Review Article
Austin J Nephrol Hypertens. 2014;1(1): 1005.
Pathogenesis of Acute Renal Failure Induced by Iodinated Radiographic Contrast Media
Michele Andreucci1*, Teresa Faga1, Antonio Pisani2, Massimo Sabbatini2 and Ashour Michael1
1Department of “Health Sciences”, Magna Graecia University, Italy
2Department of Nephrology, Federico II University, Italy
*Corresponding author: Michele Andreucci, Nephrology Unit, Department of “Health Sciences”, Campus Salvatore Venuta, “Magna Graecia” University, Viale Europa, loc. Germaneto, I-88100 Catanzaro, Italy
Received: June 10, 2014; Accepted: July 07, 2014; Published: July 09, 2014
Abstract
The use of iodinated radiographic contrast media, to improve the visibility of internal organs and structures in X-ray based imaging techniques, can cause Acute Renal Failure, commonly called Contrast-induced Nephropathy (CIN). The pathogenetic mechanisms responsible for contrast media nephrotoxicity have not been completely elucidated; knowing them, however, is very important to prevent CIN. All pathogenetic factors that have been suggested by many authors are discussed in this review, including haemodynamic changes, formation of reactive oxygen species (ROS), role of Nitric Oxide (NO), the role of adenosine and endothelin and cytotoxicity of contrast media, and the intracellular Ca2+ overload. Clinical conditions favouring the occurrence of CIN are also mentioned, including dehydration, salt depletion, reduction of ‘effective’ circulating blood volume, pre-existing chronic renal failure, and diabetes mellitus.
Keywords: Contrast-Induced acute kidney injury; Contrast-Induced nephropathy; Acute renal failure; Radiographic contrast media; Iodinated contrast material; Kidney; Tubule; Renal cell; Kinase; Reactive oxidative species; Cell death
Abbreviations
AKI: Acute Kidney Injury; ARF: Acute Renal Failure; eGFR: Estimated Glomerular Filtration Rate; CIN: Contrast-induced Nephropathy; SCr; Serum Creatinine; CrCl: Creatinine Clearance; RBF: Renal Blood Flow; CT: Computed Tomography; MDRD: Modification of Diet in Renal Disease; NO: Nitric Oxide; ROS: Reactive Oxygen Species; CRF: Chronic Renal Failure; LOCM: Low-osmolar Contrast Media; HOCM: High-osmolar Contrast Media; IOCM: Iso-osmolar Contrast Media
Introduction
The intravenous or intra-arterial injection of iodinated radiographic contrast media is performed to improve the visibility of internal organs and structures in X-ray based imaging techniques, such as radiography and computed tomography. This procedure, however, may cause impairment of renal function. We define Contrast-induced Nephropathy (CIN) or contrast-induced Acute Kidney Injury (AKI) as an Acute Renal Failure (ARF) occurring 24 to 72 hours after the intravascular injection of radiographic contrast media that cannot be explained by other causes. It is usually a nonoliguric, asymptomatic and transient decline in renal function. The impairment of renal function is mirrored by an increase of serum creatinine (SCr) by 0.5 mg/dl (or more) or by a 25% (or more) increase in SCr from baseline. SCr reaches the peak value on the third to fifth day and returns to baseline within 10–14 days. But the values of SCr vary with age, muscle mass and sex. Thus, it is always better to consider the creatinine clearance (CrCl) as derived from SCr, 24- hour urine volume and urinary concentration of creatinine [1]. But measurement of CrCl is cumbersome, impractical and inaccurate. It is better to calculate CrCl from SCr, age, body weight, and gender using either the MDRD (Modification of Diet in Renal Disease) calculation [2] or the simple Cockcroft-Gault formula: (140 - number years of age) x Kg body weight/ 72 / mg/dL of SCr; in females the result is multiplied by 0.85 [3]. This is called the estimated glomerular filtration rate (eGFR). Thus, CIN is a decrease of eGFR to 30-60 mL/ min - renal insufficiency or less. In some cases, CIN is a severe ARF with oliguria (<400 mL/24 hrs), requiring dialysis. In these patients the mortality is high [4].
The clinical features and the management of CIN is the same as that for ARF due to other causes [1,5,6].
CIN seems not to occur when renal function is normal. However, the clinical necessity for diagnostic procedures using contrast media has been increasing especially in patients with cardiovascular diseases, whose renal function is frequently impaired, hence leading to a more frequent occurrence of CIN in clinical practice.
The pathogenetic mechanisms responsible for contrast media nephrotoxicity are not completely known. Many factors have been implicated. Hereafter, we will examine all the pathogenetic factors that have been suggested by many authors in the literature.
