Case Report
Austin J Nephrol Hypertens. 2014;1(1): 1003.
Rituximab in Granulomatosis with Polyangiitis: Challenging Equilibrium Between Therapeutic Efficacy and Iatrogenic Complications
Adele Mitrotti, Luigi Rossi, Francesca Indrio, Michele Rossini, Anna Maria Di Palma, Carlo Manno, Loreto Gesualdo and Vincenzo Montinaro*
Department of Nephrology, Dialysis and Transplant, University of Bari “Aldo Moro”, Italy
*Corresponding author: Vincenzo Montinaro, Department of Nephrology, Dialysis and Transplant, University of Bari “Aldo Moro”, Azienda Ospedaliero-Univesitaria Policlinico, Bari, Piazza G. Cesare, 11, 70124 Bari, Italy
Received: June 05, 2014; Accepted: June 30, 2014; Published: July 02, 2014
Abstract
Background: Granulomatosis with Polyangiitis is a renal-pulmonary syndrome that is treated with aggressive immunosuppressive therapy including high dose corticosteroids, cyclophosphamide, and plasmapheresis. Rituximab is also effective in inducing remission. Excess of immune suppressors may provoke generalized infections. Ultimate objective of therapy is to find equilibrium between the aggressive disease and avoidance of severe adverse effects of immune suppressors.
Case presentation: We report a patient affected by rapidly evolving granulomatosis with polyangiitis, characterized by dialysis-dependent acute renal failure. The initial therapy based on high dose corticosteroids, oral cyclophosphamide and plasmapheresis was ineffective, while two infusions of 1 g of rituximab 15 days apart were effective in inducing remission. A few days after the second rituximab infusion, the patient presented cardiac arrest and tonic-clonic seizures and was admitted to the ICU. Brain MRI showed a severe and diffuse leukoencephalopathy that indicated a severe prognosis.
A progressive multifocal leukoencephalopathy (PML) was excluded, since JC virus in liquor resulted negative. After a few days from the ICU admission, the patient showed a rapid clinical improvement, and was discharged a few weeks later with a mild residual renal insufficiency and complete disappearance of the neurological signs and normalization of the brain MRI pattern.
Conclusion: In this case, it is uncertain whether the neurological and cardiac complications were a consequence of rituximab infusion or represented an evolution of the vasculitis. Anti-CD20 mab can be very effective in inducing remission of granulomatosis with polyangiitis; however, the exact dose regimen and interval between administrations need to be established.
Keywords: Granulomatosis with polyangiitis; Rituximab; adverse effects
Introduction
Granulomatosis with polyangiitis (GPA) is an antineutrophil cytoplasm antibody (ANCA)-associated small-vessel vasculitis that provokes a life-threatening renal-pulmonary syndrome and systemic involvement [1,2]. Remission can be achieved in a high percentage of cases by an aggressive immune suppressive therapy that includes high dose intravenous methylprednisolone followed by oral prednisone or intravenous methylprednisolone associated to either oral or intermittent intravenous cyclophosphamide (CYP) [3]. Intermittent intravenous CYP has the same efficacy on induction of remission compared to daily oral CYP; still it allows administering a lower cumulative dose and is associated to the same rate of leukopenia or infections as the daily oral CYP. However, there is a trend of a higher relapse rate with intravenous CYP compared to oral CYP, although the difference does not seem to be significant [4]. Therefore, especially in the nephrological practice of treating GPA, there is more confidence with oral CYP.
However, CYP can be used for a limited time, owing to its high toxicity. Maintenance of remission is attained with low dose oralsteroids and eitherazathioprine or methotrexate for a prolonged time; mycophenolate mofetil has proved to be less effective than azathioprine [5]. Nevertheless, in certain very aggressive forms, the immune suppressors do not act rapidly enough to halt the evolution of vasculitis or in some cases the disease flares are frequent and cannot be managed with CYP [6]. For these reasons, rituximab has been recently indicated as a potential alternative to CYP for induction and maintenance of remission of GPA [7]. Excessive immune suppression may also provoke invasive and life-threatening localized or systemic infections. Therefore, management of GPA requires a careful choice of the therapeutic regimen in order to establish equilibrium between the immune suppression and avoidance of potentially fatal complications.
We describe herein a case of GPA with an aggressive clinical course that was treated initially by oral CYP and, due to the rapidly deteriorating clinical conditions, with rituximab subsequently. The anti-CD20 treatment rapidly changed the disease course and induced remission, but was associated to severe, although reversible, cardiac and neurologic complications.
