
Research Article
Austin J Nanomed Nanotechnol. 2020; 8(1): 1057.
New Simultaneous Strategies for Pressure Sensitive Adhesion and Drug Delivery Action by Dynamic Chitosan-Snail Shell based Bionanocomposite Thin-Film Nanogel
Pradhan AK1,2, Sethy TR1, Biswalb SK2 and Sahoo PK1*
¹Department of Chemistry, Utkal University, India
²Department of Chemistry, Centurion University of Technology and Management, India
*Corresponding author: Prafulla K Sahooa, Department of Chemistry, Utkal University, Vani Vihar, Bhubaneswar 751004, India
Received: December 06, 2019; Accepted: January 02, 2020; Published: January 09, 2020
Graphical Abstract
Highlights
1. A novel multi-functionalized thin-film nanogel, TNG, has been prepared by emulsifier free emulsion graft copolymerization technique.
2. It has been characterized and evaluated by FTIR, FESEM, XRD, TGA and EWC.
3. The prepared TNG exhibit high porosity, super absorptivity and high biodegradability.
4. It has been significantly used as an excellent tool for pressure sensitive adhesion and transdermal drug delivery systems.
Abstract
A novel dynamic Chitosan (CS)-Snail shell based bionanocomposite Thin Film Nanogels (TNG)s were developed and optimized in order to obtain films possessing the optimal functional properties (flexibility, resistance, and bioadhesion) to be applied on skin. The functionalization of CS was designed 2-Hydroxyethyl Methacrylate (HEMA) and Acrylic Acid (AA) onto CS via redox initiator system of Ammonium Persulfate (APS) and complex catalyst CuSO4/ glycine (1:1) in the presence of foaming agent sorbitol was designed. The CS-g- P(HEMA-co-AA)/snail shell a novel thin film was prepared from the combination of CS-g-P (HEMA-co-AA) polymers with the modified snail shell, (nano-CaO) as a nanofiller, then the so prepared CS-g-P(HEMA-co-AA)/nano-CaO nanogel thin film is used to encapsulate active compounds in drug release systems. Characterization of TNGs was done by FT-IR, XRD, TGA, and FESEM. In addition to their in vitro drug release study in pH progressive media, TNGs are potentially suitable for use as Pressure-Sensitive Adhesives (PSAs) and super absorbents exhibiting good biodegradability.
Keywords: Chitosan; Nanogel; PSA; EWC; Biodegradability; Drug release
Introduction
Chitosan (CS) is a natural polysaccharide with good biomaterial properties being biocompatible, biodegradable, non-toxic, nonantigenic [1-3]. It is an ecologically interesting and promising carrier for sustained drug release [4]. All these important properties make chitosan a very interesting component of hydrogels in the medical and pharmaceutical fields. These have been applied for advanced drug delivery. Nanohydrogels are a three-dimensional swollen cross-linked network of polymer chains with particle sizes in the nanometer range [5,6]. However, nanogels with particle size between 10 and 200 nm are very efficient for showing adequate performance in intravenous drug delivery [7]. Nanohydrogels are widely categorized as chemically and physically cross-linked networks [8]. Chemically cross-linked nanohydrogels are produced by internal or external covalent cross-linking of the polymer chains through micro- or miniemulsion or from self-assembled nano aggregates [9]. The systemic drug administration through the skin holds several advantages such as the maintenance of constant drug levels in the blood, decrease of side effects and improvement of bioavailability by circumvention of hepatic first pass metabolism and increased patient compliance 10. In the present work, chitosan was selected as a starting material because of its good film-forming properties, wound-healing benefits, bacteriostatic effects and bioadhesive properties [11,12- 16]. Chitosan based thin film gel especially plays a very essential role in mucoadhesion (adhesion to the mucosal surface) or PSA. The chitosan based nanoparticle thin films are used to encapsulate active compounds in drug release systems.