Haemodynamic changes by contrast media
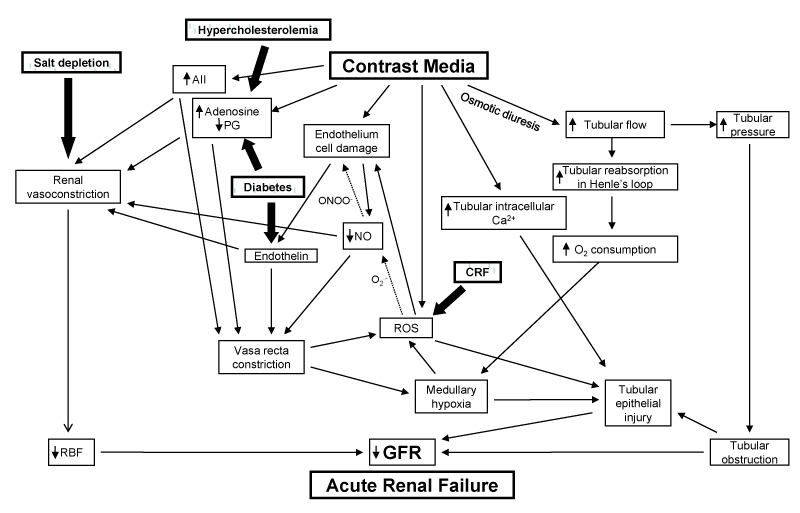
When iodinated radiographic contrast media are injected intravenously or intra-arterially, they immediately cause a haemodynamic renal biphasic response: there is an early, rapid renal vasodilatation with an initial increase in renal blood flow (RBF) that is then followed by a more prolonged vasoconstriction with an increase in intrarenal vascular resistances and a reduction in total RBF (decrease of filtration fraction). The biphasic renal blood flow response to contrast media does not occur during volume depletion; in volume depletion there is only a severe vasoconstriction [7]. The extrarenal vessels show transient vasoconstriction followed by a stable decrease in vascular peripheral resistances [8,9] (Figure).
Figure : The complex mechanisms by which radiographic contrast media cause Acute Renal Failure (CIN). Various clinical conditions, such as salt depletion, chronic renal failure (CRF), diabetes mellitus and hypercholesterolemia may aggravate the pathogenetic factors responsible for Contrast-induced ARF.
The fall in total RBF will cause per se a decrease in the glomerular filtration rate (GFR). But these haemodynamic changes will cause a renal ischaemia, which is particularly severe in the renal medulla because of its peculiar structure. Under normal physiological conditions, in fact, oxygen delivery to the outer renal medulla is poor because of its distance from the descending vasa recta; in contrast with the limited regional oxygen supply, there is a high local oxygen consumption due to the important tubular reabsorption in S3 segments of proximal renal tubules of the outer medulla and in the medullary thick ascending limbs of Henle’s loop. Prostaglandins, nitric oxide (NO), and adenosine continuously adjust medullary tubular transport activity to the limited available oxygen supply, by enhancing the regional blood flow and down regulating the tubular transport [10]. Defects in one or more of these protective mechanisms will cause medullary hypoxia. The haemodynamic changes induced by contrast media will make medullary hypoxia quite severe (Figure).
The effects of radiographic contrast media on vessels have been studied in vitro by Sendeski et al [11] to evaluate whether the contrast media modify outer medullary descending vasa recta vasoreactivity and NO production. Specimens of outer medullary descending vasa recta were isolated from rats and microperfused intraluminally with a buffered solution containing the nonionic, isosmolar (approximately 290mOsm/kg) contrast medium iodixanol (Visipaque). The authors used an iodine concentration of 23 mg/mL to simulate the dosage utilized in examinations in humans. They demonstrated that iodixanol directly constricts the descending vasa recta (causing a 52% reduction of their luminal diameter) by reducing NO, and significantly increases the vasoconstrictor response to angiotensin II. The result was a severe local hypoxia. Their conclusion was that iodixanol, in doses typically used in clinical practice for coronary interventions, constricts medullary descending vasa recta, intensifies angiotensin II induced vasoconstriction, and reduces the bioavailability of NO.
As mentioned, the ascending limb of Henle’s loop is located in the renal medulla; in this tubular segment, even in normal conditions, there is a high O2 demand due to its active ion transport. Since radiographic contrast media induce an osmotic diuresis and consequently an increase in tubular reabsorption in the Henle’s loop, the consequent increased energy need and the high O2 consumption of the ascending limb will worsen the already hypoxic environment in the renal medulla [1, 12] (Figure).