Case History
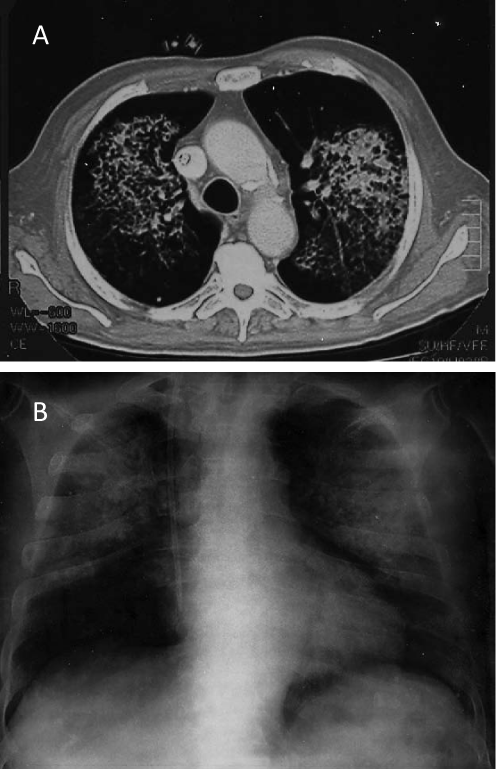
The patient was a 72 years old male, affected by hypertension that had underwent trans-ureteral resection of a bladder carcinoma 10 years before. In April 2013, a moderate renal insufficiency was detected (eGFR 44 ml/min/1.73 m2, according to CKD-EPI equation); other laboratory investigations were not performed. A month later, severe renal insufficiency supervened and was associated with fever, fatigue, dyspepsia, constipation and weight loss. Other relevant laboratory findings were anemia, hypoalbuminemia, proteinuria, HBs Ag positivity. The autoantibody evaluation by immunofluorescence assays showed positivity of c-ANCA, which was associated with anti-PR3 antibody to high titer by the ELISA assay (Table 1). The severe renal impairment required hemodialysis. After a week, renal biopsy was performed. Renal histopathologic alterations were characterized by diffuse extra capillary proliferation, crescents in various stages (cellular, fibrocellular and fibrous) and segmental fibrinoid necrosis of capillary loops. Interstitial lymphocytic and monocytic cell infiltrate was also present, associated to tubulitisand severe tubular damage. Finally, interstitial fibrosis was evident in 50% of the renal parenchyma. In consideration of the histological finding, an immunosuppressive therapy with high dose methylprednisolone bolus and oral CYP 100 mg per day was administered. Also, ten treatments of plasma exchange were performed. Given the positivity for HBs Ag, an antiviral treatment with Entecavir 0.5 mg every 72 hours was initiated. At this time point, chest CT showed bilateral diffuse parenchymal infiltrates in the upper lobes associated with calcified nodules in both lungs (Figure 1); clinically, the patient presented gross hematuria and hemoptysis; therefore, broad-spectrum antibiotics and antifungal therapy was initiated.
Figure 1: CT scan (A) and lain chest Rx (B) of the patient during the acute pulmonary vasculitic involvement. A wide pulmonary interstitial infiltrate especially in the superior lobes is evident by the two imaging techniques.
Parameter
Value
Reference range
Serum creatinine
709.0 mol/L
< 97.2
BUN
44.4mmol/L
3.33 - 8.34
Hemoglobin
87 g/L
125 – 145
Serum albumin
25 g/L
35 - 45
Proteinuria
1.5 g/24 h
< 0.2 g/24 h
c-ANCA
Positive
Negative
Anti-PR3Ab
110 I.U./ml
< 5
Table 1: Main laboratory parameters at the clinical onset.
The clinical conditions of the patient deteriorated rapidly, hypoxemia ensued and non-invasive ventilation (CPAP) was needed. After a week of therapy with CYP, steroids and broad-spectrum antibiotics, an additional evaluation with thoracic high resolution CT scan showed more severe lung impairment, characterized by new areas of parenchymal consolidation with widespread ground-glass infiltrates bilaterally. In consideration of the worsening and life-threatening clinical conditions a rescue therapy with monoclonal anti-CD-20 antibodies (rituximab) was deemed necessary. A dose of 1 g was administered and repeated after 15 days.
Following the first infusion of rituximab, the patient showed a steady improvement of clinical and radiologic conditions; also, indices of renal function ameliorated, and about twenty days after the onset, hemodialysis was discontinued.
Four days after the second rituximab infusion, the patient presented sudden cardio-respiratory arrest. The prompt cardio-pulmonary resuscitation was efficient, although a few minutes later, the patient presented tonic-clonic seizures with subsequent loss of consciousness and coma. The ECG performed after cardiac arrest showed a transiently delayed atrial-ventricular conduction and secondary repolarization abnormalities. This new condition prompted to invasive ventilatory support and admission to the ICU.