The thin film is used as adsorbent surface for drug release. PSA, designed for enhanced transdermal delivery of drugs and being compatible with drugs of different physicochemical properties does not act as a barrier to drug diffusion and is non-toxic as well [17-19]. Nanogels have potential activity similar to the natural living tissue more than any other class of artificial biomaterials due to their high water content and soft stability comparable to natural tissue [20].The high water content and large pore sizes of the most hydrogels often result in comparatively fast drug release, over few hours to a few days. The surface modification of nano scale fillers plays a significant role in the preparation of nanocomposite based thin film nanogel especially for drug delivery.
Polymer-porous nano-CaO represents a new class of materials with high performance and is of great academic and industrial interest [21].
Rashid A et al., demonstrated drug delivery of 2-hydroxyethylmethacrylate-co-acrylic acid hydrogels for greater therapeutic efficiency in PSA [22]. However, our aim is to design a hydrophilic PSA to keep the hydrophilic nature of the skin delivery system because this type of adhesive offers several advantages over the hydrophobic ones: improved skin adhesion, compatibility with a higher variety of drugs and excipients, and expanded capability to control adhesion-cohesive properties [23].
Materials and Methods
Materials
Diclofenac sodium was purchased from Ranbaxylaboratories Ltd. The deacetylated chitosan and initiator, Ammonium Persulfate (APS), purchased from Himedia Mumbai, India. HEMA and AA ware SRL India, orthophosphoric acid, ethanol, and sorbitol were purchased from Qualigen India Ltd. Snail shells were collected from the rice field and washed with thrice with distilled water, oven dried.
Diclofenac sodium based TNG
TNG possesses a degree of flexibility very similar to natural tissue, due to their significant water content. Diclofenac 0.1-5% w/v concentrations of polymeric TNG dispersions were made separately.
Preparation of copolymer, Poly(HEMA-co-AA)
Desired quantity of monomers HEMA and AA, surfactant sorbitol, complex catalystCuSO4/glycine (1:1) and distilled water were taken in a reaction vessel. There action temperature was carried out at 55oC in the N2 atmosphere. Then initiator APS was added with continuous stirring. The reaction was ceased after 3 h by quenching the reaction vessel in ice water. The sample was coagulated with the non-solvent and then washed with hot water for three times and then oven dried at 65oC for 3 h. Then the copolymer [P(HEMA-co-AA)] was kept in the desiccator for 1 h and weighed.
Preparation of nano-CaO
The clean and dry rice field snail shell was cleaned from dirt and sticking flesh, then sun dried. The oven dried sample crushed and grinded by an attritor, then the powdered snail shell was treated with ortho phosphoric acid followed by heating in a furnace at 1000oC to produce the desired nano-CaO compound. After cooling, the sample was stored in desiccators [24,25].
Preparation of chitosan-g-Poly(HEMA-co-AA)copolymer thin filmgel (TG) and chitosan-g-Poly(HEMA-co-AA)/nano-CaO Thin Film Nanogel (TNG)
Desired quantity of chitosan (1.2g), monomers HEMA and AA and sorbitol as surfactant along with distilled water were taken in a reaction vessel and heated with a temperature maintained at 55oC in an inert atmosphere. Later the initiator APS along with complex catalyst CuSO4/glycine (1:1) in the presence of sorbitol was added with constant stirring. After 3 h the reaction was stopped by quenching the experimental set up in ice water slowly. Lastly, the synthesized sample was coagulated with the non-solvent followed by washing with hot water for three times and then dried in oven at 65oC for 4 h. Then the graft copolymer chitosan-g-[Poly(HEMA-co-AA)] was kept in the desiccator for 1 h and weighed.
For preparation of the grafted nanogel (TNG13 to TNG21), chitosan (1.2g), monomers HEMA, AA, initiator (APS) and desired quantity of CaO (nanoclay), surfactant sorbitol (0.05g), and complex catalyst were added sequentially to the reaction vessels. The reaction was carried out as per the method of the homopolymer and the copolymer mentioned earlier.