Arakawa et al [13] have suggested that adenosine plays an important role in contrast-induced deterioration of renal function. They demonstrated that in dogs with normal renal function, the non-ionic contrast medium iohexol (Omnipaque 300) elicits renal vasodilatation by activating mainly the adenosine A2 receptors with an increase in RBF, whereas in dogs with impaired renal function iohexol induces both A2 and A1 activation, the former associated with the initial renal vasodilatation, the latter responsible for the sustained vasoconstriction with aggravation of renal hemodynamics [13]. They proposed that adenosine receptor antagonists (theophylline, aminophylline) could have protective effects against contrast media under such conditions. In fact, they observed, in dogs with renal insufficiency, that the contrast medium-induced renal deterioration was prevented both by a non-selective antagonist theophylline and by a selective A1 receptor antagonist KW-3902. Unfortunately, the use of the non-selective adenosine receptor antagonist’s theophylline or aminophylline in humans has given controversial results: some authors, in fact, have observed beneficial effects [14-19] others have denied any beneficial results [20,21]. Arakawa et al [13] concluded that the contrast-induced vasoconstriction is mediated by adenosine A1 receptors, whereas the activation of adenosine A2 receptors is responsible for the contrast-induced renal vasodilatation.
The intrarenal production of the vasodilators NO and prostaglandins is responsible for the maintenance of perfusion and oxygen supply in the renal medulla; therefore, reductions in the availability of these mediators, as induced by contrast media, will cause medullary hypoxia.
The formation of reactive oxygen species (ROS) and the crucial role of the fall of NO
Medullary hypoxia may lead to the formation of reactive oxygen species (ROS) [22,23] that may exert direct tubular and vascular endothelial injury and might further intensify renal parenchymal hypoxia by virtue of endothelial dysfunction and dysregulation of tubular transport [24,25] (Figure).
The decrease in NO is believed to be due to its reaction with ROS in particular superoxide [26,27]. This reaction may lead to the formation of the more powerful oxidant peroxynitrite [28] that may be more detrimental to the endothelial cells. As shown in the Figure, the dotted arrows indicate the reaction of the ROS (superoxide anions: O2.-) with NO that not only causes a reduction in NO levels, but also leads to the formation of peroxynitrite anion (ONOO-), a potent oxidant that causes cell injury.
Even under physiological conditions, tubular transport is associated with ROS formation, mostly in the renal medullary thick ascending limb of Henle’s loop, where the dense mitochondrial population represents a major source for generation of superoxide anions (O2-), and hydroxyl radicals (OH-) by NAD(P)H-oxidase [24,27]. The administration of radiographic contrast media augments ROS production and renal oxidative stress which, in turn, mediate the damage to cell membranes leading to cellular apoptosis and necrosis, particularly in medullary thick ascending limbs and in S3 segments of proximal renal tubules of the outer medulla [27].
Patients with chronic renal failure (CRF) have defective antioxidant systems [29] and increased oxidative stress associated with inflammation and endothelial dysfunction [30]. This may explain why pre-existing renal failure certainly represents the most common condition predisposing to the development of CIN.
Thus, animal and human studies have clearly demonstrated that ROS generation is enhanced following contrast administration, suggesting their important role in the pathogenesis of CIN [27].
Myers et al [31] have carried out in vivo experiments in rats, demonstrating that the decrease in cortical and medullary microvascular blood flow induced by contrast media is partly accounted for by the downregulation of endogenous renal cortical and medullary NO synthesis. Sendeski et al [11] have demonstrated that the superoxide dismutase mimetic Tempol reduced iodixanol-induced vasoconstriction, thereby supporting the role of ROS generated during contrast media administration in medullary descending vasa recta vasoconstriction. More recently Pisani et al. [32] have demonstrated that a recombinant manganese superoxide dismutase administered in vivo to rats undergoing diatrizoate treatment was able to reduce renal oxidative stress, thereby preventing the reduction of GFR and the renal histologic damage that follows contrast media administration.
Cytotoxicity of contrast media
Iodinated radiographic contrast media also possess direct cytotoxic properties, as observed on both endothelial and renal tubular cells that lead to apoptosis and cell death. The endothelial cells are the first to come in contact with intravascular injection of contrast agents. Endothelial damage, including nuclear protrusion, cell shrinkage, fenestration of the endothelial layer and formation of microvilli (‘blebbing’) on the cell membrane, and cellular apoptosis have been observed by scanning electron microscopy [33]. Thus, the decrease in NO in the vasa recta is due not only to increased ROS production, but also to the damaged endothelial cells (including apoptosis) [26].
The damaged endothelial cell may also release endothelin that causes vasoconstriction. Heyman et al [34] have in fact demonstrated that the i.v. administration of contrast media in rats induced an increase in plasma concentration of endothelin; furthermore, contrast media stimulated endothelin release from cultured bovine endothelial cells. These results suggest a direct effect of ionic and nonionic contrast agents on vascular endothelium to release endothelin.
In addition to endothelial damage, iodinated radiographic contrast media cause damage also to the epithelial tubular cells [35]. The contrast media are filtered by glomeruli and are concentrated in the renal tubules, thereby exposing the renal tubular cells to even worse direct damage. Direct tubular epithelial cell toxicity by contrast media has been observed in studies of isolated tubule segments and cultured cells substantiated by disruption of cell integrity and apoptosis [36,37].