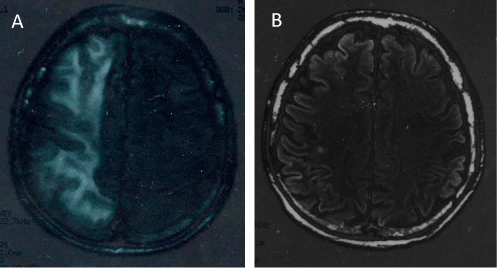
The brain MRI scans showed acute and sub-acute diffuse vascular ischemic lesions in the frontal-temporal-parietal-occipital regions of the right hemisphere (Figure 2A). Notwithstanding this MRI finding indicated a poor prognosis, the patient presented a progressive clinical improvement after 24 hours, with a regression of coma, and residual persistence of gait alterations. This condition resolved gradually in a few days. In order to exclude the unlikely possibility of a progressive multifocal leukoencephalopathy (PML), as early complication of the rituximab treatment, lumbar puncture was performed for the search of the JC virus in the cerebrospinal fluid, and the result was negative. Subsequent MRI controls showed a gradual and complete regression of the brain lesions (Figure 2B). An echocardiogram performed in the immediate post-critic period showed no alterations of the left ventricular contractibility, normal ejection fraction, and only minimal mitral and tricuspid regurgitation. After about two and a half months of hospitalization, the patient was discharged with complete regression of the pulmonary and cerebral findings. Renal function recovered with a residual moderate renal failure (eGFR 48 ml/min/1.73 m2) and mild proteinuria (0.8 g/day).
Figure 2: (A) Brain MRI gained in the immediate post-critic period showing a diffuse altered signal due to vascular-ischemic damage of the white matter in the right hemisphere. (B) MRI pattern obtained one month later that shows a complete resolution of the alterations in the white matter.
Discussion
We describe a case of GPA with a typical renal-pulmonary syndrome that evolved with neurological and cardiac involvement. The cardiorespiratory arrest associated to massive alterations of the white matter of the right hemisphere, occurred a few days after the second dose of rituximab. Anti-CD20 mab can induce acute coronary syndrome or rhythm disorders such as sinus tachycardia, atrial fibrillation and flutter or complete atrial-ventricular block during or in the immediately post-infusion period: these alterations are possibly provoked by the massive release of circulating cytokines and activation of the complement system [8-10]. ECG abnormalities suggested a possible damage from coronary vasospasm; although the second dose of rituximab was administered a few days before the cardiac arrest, a causative relationship cannot be excluded. Coronary angiogram in the present case was not performed because of the rapid amelioration of the clinical conditions and normalization of the ECG finding. Cardiac abnormalities in patients suffering from GPA have been described in 30-50% of autopsy case-series [11,12]. Heart alterations in GPA may involve conduction tissue, myocardium, pericardium, endocardium, and coronary arteries, and may occur late in the disease course even without pre-existing cardiac diseases or may also follow the improvement of the vasculitis [13,14]. Therefore, in this case the effect of rituximab could have summed up with potential pre-existing cardiac alterations caused by the vasculitis.
Concomitantly with the cardiac arrest, severe neurologic complications were recorded; MRI performed just after the cardiac arrest showed a pattern of vascular injury or PML, but the rapid improvement of the clinical conditions questioned the exact interpretation of the imaging findings.
A control MRI performed after ten days, showed a marked improvement of the damage; in the meantime the patient did not present further seizures and JC viral infection was excluded. Neurologic involvement in GPA is variable and represents generally a late event [15-19]. CNS alterations include hemorrhagic or ischemic lesions or, more rarely, leukoencephalopathy.
Progressive multifocal leukoencephalopathy is a rare complication of immune-suppressive or immune modulating therapeutic regimens and has been reported in concomitance of treatments with corticosteroids, tacrolimus, natalizumab, rituximab, efalizumab, adalimumab, infliximab, etanercept and ruxolitinib [20].
Therefore, we considered the possibility that the brain damage could be due to reversible changes in the white matter, characterized by cerebral edema due both to GPA and/or immunosuppressive therapy with rituximab. Reversible posterior leukoencephalopathy syndrome has been reported in GPA and SLE in dialysis-dependent patients. Putative pathogenic factors are endothelial dysfunction, hypertension and dysregulation of cerebral blood flow, probably consequent to the vasculitis and the concomitant immune-suppressive therapy [19,21]. Release of cytokines, activation of endothelial cells expressing CD20, in association with uremia-related factors may also contribute to the pathophysiology of this condition [21].
Rituximab is currently considered a second line treatment in GPA, due to its high cost and the lack of evidence of superiority in terms of efficacy compared to CYP [22]. In some conditions, however, characterized by an aggressive course of the vasculitis, some adverse effects of rituximab may synergize with pathologic and pathophysiologic alterations provoked by the vasculitis in target organs not typically involved in the systemic vasculitis syndrome.