Calculation of Grafting Parameters
By using the following expression the grafting parameters calculation for the chitosan copolymers is given as follows:
Grafting yield (%) = [(wt. of the graft copolymer – wt. of chitosan)/ wt. of chitosan] x100 …(1)
Scheme of TNG
Scheme 1.
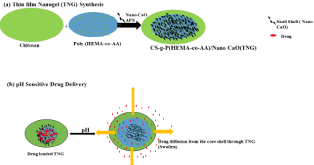
Scheme 1::
Characterization
The characterization of the pure chitosan, chitosan-copolymer and nano-CaO snail shell-based grafted bionanocomposite were carried out by FTIR spectra, XRD, FESEM and TGA etc.
Fourier Transform Infrared (FTIR) analysis was carried out for characterization of grafting of Poly(HEMA-co-AA) onto the chitosan and incorporation of nano-filler on Perkin Elmer Paragon 500 FT IR Spectrophotometer with KBr pellets in the optical range of 400-4000 cm-1.The dispersion of grafted copolymer into nano snail shell based thin film and drug incorporation in thin film was studied using the XRD with diffraction angle 2θ from10o to 90o on a Bruker D8 Discover (Germany) instrument at 25oC. A Field Emission Scanning Electron (FESEM) Microscope GEMINI®FE-SEM was used for investigation of the nanoscale pattern of the grafted samples in order to examine the surface morphology after gold coating and a low beam energy of 1 kV was operated to eradicate the possibility of any thermal damage to the prepared samples. Ultrathin section (the edge sample sheets perpendicular to the compression molds) of the grafted samples having a thickness of 100 nm was microtomed at -80oC. The Thermogravimetric Analysis (TGA) for measuring the thermal stability of the nano polymer thin films using a Shimadzu DTA-500 system was done in the inert N2-atmosphere from normal room temperature to 600oC at a 10oC/min heating rate.
Result and Discussion
FT IR Analysis of TNG
The graft binding of Poly(HEMA-co-AA ) and nanoCaO filler onto the surface of chitosan was investigated by the FTIR spectral analysis as shown in Figure 1. From the Figure. 1(E) of chitosan-g- Poly(HEMA-co-AA ) /nano-CaO segments, the ester (-CO-O-CH2-) gives C=O functional group band at 1745 cm-1,C-O binding site at 1185cm-1, NH-CH2grafting site at 1340cm-1 and Ca-O-Ca peak at 895.5 cm-1 respectively, so the intercalation of the snail shell (nano- CaO) layer into the copolymer matrix layer has been confirmed. A peak 1196.5 cm-1 for C-O stretching in the Figure 1(D) of the chitosan-copolymer indicates the spectra for amide I and amide II bands of chitosan -g- Poly(HEMA-co-AA) located at 1645 cm-1and 1593 cm-1respectively, the elongated variation of peak is due to the linkage of pure chitosan with the copolymer Poly(HEMA-co-AA ) and other interactions as well. In Figure 1(C) Spectra of the copolymer Poly(HEMA-co-AA ) shows the peak at 3150 cm-1 arising due to C-H stretching frequency and 1745 cm-1 due to ester group frequency. Hence, the shifting of the peak to lower frequency may be is assumed as the result of the hydrogen bonding formation between the –NH2 group of chitosan and the carbonyl group of the hanging bunch of the chitosan backbone. In Figure 1(C) Spectraof Poly(HEMA-co-AA) shows the peak at 3150 cm-1accounts for C-H stretching vibration and1745 cm-1for ester group. Figure 1(B) for Chitosan, it shows the peak at 3365 cm-1 which is due to the -NH2 group, 1645 cm-1 indicates the amide and 1010 cm-1 for the C-O-C linkage respectively. In Figure 1(A) for snail shell, the Ca-O bond resembles the 910 cm-1 as the stretching frequency and the bending frequency region of two frequency ranges such as 780cm-1, 460 cm-1 respectively.