The biochemical changes underlying the epithelial damage have been extended to study changes in major intracellular signalling pathways involved in cell survival, death and inflammation [23,38- 45] in vitro in cultured renal tubular cells [46]. Recent studies have clarified these aspects in primary human tubular cells as well as in HK-2 cells exposed to different contrast media. Andreucci et al [44] demonstrated a decreased cell viability, secondary to a reduced activation of Akt and of ERK 1/2, both kinases known to play a pivotal role in cell survival/proliferation, which was substantially alleviated by transfecting the HK-2 cells with a constitutively active form of Akt. The same authors have demonstrated, in HK-2 cells, that contrast media affect the activation/deactivation of transcription factors, like FoxO3a and STAT3, that control the genes involved in apoptosis and cell proliferation [38,42].
In vivo animal studies as well as in vitro studies suggest that iodinated contrast media can directly induce caspase-mediated apoptosis of renal tubular cells [47]. Contrast-induced apoptosis may also be due to the activation of shock proteins and the concurrent inhibition of cytoprotective enzymes and prostaglandins [48,49].
The tubular cell damage may be aggravated by factors such as renal hypoperfusion and hypoxia, by properties of contrast media, such as ionic strength, high osmolarity and/or viscosity, and by clinically unfavourable conditions, such as pre-existing renal impairment particularly if secondary to diabetes [1,12].
Intracellular Ca2+ overload
Under physiological conditions, the Na+/Ca2+ exchanger (NCX) can pump the Ca2+ outside the renal tubular epithelial cells using the Na+ concentration gradient across the cell membrane to keep a low intracellular Ca2+ level. In pathological conditions, such as CIN, NCX can reversely extrude Na+ for Ca2+ influx and result in intracellular Ca2+ overload. Intracellular Ca2+ overload is considered to be a key factor in ischemic cell injury and CIN [50,51].
The osmotic diuresis caused by the contrast media
Radiographic contrast media have different osmolalities (Table). Thus, ionic High-Osmolar Contrast Media (HOCM, e.g. diatrizoate) have an osmolality of 1500 to 1800 mOsm/kg, i.e. 5–8 times the osmolality of plasma; non-ionic Low-Osmolar Contrast Media (LOCM e.g. iohexol) have an osmolality of 600 to 850 mOsm/kg, i.e. 2–3 times the osmolality of plasma; non-ionic Iso-Osmolar Contrast Media (IOCM e.g. iodixanol) have an osmolality of approximately 290 mOsm/kg, i.e. same osmolality as plasma [12,52].
Name
Type
Iodine content (mg/mL)
mOsm/kg
Osmolality type
Ionic
Diatrizoate (Hypaque 50)
Monomer
300
1,550
HOCM
Metrizoate Isopaque (Conray 370)
Monomer
370
2,100
HOCM
Ioxaglate (Hexabrix)
Dimer
320
580
LOCM
Nonionic
Iopamidol (Isovist-370)
Monomer
370
796
LOCM
Iohexol (Omnipaque 350)
Monomer
350
884
LOCM
Iodixanol (Visipaque 320)
Dimer
320
290
IOCM
Table : Iodinated Contrast Media Commonly Used in Clinical Practice.
It has been observed that the use of LOCM rather than HOCM is beneficial in the prevention of CIN in patients with pre-existing CRF [53-56]. Furthermore, iodixanol (IOCM) seems less nephrotoxic than iohexol (LOCM), at least in patients subjected to intra-arterial administration of the drug and having renal insufficiency [57,58]. However, recent studies and meta-analyses have found no significant difference in the rates of CIN between IOCM and LOCM [57-62].
In addition to the osmolality of iodinated contrast media, their viscosity is very important. The low osmolality achieved with the IOCM has come at the price of considerably increased viscosity; at comparable iodine concentrations and x-ray attenuation, the non-ionic dimeric IOCM have about twice the viscosity of non-ionic monomeric LOCM [63-65].
Since most of the water filtered by the glomerulus is reabsorbed along the renal tubule, the concentration of the contrast medium increases considerably within the tubular lumen. The result will be a progressive increase in tubular fluid osmolality and, due to the exponential concentration-viscosity relationship, an overproportional increase in tubular fluid viscosity as well as in the urine viscosity [12,63]. Since the fluid flow rate through a tube increases with the pressure gradient and decreases with the flow resistance and since the resistance increases proportionally to fluid viscosity, the increased viscosity caused by a contrast medium increases the intratubular pressure [63]. Thus, the osmotic diuresis caused by the contrast media raises the intratubular pressure with a condition of tubular obstruction that contributes to the tubular epithelial damage [12].
