
Conclusions
Liver fibrosis is a wound healing process. Injury to liver cells accumulates Extracellular Matrix (ECM) proteins in the liver. During the wound healing process liver form scar tissues by removing injured tissue. Majorly fibrosis caused by heavy alcohol consumption, hepatitis B & C virus, and nonalcoholic fatty liver disease. Conventional treatment is available only for the symptomatic treatment but due to its low concentration in the systemic circulation, it does not provide sufficient treatment for liver therapy.
Currently, treatment available on the basis of nanotechnology is come up as the inventive and promising approach in treatment for liver fibrosis as an alternative to conventional therapy. Different nanoparticles have been developed for the treatment of liver fibrosis, such as inorganic, liposomal, polymeric, albumin, and nanomicelles nanoparticles. Available conventional drugs and new drug moieties are modified by conjugation/coupling with these nanoparticles for targeted drug delivery, increasing systemic bioavailability, controlled release of drug and less adverse effect. Currently, among all nanoparticle formulations explored by scientists, liposomes, and nanomicelles were considered as first generation nanoparticles with minimum toxicity and having the flexibility for further chemical and physical modifications. Now, these current therapies with nanoparticles can be helpful in the prevention and cure of liver fibrosis. Currently, there are varieties of drugs with nanoparticles under preclinical and clinical trials. Nanoparticles provide great scope in the future for the treatment of liver disease and other diseases.
Acknowledgement
SJS Flora and SN are grateful to Department of Pharmaceuticals, Ministry of Chemical and Fertilizes, Govt. of India, SN was further supported by Women Scientist Scheme (WOS-A), Department of Science and Technology, Government of India (grant no. SR/WOS-A/ LS-1224/2015). HS was supported by GPAT, AICTE scholarship.
References
- Trautwein C, Friedman SL, Schuppan D, Pinzani M. Review hepatic fibrosis : Concept to treatment. J Hepatol. 2015; 62: 15-24.
- Bataller R, Brenner DA. Liver fibrosis Find the latest version : Science in medicine Liver fibrosis. 2005; 115: 209-218.
- Zhou W, Zhang Q, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014; 20: 7312-7324.
- Yang J, Zhang Y, Luo L, Meng R, Yu C. Global mortality burden of cirrhosis and liver cancer attributable to injection drug use, 1990-2016: An age-periodcohort and spatial autocorrelation analysis. Int J Environ Res Public Health. 2018; 15:170.
- Unnati P, Patel G, Patel D, Patel S, Patel M, Dass E. A prospective study of liver cirrhosis: An overview, Prevalence,clinical manifestation and investigations in patients admitted to the medicine ward in a rural teaching hospital. Int J Sci Res. 2018; 7: 2016-2018.
- Murray JL, Lopez AL. Alternative projections of mortality and disability by cause 1998-2020: Global Burden of Disease Study. Lancet. 1997; 349: 1498- 1504.
- Giannitrapani L, Soresi M, Bondì ML, Montalto G, Cervello M. Nanotechnology applications for the therapy of liver fibrosis. World J Gastroenterol. 2014; 20: 7242-7251.
- Perz JF, Armstrong GL, Farrington LA, Hutin YJF, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006; 45: 529-538.
- Papadakis MA. Current medical diagnosis and treatment. 57th ed. Mc Graw Hill Education. 2018; 1-1953.
- Andrade ZA. Schistosomiasis and liver fibrosis. Parasite Immunol. 2009; 31: 656-663.
- Bruha R, Dvorak K, Petrtyl J. Alcoholic liver disease. World J Gastroenterol. 2012; 4: 81-90.
- Invernizzi P, Floreani A, Carbone M, Marzioni M, Craxi A, Muratori L, et al. Primary Biliary Cholangitis: advances in management and treatment of the disease. Dig Liver Dis. 2017; 49: 841-846.
- Hermosillo-sandoval JM, Rodríguez-carrizález AD, Miranda-díaz AG. Strategies in secondary biliary fibrosis. 2013; 10-18.
- Kotalova R, Jirsa M, Vavrova V, Vrabelova-Pouchla S, Macek M. Wilson disease as a cause of liver injury in cystic fibrosis. J Cyst Fibros. 2009; 8: 63-65.
- Alter MJ. Advances in hepatology vaccinating patients with chronic liver disease. 2012; 8: 120-122.