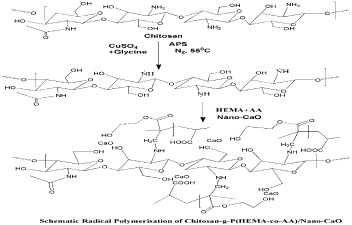
Figure 1: Fourier Transform Infrared spectra of (A) Snail shell, (B) Chitosan,
(C) P(HEMA-co-AA), (D) Chitosan-g-P(HEMA-co-AA), (E) Chitosan-g-
P(HEMA-co-AA)/nano-CaO TNG.
XRD spectra of TNG
The crystallinity of samples like nano-CaO, chitosan-g- Poly(HEMA-co-AA) and chitosan-g-Poly(HEMA-co-AA)/nano- CaO,chitosan-g-Poly(HEMA-co-AA)/nano-CaO TNG after drug delivery were investigated by XRD study. Crystallinity and the phases of the so formed powders were investigated by the powdered XRD and its Microstructures were also confirmed. In XRD analysisof the samples in the Figure 2(A) is the X-ray pattern of snail shell indicating sharp peaks due to its crystalline nature. The crystalline pattern at around 2θ=100and 200, indicates lower degree of crystallinity [26,27]. However, in Figure 2(B) chitosan-g-Poly(HEMA-co-AA), the copolymer shows a broader pattern due to gel nature of a novel material appearing as smooth broad curve. Non-appearance of sharp peak indicates its amorphous nature In Figure 2(C), the chitosan- g-Poly(HEMA-co-AA)/nano-CaO TNG confirms the intercalation of polymer matrix in the sheets of snail shell. Hence, it is proved that the grafted copolymer has been properly embedded inside the sheets of CaO matrix of the snail shell. In Figure 2(D), chitosan-g- Poly(HEMA-co-AA)/nano-CaO TNG after drug delivery, clearly indicated that the spherical porous material nano-CaO based TNG also adsorbed the drug.
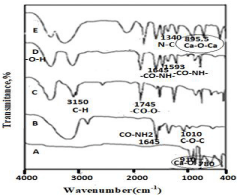
Figure 2: X-Ray Difraction of (A) Snail shell, (B) Copolymer, (C) TNG and (D)
TNG after drug delivery.
FESEM of TNG
The FESEM micrographs of the nano-CaO (I),the copolymer without nano-CaO (II) and the copolymer with nano-CaO TNG (III) and after biodegradation of Chitosan-g-P(HEMA-co-AA)/nano-CaO TNG (IV) are illustrated in Figure 3 at various magnifications like 2x102KX, 1μm 1x102KX respectively. The surface morphology graphs revealed the uniform intercalation of the synthesized copolymer into the layers of the snail shells as cleanly given in Figure 3 (E&F). The chitosan based TNG is used in biomedical application due to their microporosity. The surface modification by activated sludge water after biodegradation has been obtained as shown in Figure 3(G). It is due to the biodecomposition or the ample mass growth by the bacteria and fungi on the chitosan-g-Poly(HEMA-co-AA)/nano- CaO TNG which results in the roughness of the surface as compared to the plane surface that of before biodegradation (Figure 3). So, the synthesized unique TNG is environmental friendly in nature.
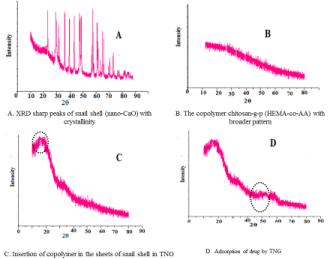
Figure 3: FESEM of (I) Nano-CaO (A&B), (II) Copolymer (C&D), (III) TNG
(E&F) and (IV) TNG after biodegradation TNG (G).