Clinical conditions favouring the occurrence of CIN
Some of the mentioned pathogenetic factors may be aggravated by various clinical conditions. Thus, dehydration, particularly in the elderly due to impaired sensation of thirst [66], and salt depletion following abnormal gastrointestinal, renal or dermal fluid losses associated with insufficient salt intake and reduction of ‘effective’ circulating blood volume aggravate renal vasoconstriction thereby predisposing to ARF [67]. The ‘effective’ circulating blood volume may be defined as the relative fullness of the arterial tree as determined by cardiac output, peripheral vascular resistance and total blood volume [5]. A reduction of ‘effective’ circulating blood volume may be due to congestive heart failure, compromised left ventricle systolic performance, prolonged hypotension or liver cirrhosis or nephrotic syndrome. Under such circumstances renal vasoconstriction is accentuated thereby making renal ischemia more severe [12].
Patients with pre-existing CRF have increased oxidative stress [29, 30], thereby predisposing to the development of CIN (Figure).
The biologically active endothelins, produced by proteolysis of the precursor prepro-endothelins under the action of endothelin-converting enzyme, are increased in circulating blood of diabetics [68]. In diabetic patients there is also a hypersensitivity of renal vessels to adenosine [69]. These factors may justify the predisposition of diabetics to the development of CIN [12] (Figure).
The renal hemodynamic changes induced by radiocontrast media are due to alteration of vasodilator and vasoconstrictor influences, mediated by local nitric oxide, prostaglandin, adenosine and endothelin systems within the kidney [70]. Evidence exists indicating that hypercholesterolemia impairs endothelium-dependent vasorelaxation [71-74]. It has been demonstrated that hypercholesterolemia makes the kidney vulnerable to iodinated contrast media by inducing disorders in intrarenal prostaglandins and renal nitric oxide system [74,75] (Figure) leading to the suggestion for use of statins as a protective measure against CIN [76-81].
References
- Andreucci M, Solomon R, Tasanarong A. Side Effects of Radiographic Contrast Media: Pathogenesis, Risk Factors, and Prevention. Biomed Res Int. 2014; 2014: 741018.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Annals of internal medicine 1999; 130: 461-470.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976; 16: 31-41.
- Andreucci M, Faga T, Sabbatini M, Pisani A, Russo D, Michael A: How to prevent Contrast-Induced Nephropathy in clinical practice. Journal of Clinical Nephrology and Research. 2014, [in press].
- Andreucci VE, Fuiano G, Russo D, Andreucci M. Vasomotor nephropathy in the elderly. Nephrol Dial Transplant. 1998; 13 Suppl 7: 17-24.
- Andreucci VE, Fuiano G, Stanziale P, Andreucci M. Role of renal biopsy in the diagnosis and prognosis of acute renal failure. Kidney Int Suppl. 1998; 66: S91-95.
- Katzberg RW, Morris TW, Schulman G, Faillace RT, Boylan LM, Foley MJ, et al. Reactions to intravenous contrast media. Part I: Severe and fatal cardiovascular reactions in a canine dehydration model. Radiology. 1983; 147: 327-330.
- Murphy SW, Barrett BJ, Parfrey PS. Contrast nephropathy. J Am Soc Nephrol. 2000; 11: 177-182.
- Detrenis S, Meschi M, Musini S, Savazzi G. Lights and shadows on the pathogenesis of contrast-induced nephropathy: state of the art. Nephrol Dial Transplant. 2005; 20: 1542-1550.
- Heyman SN, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of radiocontrast nephropathy. Clin J Am Soc Nephrol. 2008; 3: 288-296.
- Sendeski M, Patzak A, Pallone TL, Cao C, Persson AE, Persson PB, et al. Iodixanol, constriction of medullary descending vasa recta, and risk for contrast medium-induced nephropathy. Radiology. 2009; 251: 697-704.
- Andreucci M, Faga T, Pisani A, Sabbatini M, Michael A: Acute Kidney Injury by Radiographic Contrast Media: Pathogenesis and Prevention. BioMed Research Int 2014, [in press].
- Arakawa K, Suzuki H, Naitoh M, Matsumoto A, Hayashi K, Matsuda H. Role of adenosine in the renal responses to contrast medium. Kidney Int. 1996; 49: 1199-1206.
- Erley CM, Duda SH, Schlepckow S, Koehler J, Huppert PE, Strohmaier WL, et al. Adenosine antagonist theophylline prevents the reduction of glomerular filtration rate after contrast media application. Kidney Int. 1994; 45: 1425-1431.
- Erley CM, Duda SH, Rehfuss D, Scholtes B, Bock J, Müller C, et al. Prevention of radiocontrast-media-induced nephropathy in patients with pre-existing renal insufficiency by hydration in combination with the adenosine antagonist theophylline. Nephrol Dial Transplant. 1999; 14: 1146-1149.