- Malaguarnera G, Cataudella E, Giordano M, Nunnari G, Chisari G, Malaguarnera M. Toxic hepatitis in occupational exposure to solvents. World J Gastroenterol. 2012; 18: 2756-2266.
- Devarbhavi H. An Update on Drug-induced Liver Injury. J Clin Exp Hepatol. 2012; 2: 247-259.
- Benedict M, Zhang X, Benedict M, Zhang X. Non-alcoholic fatty liver disease : An expanded review. 2017; 9: 715-732.
- Son D, Le NT, Pannacciulli N, Chen K, Salbe AD, Hill JO, et al. Early Response of a2(I) Collagen to Acetaldehyde in Human Hepatic Stellate Cells is TGF-β Independent. 2005; 42: 343-352.
- Milani S, Herbst H, Schuppan D, Kim KY, Riecken EO, Stein H. Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology. 1990; 98: 175-184.
- Moreira RK. Hepatic Stellate Cells and Liver Fibrosis. Arch Pathol Lab Med. 2007; 131: 1728-1734.
- Schuppan D, Afdhal NH. Liver Cirrhosis. Lancet. 2009; 371: 838-851.
- Ferenci P. Hepatic Encephalopathy. Gastroenterol reports. 2017; 5: 138-147.
- Bloom S, Kemp WL. Portal Hypertension - Pathophysiology, Diagnosis and Management. Intern Med J. 2015; 45: 16-26.
- Moore CM, Thiel DH Van, Moore CM, Thiel DH Van, Hepa S. Cirrhotic ascites review : Pathophysiology , diagnosis and management. World J oh Hepatol. 2013; 5: 251-263.
- Ghazi AAM, Attarian H, Attarian S, Abasahl A, Daryani E, et al. Hypercalcemia and huge splenomegaly presenting in an elderly patient with B-cell non- Hodgkin ’s lymphoma : a case report. J Med Case Rep. 2010; 4: 2-5.
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. Vol. 112. Journal of Cinical Investigation. 2003. 1776-1784.
- Yu K, Li Q, Shi G, Li N. Involvement of epithelial–mesenchymal transition in liver fibrosis. Saudi J Gastroenterol. 2018; 24: 5-11.
- Kalluri R. EMT: When epithelial cells decide to become mesenchymal-like cells. Vol. 119. Journal of Cinical Investigation. 2009; 112: 1417-1419.
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. Vol. 119. Journal of Clinical Investigation. 2009; 119: 1420-1428.
- Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011; 179: 1074-1780.
- Wang W, Zhao X, Hu H, Chen D, Gu J, Deng Y, et al. Galactosylated solid lipid nanoparticles with cucurbitacin B improves the liver targetability. Drug Deliv. 2010; 17: 114-122.
- Li L, Wang H, Ong ZY, Xu K, Ee PLR, Zheng S, et al. Polymer- and lipidbased nanoparticle therapeutics for the treatment of liver diseases. Nano Today. 2010; 5: 296-312.
- Li B, Yu M, Pan X, Ren C, Peng W, Li X, et al. Artesunate reduces serum lipopolysaccharide in cecal ligation/puncture mice via enhanced lps internalization by macrophages through increased mRNA expression of scavenger receptors. Int J Mol Sci. 2014; 15: 1143-1161.
- Liu G, Bi Y, Wang R, Yang H, Zhang Y, Wang X, et al. Targeting S1P1 Receptor Protects against Murine Immunological Hepatic Injury through Myeloid-Derived Suppressor Cells. J Immunol. 2014; 192: 3068-3079.
- Beljaars L, Olinga P, Molema G, De Bleser P, Geerts A, Gmm G, et al. Characteristics of the hepatic stellate cell- selective carrier mannose 6-phosphate. Liver. 2001; 21: 320-328.
- Li F, Li QH, Wang JY, Zhan CY, Xie C, Lu WY. Effects of interferon-gamma liposomes targeted to platelet-derived growth factor receptor-beta on hepatic fibrosis in rats. J Control Release. 2012; 159: 261-270.
- Li Y, Liu F, Ding F, Chen P, Tang M. Inhibition of liver fibrosis using vitamin A-coupled liposomes to deliver matrix metalloproteinase-2 siRNA in vitro. Mol Med Rep. 2015; 12: 3453-3461.