TGA of TNG
The thermal decay of (A) nano-CaO, (B) chitosan-g-Poly (HEMAco- AA), (C) chitosan-g-P(HEMA-co-AA)/nano-CaO TNG and (D) chitosan-g-Poly(HEMA-co-AA)/nano-CaO TNG after drug delivery were considered by the thermal analysis as given in Figure 4. The primary decay of both the samples occurs which is due to the existence of ample of moisture and amorphous nature of TNG material. The decay of the chitosan-g-Poly(HEMA-co-AA) copolymer at 120oC temperature and that of the chitosan-g-Poly(HEMA-co-AA)/nano- CaO(TNG) at 160oC is examined. It is due to the fact that the higher thermal decay of the chitosan-g-Poly(HEMA-co-AA)/nano-CaO TNG has been ascribed for the intercalation of the Poly(HEMAco- AA) through the sheets of the nano-CaO layer. It is a beneficial property for this TNG as it can persist the higher temperature because the CaO is thermally stable and highly resistant.
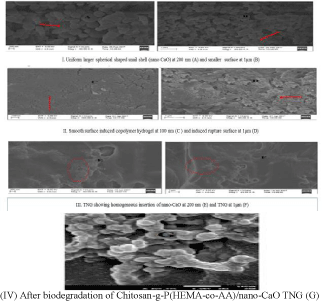
Figure 1: TGA thermograms of (A) Nano-CaO (B) Copolymer (C) TNG and
(D) TNG after drug delivery.
Equilibrium Water Content (EWC)
The crosslinked copolymer and the crosslinked TNG were dipped in separate pH buffers along the solutions at (i.e pH 3.5 to 11) loaded with different nano-CaO load (0.10,0.25, 0.5, 1.0, 1.25, 1.5 g) for 24h to reach equilibrium swelling. The samples which have got swollen were drawn from the buffers until more buffers onto the polymer matrix washed off through the filter papers, then weight of the samples were taken. Then the sample’s Equilibrium Water Content (EWC) was evaluated by the given following formula as under:
Equilibrium Water Content (EWC)(%) = [Ws – Wd/Ws ] × 100…(2) [28]
Where, Ws and Wd are the weights of the polymer sample at the equilibrium swelling and dry states accordingly. The equilibrium water content at various pH were examined in order to get the working efficiency of the TNG i.e., to evaluate the most selected hydrophilic absorption drug into the pendant copolymer graft. The sample swells off in buffer solutions and it is increased from pH 7.0 to 8.7 and then slows down as shown in Figure 5. Further, the measurement of EWC of TNG also indicates the swelling of the grafted samples is sensitive towards pH .i.e., the grafted TNG is less porous in nature at lower pH and it is maximum porous at pH 8.7. Here, the prepared TNG has shown better EWC than that of the copolymer TG. It is attributed due to the fact that the porosity of the sample is maximum at the load of 1gm nano-CaO and at a higher of pH = 8.7 (Figure 5). The water swell ability of the microsphere at pH 8.7 was better than those at pH 7 and pH 3.5. Thus, this enhancement is attributed due to the change of group from COOH in acidic condition (pH 3.5) to COO- group in alkaline condition (pH=8.7) of TNG [29,30] and also the ionic nature leads to increase in swelling of gel and drug release at higher pH 8.7 [31]. In addition, the protonated amino group changed to unionized amino group, which led to decrease in swelling of gel and drug release [32]. The higher crosslinking density of the TNG results less porosity, so it may get less space to absorb water in its template and at lower pH≤7.0 the strength of the internal gel structure may be very less to retain the water.
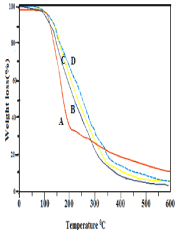
Figure 5: EWC study of chitosan-g-Poly(HEMA-co-AA)( TG) and chitosan-g-
P(HEMA-co-AA)/nano-CaO TNG ( TNG13toTNG18).