- Katholi RE, Taylor GJ, McCann WP, Woods WT Jr, Womack KA, McCoy CD, et al. Nephrotoxicity from contrast media: attenuation with theophylline. Radiology. 1995; 195: 17-22.
- Huber W, Ilgmann K, Page M, Hennig M, Schweigart U, Jeschke B, et al. Effect of theophylline on contrast material-nephropathy in patients with chronic renal insufficiency: controlled, randomized, double-blinded study. Radiology. 2002; 223: 772-779.
- Deray G, Martinez F, Cacoub P, Baumelou B, Baumelou A, Jacobs C, et al. A role for adenosine calcium and ischemia in radiocontrast-induced intrarenal vasoconstriction. Am J Nephrol. 1990; 10: 316-322.
- Arend LJ, Bakris GL, Burnett JC Jr, Megerian C, Spielman WS. Role for intrarenal adenosine in the renal hemodynamic response to contrast media. J Lab Clin Med. 1987; 110: 406-411.
- Abizaid AS, Clark CE, Mintz GS, Dosa S, Popma JJ, Pichard AD, et al. Effects of dopamine and aminophylline on contrast-induced acute renal failure after coronary angioplasty in patients with preexisting renal insufficiency. Am J Cardiol. 1999; 83: 260-263, A265.
- Shammas NW, Kapalis MJ, Harris M, McKinney D, Coyne EP. Aminophylline does not protect against radiocontrast nephropathy in patients undergoing percutaneous angiographic procedures. J Invasive Cardiol. 2001; 13: 738-740.
- Giaccia AJ, Simon MC, Johnson R. The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004; 18: 2183-2194.
- Sabbatini M, Santillo M, Pisani A, Paterno R, Uccello F, Seru R, et al. Inhibition of Ras/ERK1/2 signaling protects against postischemic renal injury. American journal of physiology Renal physiology. 2006; 290: 1408-1415.
- Heyman SN, Rosen S, Khamaisi M, Idée JM, Rosenberger C. Reactive oxygen species and the pathogenesis of radiocontrast-induced nephropathy. Invest Radiol. 2010; 45: 188-195.
- Dawson P, Harrison MJ, Weisblatt E. Effect of contrast media on red cell filtrability and morphology. Br J Radiol. 1983; 56: 707-710.
- Sendeski MM. Pathophysiology of renal tissue damage by iodinated contrast media. Clin Exp Pharmacol Physiol. 2011; 38: 292-299.
- Pisani A, Riccio E, Andreucci M, Faga T, Ashour M, Di Nuzzi A, et al. Role of reactive oxygen species in pathogenesis of radiocontrast-induced nephropathy. Biomed Res Int. 2013; 2013: 868321.
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007; 87: 315-424.
- Martín-Mateo MC, Sánchez-Portugal M, Iglesias S, de Paula A, Bustamante J. Oxidative stress in chronic renal failure. Ren Fail. 1999; 21: 155-167.
- Okamura DM, Pennathur S, Pasichnyk K, López-Guisa JM, Collins S, Febbraio M, et al. CD36 regulates oxidative stress and inflammation in hypercholesterolemic CKD. J Am Soc Nephrol. 2009; 20: 495-505.
- Myers SI, Wang L, Liu F, Bartula LL. Iodinated contrast induced renal vasoconstriction is due in part to the downregulation of renal cortical and medullary nitric oxide synthesis. J Vasc Surg. 2006; 44: 383-391.
- Pisani A, Sabbatini M, Riccio E, Rossano R, Andreucci M, Capasso C, et al. Effect of a recombinant manganese superoxide dismutase on prevention of contrast-induced acute kidney injury. Clin Exp Nephrol. 2014; 18: 424-431.
- Gospos C, Freudenberg N, Staubesand J, Mathias K, Papacharlampos X. The effect of contrast media on the aortic endothelium of rats. Radiology. 1983; 147: 685-688.
- Heyman SN, Clark BA, Kaiser N, Spokes K, Rosen S, Brezis M, et al. Radiocontrast agents induce endothelin release in vivo and in vitro. J Am Soc Nephrol. 1992; 3: 58-65.
- Caiazza A, Russo L, Sabbatini M, Russo D. Hemodynamic and tubular changes induced by contrast media. Biomed Res Int. 2014; 2014: 578974.
- Hardiek K, Katholi RE, Ramkumar V, Deitrick C. Proximal tubule cell response to radiographic contrast media. Am J Physiol Renal Physiol. 2001; 280: F61-70.
- Heinrich MC, Kuhlmann MK, Grgic A, Heckmann M, Kramann B, Uder M. Cytotoxic effects of ionic high-osmolar, nonionic monomeric and nonionic iso-osmolar dimeric iodinated contrast media on renal tubular cells in vitro. Radiology. 2005; 235: 843-849.