- Yang J, Hou Y, Ji G, Song Z, Liu Y, Dai G, et al. Targeted delivery of the RGD-labeled biodegradable polymersomes loaded with the hydrophilic drug oxymatrine on cultured hepatic stellate cells and liver fibrosis in rats. Eur J Pharm Sci. 2014; 52:180-190.
- Ramirez-Garcia A, Arteta B, Abad-Diaz-de-Cerio A, Pellon A, Antoran A, Marquez J, et al. Candida albicans Increases Tumor Cell Adhesion to Endothelial Cells In Vitro: Intraspecific Differences and Importance of the Mannose Receptor. PLoS One. 2013; 8: 1-10.
- Coombs PJ, Taylor ME, Drickamer K. Two categories of mammalian galactose-binding receptors distinguished by glycan array profiling. Glycobiology. 2006; 16: 1-12.
- Abel T, El Filali E, Waern J, Schneider IC, Yuan RCMQ, Unch MH, et al. Specific gene delivery to liver sinusoidal and artery endothelial cells. Am Soc Hematol. 2013; 122: 2030-2038.
- Chen YN, Hsu SL, Liao MY, Liu YT, Lai CH, Chen JF, et al. Ameliorative effect of curcumin-encapsulated hyaluronic acid-PLA nanoparticles on thioacetamide-induced murine hepatic fibrosis. Int J Environ Res Public Health. 2017; 14: 1-16.
- Thomas RG, Moon MJ, Kim JH, Lee JH, Jeong YY. Effectiveness of losartanloaded Hyaluronic Acid (HA) micelles for the reduction of advanced hepatic fibrosis in C3H/HeN mice model. PLoS One. 2015; 10: 1-17.
- Bataller R, Brenner DA. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001; 21: 437-451.
- Bansal R, Nagórniewicz B, Prakash J. Clinical advancements in the targeted therapies against liver fibrosis. 2016; 2016: 1-16.
- Mandorfer M, Bota S, Schwabl P, Bucsics T, Pfisterer N, Kruzik M, et al. Nonselective β blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology. 2014; 146: 1680-1690.
- Sersté T, Melot C, Francoz C, Durand F, Rautou PE, Valla D, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010; 52: 1017-1022.
- Pascal JP, Cales P. Propranolol in the preventation of first upper gastrointestinal tract hemorrhage in patients with cirrhosis of the liver and esophageal varices. N Engl J Med. 1987; 317: 856-861.
- Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: The st francis heart study randomized clinical trial. Am J Gastroenterol. 2011; 106: 71-77.
- Xue C, Gudkov A, Haber M, Norris MD. Small Molecule Drugs and Targeted Therapies for Neuroblastoma. Europe: INTECH; 2013; 300-324.
- Hoelder S, Clarke PA, Workman P. Discovery of small molecule cancer drugs: Successes, challenges and opportunities. Mol Oncol. 2012; 6: 155-176.
- Lakner AM. Hepaatic stellate cell transdiffrentiation: Pro-fibrogenic effectors. University of North Carolina at Charlotte. 2011.
- Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, et al. Ligands of peroxisome proliferator-activated receptor ϒ modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000; 119: 466-478.
- Corinaldi J, Nasrallah R, Clark J, Paris G, Miura P, Jasmin BJ, et al. Troglitazone induces extracellular matrix and cytoskeleton remodeling in mouse collecting duct cells. J Biomed Biotechnol. 2012; 2012: 1-10.
- Trivedi PJ, Hirschfield GM, Gershwin ME. Obeticholic acid for the treatment of primary biliary cirrhosis. Expert Rev Clin Pharmacol. 2016; 9: 13-26.
- Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004; 127: 1497-1512.
- Reese LJ, Tider DS, Stivala AC, Fishbein DA. Effects of angiotensin converting enzyme inhibitors on liver fibrosis in HIV and hepatitis c coinfection. AIDS Res Treat. 2012; 2012: 1-8.
- Kim MY, Baik SK, Park DH, Jang YO, Suk KT, Yea CJ, et al. Angiotensin receptor blockers are superior to angiotensin-converting enzyme inhibitors in the suppression of hepatic fibrosis in a bile duct-ligated rat model. J Gastroenterol. 2008; 43: 889-896.
- Basu PP, Aloysius MM, Shah NJ, Brown Jr RS. Review article: the endocannabinoid system in liver disease, a potential therapeutic target. Aliment Pharmacol Ther. 2014; 39: 790-801.