Biodegradation by activated sludge
Biodegradation of cross-linked copolymer hydrogel and crosslinked TNG were studied under sludge water in order to compare the extent of biodegradation at different conditions. The sludge water having various microbes those are responsible degradation the TNGs. A polypropylene container was used to collect the sludge in which sludge water was fully filled and then it was closed [33,34]. The samples (0.5g) were dipped in the sludge water and kept an in incubator together in a vessel sterilized well at room temperature (27±2oC) for 15, 45, 90 days.
It can see that the nano-CaO consisting TNGs showed better biodegradability those of the copolymer sample TGs. It may be due to the developed hydrophilicity and porosity in the snail shell based TNG that could enhance the water insertion along with various microbes into the biopolymer graft. It was further confirmed from the FESEM (Figure 3G) micrographs. Hence, the TNG in Figure 3(G) has given better biodegradability than the other TGs comparatively because of more hydrophilicity and massive colony growth of the microbes. From Figure 6, the TNG16 sample showed maximum degradation among the samples.
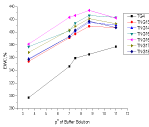
Figure 6: Biodegradation of chitosan-g-Poly(HEMA-co-AA)(TG4) and
chitosan-g-Poly(HEMA-co-AA)/nano-CaO TNG ( TNG13to TNG18).
Peel adhesion study
TNGs have applications as pressure sensitive tapes on aluminium, tin and skin as shown in (Table 2). It has good results for pressure sensitive adhesion to higher energetic surfaces like aluminium and skin. The surface adhesion is mainly due to its polar carbonyl group. On wet aluminium and tin surfaces the forces of adhesion is not same as that of the skin surface. There is no correlation between these three surfaces. It is because both the aluminium and tin surfaces are flat and rigid and thus the initial contact with such surfaces is very much unusual than that with skin, which is a soft tissue. The TNG16 sample shows good adhesion over skin than other monomer feed ratio TNGs. It is endorsed to the extra addition of the acrylic acid monomer and chitosan composition ratio attracting the hydrophilic nature of the Pressure sensitive adhesive films, so it can bind the skin more easily than other biopolymers. On contact with the skin, a better force of adhesion is produced at surface of the interface, which needs a greater tensile strength to cleavage the bond.
TABLECREATED
Table 1: Variation of [HEMA],[AA] in chitosan-g-Poly(HEMA-co-AA) copolymer thin film gel(TG) and chitosan-g-Poly(HEMA-co-AA)/nano-CaO thin film nanogel (TNG), their % grafting and % EWC at pH=8.
TABLECREATED
Table 2: Monomer composition ratio and corresponding adhesive forces at contact time 60s.
Evaluation of in vitro drug release by dynamic method
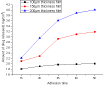
Separate three films of various thicknesses, i.e., 100μm, 150μm and 200μm (Figure 7) are prepared for 1.5w/w of drug, Diclofenac sodium, on 300μm thick skin between each application. Stabilizing adhesion methodology is being carried out with a period of 1 h. During adhesion, it is seen that the release of drug is stable for 100μm thick film, 1.8μg/cm2 to 1.92μg/cm2 for 150μm thick film, as shown in figure 7 the drug release is moderate, 2.1μg/cm2 to 3.1 μg/cm2 for 200μm thick film, the drug release study shows an interesting result, 2.2μg/cm2 to 4.12μg/cm2 i.e., large increase in drug release occurred during both the 2nd and 3rd hour and then a stable curve is obtained. It is attributed to the increase of film thickness, the reason for higher quantities of drug release.
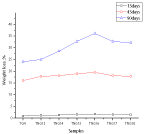
Figure 7: Drug release in the skin during adhesion with time intervals for TNG
of different thickness.