- Andreucci M, Faga T, Russo D, Bertucci B, Tamburrini O, Pisani A, et al. Differential activation of signaling pathways by low-osmolar and iso-osmolar radiocontrast agents in human renal tubular cells. J Cell Biochem. 2014; 115: 281-289.
- Michael A, Faga T, Pisani A, Riccio E, Bramanti P, Sabbatini M, et al. Molecular mechanisms of renal cellular nephrotoxicity due to radiocontrast media. Biomed Res Int. 2014; 2014: 249810.
- Andreucci M, Michael A, Kramers C, Park KM, Chen A, Matthaeus T. Renal ischemia/reperfusion and ATP depletion/repletion in LLC-PK (1) cells result in phosphorylation of FKHR and FKHRL1. Kidney Int. 2003; 64: 1189-1198.
- Andreucci M, Fuiano G, Presta P, Lucisano G, Leone F, Fuiano L, et al. Downregulation of cell survival signalling pathways and increased cell damage in hydrogen peroxide-treated human renal proximal tubular cells by alpha-erythropoietin. Cell Prolif. 2009; 42: 554-561.
- Andreucci M, Lucisano G, Faga T, Bertucci B, Tamburrini O, Pisani A, et al. Differential activation of signaling pathways involved in cell death, survival and inflammation by radiocontrast media in human renal proximal tubular cells. Toxicol Sci. 2011; 119: 408-416.
- Andreucci M [Contrast media and nephrotoxicity: a molecular conundrum]. G Ital Nefrol. 2011; 28: 355.
- Andreucci M, Fuiano G, Presta P, Esposito P, Faga T, Bisesti V, et al. Radiocontrast media cause dephosphorylation of Akt and downstream signaling targets in human renal proximal tubular cells. Biochem Pharmacol. 2006; 72: 1334-1342.
- Quintavalle C, Brenca M, De Micco F, Fiore D, Romano S, Romano MF, et al. In vivo and in vitro assessment of pathways involved in contrast media-induced renal cells apoptosis. Cell Death Dis. 2011; 2: e155.
- Andreucci M, Faga T, Lucisano G, Uccello F, Pisani A, Memoli B, et al. Mycophenolic acid inhibits the phosphorylation of NF-kappaB and JNKs and causes a decrease in IL-8 release in H2O2-treated human renal proximal tubular cells. Chem Biol Interact. 2010; 185: 253-262.
- Lee HC, Chang JG, Yen HW, Liu IH, Lai WT, Sheu SH. Ionic contrast media induced more apoptosis in diabetic kidney than nonionic contrast media. J Nephrol. 2011; 24: 376-380.
- Cunha MA, Schor N. Effects of gentamicin, lipopolysaccharide, and contrast media on immortalized proximal tubular cells. Ren Fail. 2002; 24: 687-690.
- Peer A, Averbukh Z, Berman S, Modai D, Averbukh M, Weissgarten J, et al. Contrast media augmented apoptosis of cultured renal mesangial, tubular, epithelial, endothelial, and hepatic cells. Invest Radiol. 2003; 38: 177-182.
- Yang D, Yang D, Jia R, Tan J. Na+/Ca2+ exchange inhibitor, KB-R7943, attenuates contrast-induced acute kidney injury. J Nephrol. 2013; 26: 877-885.
- Duan SB, Liu FY, Luo JA, Wu HW, Liu RH, Peng YM, et al. Nephrotoxicity of high- and low-osmolar contrast media. The protective role of amlodipine in a rat model. Acta Radiol. 2000; 41: 503-507.
- Katzberg RW. Urography into the 21st century: new contrast media, renal handling, imaging characteristics, and nephrotoxicity. Radiology. 1997; 204: 297-312.
- Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ. Nephrotoxicity in High-Risk Patients Study of Iso-Osmolar and Low-Osmolar Non-Ionic Contrast Media Study Investigators. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003; 348: 491-499.
- Taliercio CP, Vlietstra RE, Ilstrup DM, Burnett JC, Menke KK, Stensrud SL, et al. A randomized comparison of the nephrotoxicity of iopamidol and diatrizoate in high risk patients undergoing cardiac angiography. Journal of the American College of Cardiology. 1991; 17: 384-390.
- Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology. 1993; 188: 171-178.
- Barrett BJ. Contrast nephrotoxicity. J Am Soc Nephrol. 1994; 5: 125-137.
- Dong M, Jiao Z, Liu T, Guo F, Li G. Effect of administration route on the renal safety of contrast agents: a meta-analysis of randomized controlled trials. J Nephrol. 2012; 25: 290-301.
- Heinrich MC, Häberle L, Müller V, Bautz W, Uder M. Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: meta-analysis of randomized controlled trials. Radiology. 2009; 250: 68-86.
- Kuhn MJ, Chen N, Sahani DV, Reimer D, van Beek EJ, Heiken JP, et al. The PREDICT study: a randomized double-blind comparison of contrast-induced nephropathy after low- or isoosmolar contrast agent exposure. AJR Am J Roentgenol. 2008; 191: 151-157.