A final important consideration is about the therapeutic regimens of rituximab administration. In this case, the dramatic worsening of respiratory function forced us to use a dose of 1 gram of rituximab and the second dose after fifteen days, rather than the more used four doses of 375 mg/m2 at intervals of one week [23,24]. The therapeutic results, in terms of improvement of lung and kidney function were impressive, even though it is no possible to exclude that the occurrence of cardiac and neurological involvement was, at least in part, caused by the drug or to its mode of administration. More studies in order to define the better therapeutic tailoring of this drug are required.
Consent
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
References
- Fauci AS, Wolff SM. Wegener's granulomatosis: studies in eighteen patients and a review of the literature. 1973. Medicine (Baltimore). 1994; 73: 315-324.
- Jennette JC, Falk RJ. Antineutrophil cytoplasmic autoantibodies and associated diseases: a review. Am J Kidney Dis. 1990; 15: 517-529.
- Langford CA. Cyclophosphamide as induction therapy for Wegener's granulomatosis and microscopic polyangiitis. Clin Exp Immunol. 2011; 164 Suppl 1: 31-34.
- de Groot K, Harper L, Jayne DR, Flores Suarez LF, Gregorini G, Gross WL, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009; 150: 670-680.
- Hiemstra TF, Walsh M, Mahr A, Savage CO, de Groot K, Harper L, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized controlled trial. JAMA. 2010; 304: 2381-2388.
- Miloslavsky EM, Specks U, Merkel PA, Seo P, Spiera R, Langford CA, et al. Clinical outcomes of remission induction therapy for severe antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2013; 65: 2441-2449.
- Cartin-Ceba R, Golbin JM, Keogh KA, Peikert T, Sánchez-Menéndez M, Ytterberg SR, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener's): ten-year experience at a single center. Arthritis Rheum. 2012; 64: 3770-3778.
- van der Kolk LE, Grillo-López AJ, Baars JW, Hack CE, van Oers MH. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001; 115: 807-811.
- Cervera Grau JM, Esquerdo Galiana G, Belso Candela A, Llorca Ferrándiz C, Juárez Marroquí A, Maciá Escalante S, et al. Complete atrioventricular block induced by rituximab in monotherapy in an aged patient with non-Hodgkin's diffuse large B-cell lymphoma. Clin Transl Oncol. 2008; 10: 298-299.
- Lee L, Kukreti V. Rituximab-induced coronary vasospasm. Case Rep Hematol. 2012; 2012: 984986.
- Knockaert DC. Cardiac involvement in systemic inflammatory diseases. Eur Heart J. 2007; 28: 1797-1804.
- To A, De Zoysa J, Christiansen JP. Cardiomyopathy associated with Wegener's granulomatosis. Heart. 2007; 93: 984.
- Korantzopoulos P, Papaioannides D, Siogas K. The heart in Wegener's granulomatosis. Cardiology. 2004; 102: 7-10.
- Salazar-Exaire D, Ramos-Gordillo M, Vela-Ojeda J, Salazar-Cabrera CE, Sanchez-Uribe M, Calleja-Romero MC. Silent ischemic heart disease in a patient with necrotizing glomerulonephritis due to Wegener's granulomatosis. Cardiorenal Med. 2012; 2: 218–224
- Drachman DA. Neurologic involvement in Wegener's granulomatosis. Arch Neurol. 1963; 8: 145–155.
- Siva A. Vasculitis of the nervous system. J Neurol. 2001; 248: 451-468.
- Weinberger LM, Cohen ML, Remler BF, Naheedy MH, Leigh RJ. Intracranial Wegener's granulomatosis. Neurology. 1993; 43: 1831-1834.
- Kraemer M, Berlit P. Systemic, secondary and infectious causes for cerebral vasculitis: clinical experience with 16 new European cases. Rheumatol Int. 2010; 30: 1471-1476.
- Ohta T, Sakano T, Shiotsu M, Furue T, Ohtani H, Kinoshita Y, et al. Reversible posterior leukoencephalopathy in a patient with Wegener granulomatosis. Pediatr Nephrol. 2004; 19: 442-444.
- Lima MA. Progressive multifocal leukoencephalopathy: new concepts. Arq Neuropsiquiatr. 2013; 71: 699-702.
- Mavragani CP, Vlachoyiannopoulos PG, Kosmas N, Boletis I, Tzioufas AG, Voulgarelis M, et al. A case of reversible posterior leucoencephalopathy syndrome after rituximab infusion. Rheumatology (Oxford). 2004; 43: 1450-1451.
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidneyinter. Suppl. 2012; 2: 139–274.
- Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010; 363: 221-232.
- Charles P, Néel A, Tieulié N, Hot A, Pugnet G, Decaux O, et al. Rituximab for induction and maintenance treatment of ANCA-associated vasculitides: a multicentre retrospective study on 80 patients. Rheumatology (Oxford). 2014; 53: 532-539.