Graphical Abstract::
Conclusions
The chitosan-g-Poly(HEMA-co-AA)/nano-CaO of snail shell based TNGs were prepared with persulphate initiator under inert atmosphere and the samples were characterized by different instrumental techniques. Finally, the TNG being intelligently sensitive to pH might be considered as an excellent tool to design novel material in surgical implantation, spherical skin development culture and drug delivery. The spherical porous size exhibits maximum pressure sensitive adhesion and increases the water uptake in a polymeric nano matrix in thin film. Preliminary results of the synthesized TNGs are studied with a view to their novel applications as pressure sensitive adhesive and in drug delivery systems in future.
Acknowledgement
AKP and TRS highly acknowledge the funding assistance by CSIR & UGC, New Delhi, India respectively to carry out the research work.
References
- Peppas NA, Bures P, Leobandung W, Ichikawa HJ. Hydrogels in pharmaceutical formulations. Pharm. Biopharm. 2000; 50: 27-46.
- Berger J, Reist M, Mayer JM, Felt O, Gurny R, Euro J. Structure and interactions in chitosan hydrogels formed by complexation or aggregation for biomedical applications. Pharm. Biopharm. 2004; 57: 35-52.
- Berger J, Reist M, Mayer JM, Felt O, Gurny, Euro RJ. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Pharm. Biopharm. 2004; 57: 19-34.
- Lee JW, Kim SY, Kim SS, Lee YM, Lee KH, Kim SJJ. Synthesis and characteristics of interpenetrating polymer network hydrogel composed of chitosan and poly(acrylic acid). Appl. Polym.Sci. 1999; 73: 113-120.
- Merino S, Martín C, Kostarelos K, Prato M, Vazquez E. Nanocomposite Hydrogels: 3D Polymer-Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano. 2015; 9: 4686-4697.
- Goncalves M, Maciel D, Capelo D, Xiao S, Sun W, Shi X, et al. Dendrimerassisted formation of fluorescent nanogels for drug delivery and intracellular imaging. Biomacromolecules. 2014; 15: 492-499.
- Cheng Y, Hao J, Lee LA, Biewer MC, Wang Q, Stefan MC. Thermally controlled release of anticancer drug from self-assembled γ-substituted amphiphilic poly(e-caprolactone) micellar nanoparticles. Biomacromolecules. 2012; 13: 2163-2173.
- Oh JK, Drumright R, Polym MK. The development of microgels/nanogels for drug delivery applications. Sci. 2008; 33: 448-477.
- Jung Kwon. Biodegradable Nanogels Prepared by Atom Transfer Radical Polymerization as Potential Drug Delivery Carriers: Synthesis, Biodegradation, in Vitro Release, and Bioconjugation. J ACS. 2007; 129: 5939.
- Brown M, Martin G, Jones S, Akomeah F. Dermal and transdermal drug delivery systems: current and future prospects. Drug Delivery. 2006; 13: 175- 187.
- Denuziere A, Ferrier D, Damour O, Domard A. Chitosan-chondroitin sulfate and chitosan-hyaluronate polyelectrolyte complexes: biological properties. Biomaterials. 1998; 19: 1275-1285.
- Rossi S, Sandri G, Ferrari F, Bonferoni MC, Caramella C. Buccal delivery of acyclovir from films based on chitosan and polyacrylic acid. Pharm. Dev. Technol. 2003; 8: 199-208.
- Torre P, Enobakhare Y, Torrado G, Torrado S. Biomaterials. Release of amoxicillin from polyionic complexes of chitosan and poly(acrylic acid). Study of polymer/polymer and polymer/drug interactions within the network structure. 2003; 24: 1499-1506.
- Cerchiara T, Luppi B, Bigucci F, Orienti I, Zecchi VJ. Physically crosslinked chitosan hydrogels as topical vehicles for hydrophilic drugs. Pharm. Pharmacol. 2002; 54: 1453-1459.
- Yusof NL, Wee A, Lim LY, Khor EJ. Flexible chitin films as potential wounddressing materials: wound model studies. Biomed. Mater. Res. 2003; 66A: 224-232.