- Tam Joseph, Jie L, Resat C, George K. Endocannabinoids in Liver Disease. Hepatology. 2012; 53: 346-355.
- Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: A translational success story. Gut. 2015; 64: 830-841.
- Moghimi SM. Nanomedicine: current status and future prospects. FASEB J. 2005; 19: 311-330.
- Kumar Teli M, Mutalik S, Rajanikant GK. Nanotechnology and nanomedicine: Going small means aiming big. Curr Pharm Des. 2010; 16: 1882-1892.
- Poilil Surendran S, George Thomas R, Moon M-J, Jeong YY. Nanoparticles for the treatment of liver fibrosis. Int J Nanomedicine. 2017; 12: 6997-7006.
- Hendi A. Silver nanoparticles mediate differential responses in some of liver and kidney functions during skin wound healing. J King Saud Univ - Sci. 2011; 23: 47-52.
- Tomuleasa C, Soritau O, Orza A, Dudea M, Petrushev B, Mosteanu O, et al. Gold nanoparticles conjugated with cisplatin/doxorubicin/capecitabine lower the chemoresistance of hepatocellular carcinoma-derived cancer cells. J Gastrointest Liver Dis. 2012; 21: 188-196.
- Oró D, Yudina T, Fernández-Varo G, Casals E, Reichenbach V, Casals G, et al. Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J Hepatol. 2016; 64: 691-698.
- Kabir N, Ali H, Ateeq M, Bertino MF, Shah MR, Franzel L. Silymarin coated gold nanoparticles ameliorates CCl4-induced hepatic injury and cirrhosis through down regulation of hepatic stellate cells and attenuation of Kupffer cells. RSC Adv. 2014; 4: 9012-9020.
- Wang Z, Chang Z, Lu M, Shao D, Yue J, Yang D, et al. Janus Silver/Silica Nanoplatforms for Light-Activated Liver Cancer Chemo/Photothermal Therapy. ACS Appl Mater Interfaces. 2017; 9: 30306-30317.
- Bartneck M, Scheyda KM, Warzecha KT, Rizzo LY, Hittatiya K, Luedde T, et al. Fluorescent cell-traceable dexamethasone-loaded liposomes for the treatment of inflammatory liver diseases. Biomaterials. 2015; 37: 367-82.
- Zhang F, Kong D, Lu Y, Zheng S. Peroxisome proliferator-Activated receptor-γ as a therapeutic target for hepatic fibrosis: From bench to bedside. Cell Mol Life Sci. 2013; 70: 259-276.
- Kumar V. Polymeric nanocarriers for delivery of small molecules and miRNAs for the treatment of liver fibrosis and pancreatic cancer. University of Nebraska Medical Center. 2016.
- Lin TT, Gao DY, Liu YC, Sung YC, Wan D, Liu JY, et al. Development and characterization of sorafenib-loaded PLGA nanoparticles for the systemic treatment of liver fibrosis. J Control Release. 2016; 221: 62-70.
- Lam PL, Kok SHL, Gambari R, Kok TW, Leung HY, Choi KL, et al. Evaluation of berberine/bovine serum albumin nanoparticles for liver fibrosis therapy. Green Chem. 2015; 17: 1640-1646.
- Üner M, Yener G. Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspective. Int J Nanomedicine. 2007; 2: 289-300.
- Yang D, Gao YH, Tan KB, Zuo ZX, Yang WX, Hua X, et al. Inhibition of hepatic fibrosis with artificial microRNA using ultrasound and cationic liposome-bearing microbubbles. Gene Ther. 2013; 20: 1140-1148.
- Abe T, Masuda T, Satodate R. Phagocytic activity of Kupffer cells in splenectomized rats. Virchows Arch A Pathol Anat Histopathol. 1988; 413: 457-462.
- Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008; 25: 1815-1821.
- Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008; 3:145-150.
- Banquy X, Suarez F, Argaw A, Rabanel JM, Grutter P, Bouchard JF, et al. Effect of mechanical properties of hydrogel nanoparticles on macrophage cell uptake. Soft Matter. 2009; 5: 3984-3991.
- You JO, Auguste DT. Nanocarrier cross-linking density and pH sensitivity regulate intracellular gene transfer. Nano Lett. 2009; 9: 4467-4473.
- He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010; 31: 3657-3666.