- Solomon RJ, Natarajan MK, Doucet S, Sharma SK, Staniloae CS, Katholi RE, et al. Cardiac Angiography in Renally Impaired Patients (CARE) study: a randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation. 2007; 115: 3189-3196.
- Reed M, Meier P, Tamhane UU, Welch KB, Moscucci M, Gurm HS, et al. The relative renal safety of iodixanol compared with low-osmolar contrast media: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2009; 2: 645-654.
- Bolognese L, Falsini G, Schwenke C, Grotti S, Limbruno U, Liistro F, et al. Impact of iso-osmolar versus low-osmolar contrast agents on contrast-induced nephropathy and tissue reperfusion in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention (from the Contrast Media and Nephrotoxicity Following Primary Angioplasty for Acute Myocardial Infarction [CONTRAST-AMI] Trial). Am J Cardiol. 2012; 109: 67-74.
- Seeliger E, Lenhard DC, Persson PB. Contrast media viscosity versus osmolality in kidney injury: lessons from animal studies. Biomed Res Int. 2014; 2014: 358136.
- Jost G, Pietsch H, Sommer J, Sandner P, Lengsfeld P, Seidensticker P. Retention of iodine and expression of biomarkers for renal damage in the kidney after application of iodinated contrast media in rats. Invest Radiol. 2009; 44: 114-123.
- Dyvik K, Dyrstad K, Tronstad A. Relationship between viscosity and determined injection pressure in angiography catheters for common roentgen contrast media. Acta Radiol Suppl. 1995; 399: 43-49.
- Andreucci VE, Russo D, Cianciaruso B, Andreucci M. Some sodium, potassium and water changes in the elderly and their treatment. Nephrol Dial Transplant. 1996; 11 Suppl 9: 9-17.
- Andreucci M, Federico S, Andreucci VE. Edema and acute renal failure. Semin Nephrol. 2001; 21: 251-256.
- Khamaisi M, Raz I, Shilo V, Shina A, Rosenberger C, Dahan R, et al. Diabetes and radiocontrast media increase endothelin converting enzyme-1 in the kidney. Kidney Int. 2008; 74: 91-100.
- Pflueger A, Larson TS, Nath KA, King BF, Gross JM, Knox FG, et al. Role of adenosine in contrast media-induced acute renal failure in diabetes mellitus. Mayo Clin Proc. 2000; 75: 1275-1283.
- Morcos SK, Epstein FH, Haylor J, Dobrota M. Aspects of contrast media nephrotoxicity. Eur J Radiol. 1996; 23: 178-184.
- Chowienczyk PJ, Watts GF, Cockcroft JR, Ritter JM. Impaired endothelium-dependent vasodilation of forearm resistance vessels in hypercholesterolaemia. Lancet. 1992; 340: 1430-1432.
- Creager MA, Cooke JP, Mendelsohn ME, Gallagher SJ, Coleman SM, Loscalzo J, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990; 86: 228-234.
- Drexler H, Zeiher AM, Meinzer K, Just H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by L-arginine. Lancet. 1991; 338: 1546-1550.
- Andrade L, Campos SB, Seguro AC. Hypercholesterolemia aggravates radiocontrast nephrotoxicity: protective role of L-arginine. Kidney Int. 1998; 53: 1736-1742.
- Yang D, Lin S, Yang D, Wei L, Shang W. Effects of short- and long-term hypercholesterolemia on contrast-induced acute kidney injury. Am J Nephrol. 2012; 35: 80-89.
- Andreucci M. [Statins in CIN: a problem at least partly solved?]. G Ital Nefrol. 2013; 30.
- Sabbatini M, Pisani A, Uccello F, Serio V, Serù R, Paternò R. Atorvastatin improves the course of ischemic acute renal failure in aging rats. J Am Soc Nephrol. 2004; 15: 901-909.
- Khanal S, Attallah N, Smith DE, Kline-Rogers E, Share D, O'Donnell MJ, et al . Statin therapy reduces contrast-induced nephropathy: an analysis of contemporary percutaneous interventions. Am J Med. 2005; 118: 843-849.
- Patti G, Nusca A, Chello M, Pasceri V, D'Ambrosio A, Vetrovec GW, et al. Usefulness of statin pretreatment to prevent contrast-induced nephropathy and to improve long-term outcome in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2008; 101: 279-285.
- Zhang BC, Li WM, Xu YW. High-dose statin pretreatment for the prevention of contrast-induced nephropathy: a meta-analysis. Can J Cardiol. 2011; 27: 851-858.
- Leoncini M, Toso A, Maioli M, Tropeano F, Bellandi F. Statin treatment before percutaneous cononary intervention. J Thorac Dis. 2013; 5: 335-342.