- Azad AK, Sermsintham N, Chandrkrachang S, Stevens WF. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2004; 69B: 216- 222.
- Feldstein MM, Raigorodskii IM, Iordanskii AL, Hadgraft J. Modeling of percutaneous drug transport in vitro using skin-imitating Carbosil membrane. J Control. Release. 1998; 52: 25-40.
- Iordanskii AL. Modeling of the drug delivery from a hydrophilic transdermal therapeutic system across polymer membrane. Eur J. Pharm. Biopharm. 2000; 49: 287-293.
- Feldstein MM, Tohmakhch VN, Malkhazov LB, Vasiliev AE, Plate NA. Hydrophilic polymeric matrices for enhanced transdermal drug delivery. Intl. J. Pharm. 1996; 131: 229-242.
- Ratner BD, Hoffman AS. Synthetic Hydrogels for Biomedical Applications. ACS Symposium series. 1976; 31: 1.
- Cho JW, Paul DR. Nylon 6 nanocomposites by melt compounding. Polymer. 2001; 42: 1083-1094.
- Ahmad A, Tulain U, Iqbal FM. Fabrication and evaluation of 2-hydroxyethyl methacrylate-co-acrylic acid hydrogels for sustained nicorandil delivery. Tropical J. Pharm. Research. 2015; 14: 1121.
- Chalykh AA, Chalykh AE, Novikov MB, Feldstein MM. Pressure-sensitive adhesion in the blends of poly(N-vinyl pyrrolidine) and poly(ethyleneglycol) of disparate chain lengths. J. Adhes. 2002; 78: 667-694.
- Cao YM, Sun J, Yu DH. Preparation and properties of nano-Al2O3 particles/ polyester/epoxy resin ternary composites. J. Appl. Polym. Sci. 2002; 83: 70- 77.
- Suparto IH, Putri DK. Synthesis of Hydroxyapatite from Rice Fields Snail Shell (Bellamya javanica) through Wet Method and Pore Modification Using Chitosan. Procedia Chemistry. 2015; 17: 27-35.
- Youling Y. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials. 2011; 4: 1399-1416.
- Harper EJ, Bonfield WJ. Tensile characteristics of ten commercial acrylic bone cements. Biomed. Mater. Res. (Appl.Biomater.) 2000; 53: 605-616.
- Ranjha MN, Mudassir JZ, Sheikj Z. Iran. Polym. J. 2011; 20: 147.
- Mourya VK, Inamdar NN, Tiwari A. Carboxymethyl chitosan and its applications. Adv. Mater. Lett. 2010; 1: 11-33.
- Tungtong S, Okonogi S, Chowwanapoonpohn S, Phutdhawong W, Yotsawimonwat S. Solubility, Viscosity and rheologial properties of watersoluble chitosan derivatives. Maejo Int. J. Sci. Tech. 2012; 6: 315-322.
- Esmaiel J, Samyra N. Swelling Behavior of Acrylic Acid Hydrogels Prepared by γ-Radiation Crosslinking of Polyacrylic Acid in Aqueous SolutionEuro. Polym. J. 2000; 36: 2685-2692.
- Rodkat N, Rutnakornpituk M. Multi-responsive magnetic microsphere of poly(N-isopropylacrylamide)/carboxymethylchitosan hydrogel for drug controlled release. Carbo. 2016; 151: 251-259.
- Federle TW, Barlaz MA, Pettigrew CA, Kerr JJ, Kemper BA, Nuck LA. Response to Statement by the Biodegradable Products Institute about the biodegradability paper. Biomacromolecules. 2002; 3: 813-822.
- Jena DK, Sahoo PK. Synthesis and study of mechanical and fire retardant properties of (carboxymethyl cellulose-g-polyacrylonitrile)/montmorillonite biodegradable nanocomposite. J. Appl. Polym. Sci. 2018; 135: 45968-45975.